Back to Journals » Infection and Drug Resistance » Volume 11
Carbapenem-resistant and cephalosporin-susceptible Pseudomonas aeruginosa: a notable phenotype in patients with bacteremia
Authors Li S , Jia X , Li C, Zou H, Liu H, Guo Y , Zhang L
Received 21 May 2018
Accepted for publication 6 July 2018
Published 20 August 2018 Volume 2018:11 Pages 1225—1235
DOI https://doi.org/10.2147/IDR.S174876
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Joachim Wink
Shuang Li,1,* Xiaojiong Jia,1,* Congya Li,1 Hua Zou,1 Hang Liu,1 Yuanbiao Guo,2 Liping Zhang1
1Department of Laboratory Medicine, The First Affiliated Hospital of Chongqing Medical University, Chongqing, China; 2Medical Research Center, The Second Chengdu Hospital Affiliated to Chongqing Medical University, Chengdu, China
*These authors contributed equally to this work
Purpose: Pseudomonas aeruginosa is recognized as a major cause of severe and potentially life-threatening infection. However, P. aeruginosa isolates with the phenotype of being carbapenem resistant and cephalosporin susceptible (Carb-R/Ceph-S) have not been thoroughly characterized to date. The aim of this study was to assess the mechanisms, risk factors, and clinical impact of Carb-R/Ceph-S P. aeruginosa bacteremia on mortality.
Patients and methods: We conducted a retrospective case–case–control study of the risk factors and clinical outcomes of hospitalized patients with Carb-R/Ceph-S P. aeruginosa bacteremia from 2011 to 2017 in Chongqing, China. Case patients infected with Carb-R/Ceph-S P. aeruginosa, carbapenem-susceptible and cephalosporin-susceptible (Carb-S/Ceph-S) P. aeruginosa, and controls with no P. aeruginosa bacteremia were compared at a ratio of 1:1:2. Real-time reverse transcription polymerase chain reaction was performed to assess resistance mechanisms. A multivariate logistic regression model was performed to investigate several potential predictors for mortality.
Results: We collected 63 Carb-R/Ceph-S P. aeruginosa isolates during the study period. None of these isolates possessed carbapenemase or extended-spectrum β-lactamase-encoding genes. The overall 30-day mortality rate was 27.0%. Real-time reverse transcription polymerase chain reaction analysis showed that an overexpression of efflux systems and decreased expression of OprD were associated with Carb-R/Ceph-S P. aeruginosa. Multivariate analysis indicated that 30-day readmission, central venous catheters, and exposure to carbapenems were unique independent predictors for acquiring Carb-R/Ceph-S P. aeruginosa bacteremia. Additionally, hematologic malignancy was a peculiar predictor for Carb-S/Ceph-S P. aeruginosa bacteremia. Notably, total parenteral nutrition was the only common factor of both Carb-R/Ceph-S and Carb-S/Ceph-S groups compared to controls. In a multivariate analysis for the outcome, intensive care unit admission and septic shock were identified as the independent predictors for mortality.
Conclusion: Our findings can potentially improve the ability of physicians to identify the high-risk patients, and carbapenems were noted to potentially increase the risk of Carb-R/Ceph-S P. aeruginosa. Additionally, cephalosporin should be considered a valuable therapeutic option for such cases of bacteremia.
