Back to Journals » International Journal of Nanomedicine » Volume 11
Capturing and concentrating adenovirus using magnetic anionic nanobeads
Authors Sakudo A, Baba K, Ikuta K
Received 23 January 2016
Accepted for publication 28 February 2016
Published 9 May 2016 Volume 2016:11 Pages 1847—1857
DOI https://doi.org/10.2147/IJN.S104926
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Thomas Webster
Akikazu Sakudo,1 Koichi Baba,2 Kazuyoshi Ikuta1,3
1Department of Virology, Research Institute for Microbial Diseases, Osaka University, Yamadaoka, Suita, Osaka, Japan; 2Baba Pediatric Clinic, Kadoma, Osaka, Japan; 3Kanonji Institute, The Research Foundation for Microbial Diseases of Osaka University, Kanonji, Kagawa, Japan
Abstract: We recently demonstrated how various enveloped viruses can be efficiently concentrated using magnetic beads coated with an anionic polymer, poly(methyl vinyl ether-maleic anhydrate). However, the exact mechanism of interaction between the virus particles and anionic beads remains unclear. To further investigate whether these magnetic anionic beads specifically bind to the viral envelope, we examined their potential interaction with a nonenveloped virus (adenovirus). The beads were incubated with either adenovirus-infected cell culture medium or nasal aspirates from adenovirus-infected individuals and then separated from the supernatant by applying a magnetic field. After thoroughly washing the beads, adsorption of adenovirus was confirmed by a variety of techniques, including immunochromatography, polymerase chain reaction, Western blotting, and cell culture infection assays. These detection methods positively identified the hexon and penton capsid proteins of adenovirus along with the viral genome on the magnetic beads. Furthermore, various types of adenovirus including Types 5, 6, 11, 19, and 41 were captured using the magnetic bead procedure. Our bead capture method was also found to increase the sensitivity of viral detection. Adenovirus below the detectable limit for immunochromatography was efficiently concentrated using the magnetic bead procedure, allowing the virus to be successfully detected using this methodology. Moreover, these findings clearly demonstrate that a viral envelope is not required for binding to the anionic magnetic beads. Taken together, our results show that this capture procedure increases the sensitivity of detection of adenovirus and would, therefore, be a valuable tool for analyzing both clinical and experimental samples.
Keywords: anionic polymer, virus concentration, adenovirus, capture, magnetic beads, poly(methyl vinyl ether-maleic anhydrate)
Introduction
Adenoviruses, which belong to the family Adenoviridae, are nonenveloped viruses with an icosahedral nucleocapsid and double-stranded DNA genome. The Mastadenovirus genus infects mammals and includes human, simian, equine, bovine, porcine, ovine, canine, and opossum viruses. In humans, 51 distinct adenoviral serotypes have been identified that can cause a wide range of illnesses including respiratory infections, gastroenteritis, acute febrile pharyngitis, pharyngoconjunctival fever, and epidemic keratoconjunctivitis.1,2
Currently, the 51 serotypes of human adenovirus are classified into subgroups A–F,3 although recent studies have reported an additional new serotype.4 Each type of adenovirus infects via a different route5,6 as follows: respiratory tract infection is caused by Types 1–7 (subgroups B, C, and E); ocular infection is caused by Types 8, 19, and 37 (subgroup D); and urinary tract infection is caused by Types 11 and 21 (subgroup B). A common causative agent of infantile diarrhea is Types 40 and 41 adenovirus (subgroup F).
Recent developments in immunochromatography have facilitated the convenient detection of adenovirus in biological samples, which has considerably improved clinical diagnosis.7–9 Nonetheless, immunochromatography is sometimes insufficiently sensitive to detect low levels of adenovirus in clinical samples.9 Although the polymerase chain reaction (PCR) is a highly sensitive means of detecting adenovirus, the method is time consuming and is generally performed in the laboratory rather than the clinic. Thus, novel methods are needed for the early and sensitive detection of adenovirus in clinical samples.
Rapid and sensitive detection of adenoviruses is crucially important for both restricting the spread of disease and improving therapeutic outcome. Pretreatment of clinical samples to concentrate adenovirus considerably increases the sensitivity of viral detection. There are two major considerations when developing a novel technique to concentrate adenovirus; first, the method should be compatible with current detection procedures, and second, it should be simple and straightforward to perform. Several approaches have been used to increase the concentration of viruses from clinical samples to enhance the sensitivity of detection.10–12 For example, ultracentrifugation and polyethylene glycol (PEG)-mediated precipitation have been used to concentrate a number of different viruses, including adenovirus. Although ultracentrifugation is a well-established procedure for concentrating viruses, it is time consuming and can be incompatible with PCR (ie, increases the number of false-positives).13,14 Moreover, while PEG precipitation of virus particles is simple and easy to perform, the PEG can sometimes interfere with the subsequent PCR amplification step.15 In addition, both ultracentrifugation and PCR reduce infectivity of viruses. An alternative approach for concentrating viruses in clinical samples is to use magnetic beads coated with molecules that efficiently bind viral particles such as organic chemicals and biomolecules. Indeed, we and other groups have reported that magnetic beads coated with an anionic polymer, poly(methyl vinyl ether-maleic anhydrate) (poly(MVE-MA)), can be used to efficiently capture different viral types.16,17 These include human immunodeficiency virus Type 1 (HIV-1),18 respiratory syncytial virus (RSV),19 Borna disease virus,20 influenza virus,21 and dengue virus,22 which are all enveloped viruses. In addition, antibodies targeting the viral envelope proteins have been shown to interfere with the binding of virus particles to the anionic magnetic beads.18,19 The results of this study imply that the anionic magnetic beads might bind viruses via the viral envelope, but it remains unclear whether the envelope is essential for the observed interaction. These considerations prompted us to further investigate whether the anionic magnetic beads are able to bind a nonenveloped virus.