Keywords: Pseudomonas aeruginosa, carbapenem resistance, bacteremia, risk factor
Introduction
Among the nosocomial pathogens, Pseudomonas aeruginosa, a gram-negative non-fermenting bacillus, is particularly worrisome because of its intrinsic resistance against multiple antimicrobial agents which reduces treatment options.1,2 Carbapenems are widely used as first-line drugs to treat nosocomial infections and are effective against multidrug-resistant P. aeruginosa and other bacterial infections producing the cephalosporinase AmpC or extended-spectrum β-lactamases.3 Nevertheless, carbapenem-resistant P. aeruginosa (CRPA) isolates are increasingly observed, probably due to the global clinical use of carbapenem.4–6 In 2017, surveys reported by the World Health Organization on multi-country antibiotic resistance revealed that CRPA is regarded as the second ranked critical-priority bacteria among 20 bacterial species with 25 patterns of acquired resistance.7 CRPA strains often exhibit cross-resistance to other antibiotics such as cephalosporins, quinolones, and aminoglycosides, which are partially responsible for the multidrug resistance characteristics.8 Therefore, physicians have to make more difficult decisions for empirically treating the patients infected with CRPA. Of note, we observed some clinical P. aeruginosa isolates that exhibited a phenotype of antibiotic resistance: resistance to carbapenems, but susceptibility to broad-spectrum cephalosporins (Carb-R/Ceph-S). In these cases, cephalosporins could be used as an alternative drug to treat these patients, especially in some countries (including China) where polymyxins are not commercially available. In addition, polymyxins have a narrow therapeutic window, and major adverse effects related to their parenteral use are neurotoxicity and nephrotoxicity. To date, only two previous studies have focused on the resistance mechanisms of Carb-R/Ceph-S P. aeruginosa infections.9,10 However, the clinical significance of this phenomenon is currently unknown. Data regarding the identification of patients with Carb-R/Ceph-S P. aeruginosa bacteremia may assist clinicians in deciding upon treatments. To the best of our knowledge, this retrospective analysis is the first comprehensive study to evaluate the mechanisms, risk factors, and clinical outcomes for nosocomial patients with Carb-R/Ceph-S P. aeruginosa bacteremia. It should be noted that we used the case–case–control study design to explore the factors associated with Carb-R/Ceph-S P. aeruginosa bacteremia, which could be an effective and more accurate method to reduce the biases caused by comparing traditional resistant and susceptible isolates. Moreover, predictors for mortality in patients with Carb-R/Ceph-S P. aeruginosa infections were also identified in our study.
Patients and methods
Study setting and study design
This retrospective case–case–control study was conducted in a 3,200-bed tertiary university-affiliated hospital located in Southwest China. We searched the clinical microbiology database of our hospital to identify the cases with the following characteristics: P. aeruginosa bacteremia diagnosed between January 2011 and December 2017; only the first P. aeruginosa bacteremia episode was considered in our study; if bacteremia occurred >14 days after the first positive blood culture, it was considered as a new episode; patients with polymicrobial bacteremia were excluded; and patients in all groups admitted for <48 hours and duplicate isolates were also excluded.
The three study groups were defined as follows: the first case group consisted of patients with Carb-R/Ceph-S P. aeruginosa bacteremia during hospitalization (case I group); the second case group consisted of patients with a positive blood culture for Carb-S/Ceph-S P. aeruginosa during hospitalization (case II group); and the control group consisted of randomly selected patients hospitalized during the same period of time (at a 2:1 ratio to the case group) with non-P. aeruginosa bloodstream infections.
In our study, we conducted a three-part analysis: 1) a retrospective case–case–control study in which the Carb-R/Ceph-S and the Carb-S/Ceph-S groups were compared with the control group (no P. aeruginosa bacteremia) to evaluate the risk factors for the isolation of Carb-R/Ceph-S and Carb-S/Ceph-S P. aeruginosa; 2) a retrospective cohort study in which the Carb-R/Ceph-S group and the Carb-S/Ceph-S group were compared to provide the potential predictors specifically associated with the acquisition of Carb-R/Ceph-S P. aeruginosa bacteremia; and 3) a retrospective study to determine the 30-day mortality associated with the isolation of Carb-R/Ceph-S P. aeruginosa bacteremia.
Isolates identification and antimicrobial susceptibility testing
Blood cultures were processed in the clinical microbiology laboratory at our hospital using an automated blood culture system (BACTEC 860 system; Becton Dickinson Diagnostic Instrument Systems, Sparks, MD, USA). P. aeruginosa was identified using the VITEK 2 Compact system or the VITEK MS system (bioMérieux, Marcy l’Etoile, France). Antimicrobial susceptibilities were determined in vitro using a VITEK 2 Compact AST-GN09 card (bioMérieux). All the carbapenem-resistant (resistant to at least one carbapenem drug, including imipenem and meropenem) and cephalosporin-susceptible (susceptible to both ceftazidime and cefepime) isolates were confirmed manually by the standard broth microdilution method. P. aeruginosa ATCC 27853 was used as a quality control strain during the antimicrobial susceptibility testing, and the results were interpreted using criteria based on the Clinical and Laboratory Standards Institute guidelines.11
Molecular characterization and resistance mechanisms
Polymerase chain reaction analyses for the presence of carbapenemase-encoding genes (blaKPC, blaVIM, blaIMP, blaNDM, and blaOXA-48-like) and β-lactamase-encoding genes (blaCTX-M, blaTEM, and blaSHV) was performed by using primers and conditions described previously.12,13 In addition, the resistance mechanisms were analyzed with four representative Carb-R/Ceph-S P. aeruginosa isolates by real-time reverse transcription polymerase chain reaction, as described in a previous study.10
Data collection
Patient demographics and clinical data were obtained from their electronic medical records and clinical microbiology laboratory databases. Cases and controls were compared using the following parameters as potential risk factors: 1) demographics (age and sex); 2) hospitalization characteristics (surgical ward, transfer from another hospital, 30-day readmission, and intensive care unit [ICU] admission); 3) primary source of infection (urinary tract infection, respiratory tract infection, intra-abdominal infection, and unknown source), underlying diseases, and primary admission diagnosis (diabetes, hypertension, lung disease, heart disease, hepatobiliary disease, gastrointestinal disease, renal disease, and malignancy); 4) previous surgery or other invasive devices before a positive culture (mechanical ventilation, total parenteral nutrition, urinary catheter, drainage tube, nasogastric tube, tracheal intubation, central venous catheter, and blood product transfusion); 5) the recent receipt of antibiotics (patients received antibiotics within 3 months before a first positive culture was obtained) and antifungal agents (including fluconazole, voriconazole, itraconazole, amphotericin B, and flucytosine); and 6) patient outcomes (length of stay before a positive culture, total length of hospital stay, 30-day mortality, and functional status deterioration).
Definitions
Carb-R/Ceph-S P. aeruginosa bacteremia was defined by the blood culture positivity for a P. aeruginosa strain that was resistant to at least one carbapenem (imipenem and/or meropenem), but susceptible to broad-spectrum cephalosporins (both ceftazidime and cefepime). Septic shock was defined as sepsis associated with organ dysfunction, accompanied by persistent hypotension following volume replacement.14 Severe anemia was defined as a hemoglobin level <60 g/L. Hypoproteinemia was defined as serum total protein or albumin levels of <60 and <25 g/L, respectively. Hypokalemia was defined as a serum potassium level <3.5 mmol/L. Neutropenia was defined as an absolute neutrophil count of <500 cells/µL. Corticosteroid use was defined as administration of >20 mg/day of prednisone or methylprednisolone for at least 4 weeks. The source of the bacteremia was determined on the basis of the isolation of P. aeruginosa from the presumed portal of entry and clinical evaluation. The primary site of bacteremia was classified into two categories: high-risk source (respiratory tract infection, intra-abdominal infection, and unknown origin) and low-risk source (urinary tract infection).15Adequate treatment was considered when an antimicrobial regimen that included an active antimicrobial was administered within 24 hours after the blood culture samples were obtained; otherwise, if the antimicrobials used empirically were not sensitive, it was considered as inadequate treatment. In addition, the patients who had not received antimicrobial therapy (either empirical or definitive) were also classified into inadequate treatment.
Statistical analysis
All analyses were performed using SPSS statistical software (version 22.0; IBM Corporation, Armonk, NY, USA). Categorical variables were presented as frequencies and percentages and were compared using a McNemar test. Continuous variables were presented as medians and interquartile ranges and were compared using Student’s t-test (normally distributed variables) and Wilcoxon rank sum test (non-normally distributed variables). Odds ratios (ORs) and 95% CIs were calculated to evaluate the strength of any association. To eliminate confounding variables in predicting the risk factors for developing Carb-R/Ceph-S P. aeruginosa bacteremia, all variables with P<0.1 by univariate analysis were enrolled into a multivariate logistic regression model for further assessment. Survival curves were constructed using the Kaplan–Meier method, and the log-rank test was used for comparisons between deceased and survived groups. All P-values were two-tailed, and P<0.05 was considered significant.
Ethical considerations
The data and the samples analyzed in the present study were obtained in accordance with the standards and approval of the Chongqing Medical University Institutional Review Board and Biomedical Ethics Committee. The ethics committee waived the need for written informed consent provided by participants due to the retrospective nature of the study. Because all patient data were analyzed in anonymity, no additional informed consent was required.
Results
Patient characteristics
During the study period, 203 episodes of P. aeruginosa nosocomial bloodstream infections occurred in 180 patients. Sixty-three patients were identified to have Carb-R/Ceph-S P. aeruginosa bacteremia (case I group), who were matched to 63 patients with Carb-S/Ceph-S P. aeruginosa bacteremia (case II group). In addition, 126 control patients with bloodstream infections other than P. aeruginosa were randomly matched to each of the case I and case II groups at a 2:1 ratio. Thus, 252 patients were available for evaluation in the final study cohort (Figure 1). The epidemiological and baseline characteristics of the patients are listed in Table 1. Of the patients exhibiting Carb-R/Ceph-S P. aeruginosa bacteremia, most patients (69.8%) were male, the median age was 63 years, and infections were more frequent in surgical wards (57.1%). All the episodes resulted from nosocomial acquisition, and the median duration of the total hospital stay was 30 days. Notably, hepatobiliary disease and hematologic malignancy were the most frequent underlying diseases in Carb-R/Ceph-S and Carb-S/Ceph-S P. aeruginosa bacteremia, respectively.
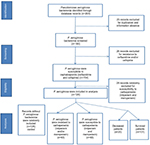  | Figure 1 Flow chart of the study selection. |
Antimicrobial susceptibility testing
Of the 63 Carb-R/Ceph-S P. aeruginosa strains evaluated, all isolates displayed resistance to imipenem and 45 (71.4%) strains showed resistance to meropenem. In addition, all isolates were susceptible to both ceftazidime and cefepime. For fluoroquinolones, 15 (23.8%) isolates were resistant to ciprofloxacin and 15 (23.8%) were resistant to levofloxacin. For aminoglycosides, 14 (22.2%) isolates were resistant to gentamicin and 10 (15.9%) were resistant to tobramycin. Moreover, 17.5% (11/63) of Carb-R/Ceph-S P. aeruginosa isolates were identified as being multidrug resistant, as they were resistant to three or more classes of antimicrobials.
Resistance mechanism of Carb-R/Ceph-S P. aeruginosa
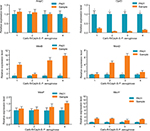
For resistance mechanisms regarding the Carb-R/Ceph-S P. aeruginosa, none of the isolates collected in our study possessed carbapenemases or extended-spectrum β-lactamase-encoding genes. Of these non-MBL-producing isolates, four isolates were selected to search for the presence of other resistance mechanisms, such as AmpC, the efflux pump, and OprD porin. As shown in Figure 2, our results revealed that overexpression of efflux systems (primarily related to the mexB and mexD efflux genes) and decreased expression of OprD could have contributed to the Carb-R/Ceph-S phenotype of P. aeruginosa isolates.
Analysis of case I group vs controls
The risk factors for the acquisition of Carb-R/Ceph-S P. aeruginosa (case I group) are shown in Table 1. In the univariate analysis, patients with Carb-R/Ceph-S P. aeruginosa bacteremia had higher frequencies in 30-day readmission than the uninfected controls. For underlying diseases and conditions, the case I group showed a higher incidence of having hematologic malignancy and hepatobiliary disease compared with controls. Moreover, the primary site of bacteremia was more likely to have been intra-abdominally derived for case patients. Compared to the uninfected controls, patients with Carb-R/Ceph-S P. aeruginosa bacteremia were frequently subjected to invasive procedures such as mechanical ventilation, total parenteral nutrition, and central venous catheterization. Additionally, hypoproteinemia and hypokalemia were significantly more common among case patients than among uninfected controls. Regarding previous antibiotic exposure, carbapenems and antifungal agents were significantly associated with the acquisition of Carb-R/Ceph-S P. aeruginosa bacteremia. In the multivariate analysis, 30-day readmission (OR, 11.03; 95% CI, 3.27–37.15; P<0.001), total parenteral nutrition (OR, 18.98; 95% CI, 3.94–91.54; P<0.001), central venous catheterization (OR, 5.85; 95% CI, 2.09–16.33; P=0.001), and exposure to carbapenems (OR, 11.41; 95% CI, 3.38–38.56; P<0.001) were independently associated with Carb-R/Ceph-S P. aeruginosa bacteremia when compared with the control group (Table 2).
Analysis of case II group vs controls
In univariate analyses listed in Table 1, risk factors for Carb-S/Ceph-S P. aeruginosa bacteremia (case II group) were observed to be significant in underlying diseases, such as hematologic malignancy and hepatobiliary disease. Devices and invasive procedures indicated increased risk of bacteremia, but only the receipt of total parenteral nutrition, central venous catheterization, and nasogastric tube were statistically significant. Moreover, case patients were more likely to receive chemoradiotherapy than uninfected controls. In contrast with the case I group, no significant difference was observed in the primary site of bacteremia and antibiotics exposure between case II group and controls. Multivariate analysis showed that hematologic malignancy (OR, 3.98; 95% CI, 1.56–10.17; P=0.004) and total parenteral nutrition (OR, 19.89; 95% CI, 5.94–66.58; P<0.001) were independently associated with Carb-S/Ceph-S P. aeruginosa bacteremia when compared with the control group (Table 2).
Analysis of case I group vs case II group
When the third model examining the case I and case II groups was analyzed to specifically evaluate the acquisition of Carb-R/Ceph-S P. aeruginosa bacteremia, the results showed that intra-abdominal–related bacteremia, mechanical ventilation, central venous catheterization, nasogastric tube, and exposure to aminoglycosides and carbapenems were significant in the univariate analysis. We observed that exposure to carbapenems (OR, 2.65; 95% CI, 1.17–6.01; P=0.020) was the only independent risk factor associated with the isolation of Carb-R/Ceph-S P. aeruginosa in the multivariate analysis (Tables 1 and 2).
Outcome study: in-hospital mortality
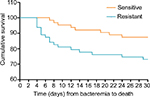
The results of the univariate and multivariate analyses of predictors for mortality are shown in Table 3. Elderly (≥60 years) patients, admission to ICU, multiple organ dysfunction syndrome, and septic shock were significant in univariate analysis. In the multivariate model, admission to an ICU (OR, 3.16; 95% CI, 1.11–8.95; P=0.031) and septic shock (OR, 4.22; 95% CI, 1.45–12.32; P=0.008) were independent risk factors associated with mortality. Additionally, the Kaplan–Meier survival analysis (Figure 3) showed that the cumulative probability of death in the 30 days after bacteremia was significantly different (P=0.035), as the 30-day mortality cumulative probability of death was 27.0% (17/63) for Carb-R/Ceph-S P. aeruginosa bacteremia and 12.7% (8/63) for Carb-S/Ceph-S P. aeruginosa bacteremia.
Discussion
Considering that CRPA bacteremia is a life-threatening illness with higher costs and increased mortality, the phenotype of Carb-R/Ceph-S P. aeruginosa deserves particular attention, especially in some countries where polymyxins are not available for clinical practice. To the best of our knowledge, this is the first analysis to investigate the risk factors and clinical outcomes in patients with Carb-R/Ceph-S P. aeruginosa bacteremia by using a case–case–control study design. A key finding in this study is that 30-day readmission is an independent risk factor for Carb-R/Ceph-S P. aeruginosa isolation. Moreover, another important finding is that a high prevalence of hematologic malignancy could be significantly associated with Carb-S/Ceph-S P. aeruginosa bacteremia.
In the present study, central venous catheterization was observed to be independently associated with Carb-R/Ceph-S P. aeruginosa bacteremia, and this association has been well established by other studies with respect to CRPA bacteremia.16 This finding highlights the importance of infection control practices to prevent the extraluminal microbial colonization of the insertion site via some invasive procedures into the bloodstream. Interestingly, our data identified 30-day readmission as a novel independent predictor associated with Carb-R/Ceph-S P. aeruginosa bacteremia, a factor for which no other study has reported significance. One possibility is that compared with other patients with an initial admission, patients with 30-day readmission, compared with other patients with an initial admission, could have more severe and multiple comorbidities that put them at a higher risk for the development of Carb-R/Ceph-S P. aeruginosa bacteremia. On the other hand, it is also possible that these patients are more vulnerable to acquiring nosocomial pathogens in the same environment due to their 30-day readmission. This observation should advocate for physicians to provide more attentive care to patients who are readmitted in the short term.
Interestingly, parenteral nutrition was the only predictor identified in both the Carb-R/Ceph-S and Carb-S/Ceph-S P. aeruginosa groups compared with the controls. However, no significant difference was observed in the two case groups, revealing that parenteral nutrition could be associated with P. aeruginosa bacteremia in general. Multiple factors could contribute to this association. First, patients receiving parenteral nutrition may have impaired intestinal mucosal structure, disrupted gut barrier functions, and increased microbe translocation, thus providing opportunities for the occurrence of bacteremia.17 Second, most parenteral nutrition formulas contain some glucose, an important carbohydrate that is essential to the body. Hyperglycemia may increase bacterial adherence and biofilm formation, thus increasing bacterial infections rates, especially for certain microorganisms associated with invasive procedures, such as P. aeruginosa.18 Third, intestinal mucosal atrophy, which can be caused by parenteral nutrition, is postulated to promote the translocation of bowel endotoxin and may change the host immune responses to infection, thus accelerating the permeability of intestinal bacteria into the bloodstream.19
Compared with previous studies on CRPA, our study is the first to demonstrate that hematologic malignancy increases the risk of Carb-S/Ceph-S P. aeruginosa bacteremia. Certain predisposing factors for the association with hematologic malignancy, such as chemotherapeutic treatment and intravenous catheterization, often cause an intestinal mucosal break or ulceration that may potentially result in bacteremia,20 while others, such as neutropenia and lymphopenia, may reduce the host cellular and humoral immunity and increase the risk of acquiring various pathogens, including P. aeruginosa.21 Therefore, in patients receiving chemotherapy, for whom hematologic malignancy was diagnosed, P. aeruginosa bacteremia should be considered with special attention.
Not surprisingly, our study confirmed the association between antibiotic use and the appearance and spread of antimicrobial-resistant bacteria.22 Although exposure to carbapenems was reported as a predictor of CRPA by other investigators,16,23 none of these studies evaluated Carb-R/Ceph-S P. aeruginosa specifically. One potential explanation is that the selection pressure exerted by previous carbapenem exposure may disrupt the gastrointestinal microflora and eradicate susceptible competing strains, thus elevating the chances of isolating Carb-R/Ceph-S P. aeruginosa. On the other hand, carbapenem resistance in these patients could be attributed to a mechanism involving a combination of efflux system overexpression and decreased OprD expression, as was observed in the present study. Considering that patients receiving carbapenem treatment may be placed at further risk for Carb-R/Ceph-S P. aeruginosa bacteremia, prudent use of antimicrobial agents is crucial to avoiding their further spread.
For clinical outcomes, we observed that the overall 30-day mortality rate was 27.0%, which was much higher than that reported from previous studies on CRPA.24 In addition, the mortality rate of Carb-R/Ceph-S vs Carb-S/Ceph-S P. aeruginosa bacteremia was significantly different in these patients. Of the factors associated with mortality, including ICU admission and septic shock, were significantly linked to an increased risk of death. This could be explained by ICU patients having more severe underlying diseases and longer hospital stays, placing them at higher risk for poor outcomes. Similarly, septic shock, a very dangerous and potentially life-threatening infection, is physiologically regarded as a proinflammatory and procoagulant response against invading pathogens, resulting in a high mortality in these patients.25 Unexpectedly, inadequate antibiotic therapy did not exhibit a significant relationship with mortality, probably due to the limited number of non-survivors in our study.
It is important to stress that because our analysis was conducted at a tertiary center, and the sample size was relatively small, our results might not be applicable to other settings. Moreover, the clonality of the resistant isolates was not investigated in our study, so potential outbreaks might not be ruled out. Nevertheless, our findings could provide some clinical predictors for physicians to identify some high-risk patients. In addition, this phenotype of Carb-R/Ceph-S P. aeruginosa deserves special concern, and the use of cephalosporin should be encouraged as a valuable therapeutic option for such infections based on the microbiology results of the patient.
In summary, this was the first comprehensive study to analyze the phenotype of Carb-R/Ceph-S P. aeruginosa bacteremia. Our findings showed that the decreased OprD expression and efflux system overexpression could be associated with Carb-R/Ceph-S P. aeruginosa. In addition, we identified some peculiar risk factors for Carb-R/Ceph-S and Carb-S/Ceph-S P. aeruginosa bacteremia, respectively. Therefore, strict control of carbapenem use and standard antibiotic stewardship are crucial to reduce the frequency of CRPA bacteremia.
Acknowledgments
This study was supported in part by the National Natural Science Foundation of China (grant no. 81471992 and 81772239), and the Postgraduate Scientific Innovation Research Foundation of Chongqing (grant no. CYB17106).
Author contributions
All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Lister PD, Wolter DJ, Hanson ND. Antibacterial-resistant Pseudomonas aeruginosa: clinical impact and complex regulation of chromosomally encoded resistance mechanisms. Clin Microbiol Rev. 2009;22(4):582–610. | ||
Poole K. Pseudomonas aeruginosa: resistance to the max. Front Microbiol. 2011;2:65. | ||
Rodríguez-Baño J, Gutiérrez-Gutiérrez B, Machuca I, Pascual A. Treatment of infections caused by extended-spectrum-beta-lactamase-, AmpC-, and carbapenemase-producing Enterobacteriaceae. Clin Microbiol Rev. 2018;31(2):e00079-17. | ||
Mcdougall DA, Morton AP, Playford EG. Association of ertapenem and antipseudomonal carbapenem usage and carbapenem resistance in Pseudomonas aeruginosa among 12 hospitals in Queensland, Australia. J Antimicrob Chemother. 2013;68(2):457–460. | ||
Buehrle DJ, Shields RK, Clarke LG, et al. Carbapenem-resistant Pseudomonas aeruginosa bacteremia: risk factors for mortality and microbiologic treatment failure. Antimicrob Agents Chemother. 2017;61(1):e01243-16. | ||
Castanheira M, Deshpande LM, Costello A, Davies TA, Jones RN. Epidemiology and carbapenem resistance mechanisms of carbapenem-non-susceptible Pseudomonas aeruginosa collected during 2009-11 in 14 European and Mediterranean countries. J Antimicrob Chemother. 2014;69(7):1804–1814. | ||
Tacconelli E, Carrara E, Savoldi A, et al. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis. 2018;18(3):318–327. | ||
Potron A, Poirel L, Nordmann P. Emerging broad-spectrum resistance in Pseudomonas aeruginosa and Acinetobacter baumannii: mechanisms and epidemiology. Int J Antimicrob Agents. 2015;45(6):568–585. | ||
Campana EH, Xavier DE, Petrolini FV, et al. Carbapenem-resistant and cephalosporin-susceptible: a worrisome phenotype among Pseudomonas aeruginosa clinical isolates in Brazil. Braz J Infect Dis. 2017;21(1):57–62. | ||
Zeng ZR, Wang WP, Huang M, et al. Mechanisms of carbapenem resistance in cephalosporin-susceptible Pseudomonas aeruginosa in China. Diagn Microbiol Infect Dis. 2014;78(3):268–270. | ||
CLSI. Performance Standards for Antimicrobial Susceptibility Testing. 25th Informational Supplement. Wayne, PA; 2015: Clinical and Laboratory Standards Institute. | ||
Yan J, Pu S, Jia X, et al. Multidrug resistance mechanisms of carbapenem resistant Klebsiella pneumoniae strains isolated in Chongqing, China. Ann Lab Med. 2017;37(5):398–407. | ||
Gutiérrez O, Juan C, Cercenado E, et al. Molecular epidemiology and mechanisms of carbapenem resistance in Pseudomonas aeruginosa isolates from Spanish hospitals. Antimicrob Agents Chemother. 2007;51(12):4329–4335. | ||
Angus DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med. 2013;369(9):840–851. | ||
Kang CI, Kim SH, Park WB, et al. Bloodstream infections caused by antibiotic-resistant gram-negative bacilli: risk factors for mortality and impact of inappropriate initial antimicrobial therapy on outcome. Antimicrob Agents Chemother. 2005;49(2):760–766. | ||
Tuon FF, Gortz LW, Rocha JL. Risk factors for pan-resistant Pseudomonas aeruginosa bacteremia and the adequacy of antibiotic therapy. Braz J Infect Dis. 2012;16(4):351–356. | ||
Ziegler TR. Parenteral nutrition in the critically ill patient. N Engl J Med. 2009;361(11):1088–1097. | ||
Zaloga GP. Parenteral nutrition in adult inpatients with functioning gastrointestinal tracts: assessment of outcomes. Lancet. 2006;367(9516):1101–1111. | ||
Fong YM, Marano MA, Barber A, et al. Total parenteral nutrition and bowel rest modify the metabolic response to endotoxin in humans. Ann Surg. 1989;210(4):449–457. | ||
Zakhour R, Chaftari AM, Raad II. Catheter-related infections in patients with haematological malignancies: novel preventive and therapeutic strategies. Lancet Infect Dis. 2016;16(11):e241–e250. | ||
Cattaneo C, Antoniazzi F, Casari S, et al. P. aeruginosa bloodstream infections among hematological patients: an old or new question? Ann Hematol. 2012;91(8):1299–1304. | ||
Baquero F, Negri MC, Morosini MI, Blázquez J. Antibiotic-selective environments. Clin Infect Dis. 1998;27(Suppl 1):S5–S11. | ||
Plüss-Suard C, Pannatier A, Kronenberg A, Mühlemann K, Zanetti G. Impact of antibiotic use on carbapenem resistance in Pseudomonas aeruginosa: is there a role for antibiotic diversity? Antimicrob Agents Chemother. 2013;57(4):1709–1713. | ||
Zhang Y, Chen XL, Huang AW, et al. Mortality attributable to carbapenem-resistant Pseudomonas aeruginosa bacteremia: a meta-analysis of cohort studies. Emerg Microbes Infect. 2016;5(5):e27. | ||
Wenzel RP. Treating sepsis. N Engl J Med. 2002;347(13):966–967. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.