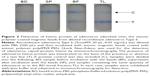
Here, we determined whether anionic magnetic beads coated with poly(MVE-MA) are able to efficiently capture adenovirus, which is a representative nonenveloped virus (Figure 1). Furthermore, the applicability of this bead-concentrating method was examined using various types of adenovirus.
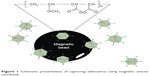  | Figure 1 Schematic presentation of capturing adenovirus using magnetic anionic nanobead. |
Materials and methods
Reagents
Unless otherwise specified, chemical reagents were obtained from Sigma-Aldrich (St Louis, MO, USA) or Wako Pure Chemical Industries (Osaka, Japan). Magnetic particles (300 nm in diameter) with a high ferrite content were used in this study. The poly(MVE-MA) coating was grafted onto the magnetic particles in 5% dimethyl sulfoxide and phosphate buffer for 3 hours at 37°C as described previously.16
Adenovirus
Purified recombinant adenovirus Type 6 (strain Tonsil99) was purchased from Hytest, Ltd. (Turku, Finland). Genetic recombinants of adenovirus Type 5, lacking the ability to grow, were purchased from Takara Bio Inc. (Otsu, Japan). Axcw2 is adenovirus Type 5 alone, while AxCAwt2 contains adenovirus Type 5, CAG promoter, cytomegalovirus enhancer, chicken β-actin promoter, and rabbit β-globin polyA site. AxCAiLacZ contains adenovirus Type 5, CAG promoter, cytomegalovirus enhancer, chicken β-actin promoter, rabbit β-globin polyA site, and Escherichia coli β-Gal gene (Takara Bio Inc.). Adenoviruses such as Adenoid75 (adenovirus Type 5, ATCC VR-5), Tonsil99 (adenovirus Type 6, ATCC VR-6), Slobitski (adenovirus Type 11, ATCC VR12), AV-587[3911] (adenovirus Type 19, ATCC VR-254), and Tak (73-3544) (adenovirus Type 41, ATCC VR930) were purchased from American Type Culture Collection (Manassas, VA, USA).
Collection of nasal aspirates
Clinical nasal aspirates were collected from pediatric patients at the Baba Pediatric Clinic (Osaka, Japan). The method used to collect the samples was described previously.17,23 Briefly, saline was introduced into the nasal cavity, and fluid was collected using a nasal aspirator, Belvital (Melisana, Nogent-sur-Marne, France). The nasal fluid was then filtered through a stainless steel mesh (200 grids per inch [25.4 mm]) to remove cell debris. The nasal aspirates were subsequently subjected to immunochromatography for adenovirus as described in the “Immunochromatography” section. As a negative control, nasal aspirates were also collected from healthy donors using the same procedure. The research project was approved by the Ethics Committee of the Research Institute for Microbial Diseases at Osaka University. Written informed consent was obtained from all the individuals who participated in this study.
Capture of adenovirus
Viral capture was performed as described previously.17 Briefly, the magnetic beads (volume of 50 μL) were washed twice with binding buffer and then twice more with phosphate-buffered saline (PBS). A 20 μL aliquot of nasal aspirate or cell culture medium infected with adenovirus was diluted with 500 μL of PBS and incubated with the washed magnetic beads for 20 minutes at room temperature. After applying a magnetic field, the supernatant was discarded. The beads were then washed three more times with PBS. Finally, the washed beads were resuspended in PBS and analyzed by Western blotting, immunochromatography, viral DNA extraction, or viral titration assay.
Immunochromatography
A rapid test for the detection of adenovirus antigens was performed using Quick Navi-Adeno (Denka Seiken Co., Ltd., Tokyo, Japan), which recognizes the adenovirus hexon protein.24 The experimental procedure was conducted according to the manufacturer’s instructions.
Enzyme-linked immunosorbent assay
An adenovirus-X™ rapid titer kit (Clontech Laboratories Inc., Mountain View, CA, USA) was used to assay for adenovirus hexon protein. Briefly, samples were incubated with HEK293 cells for 48 hours, and the cells were then fixed with ice-cold 100% methanol and stained with mouse anti-adenovirus hexon antibody. The signal was detected with a rat anti-mouse antibody conjugated to horseradish peroxidase and developed with metal-enhanced 3,3′-diaminobenzidine tetrahydrochloride. Brown/black staining, indicating infection with adenovirus, was visualized by optical microscopy.
Viral titration assay
The number of plaque forming units (PFUs) per mL was determined by performing twofold serial dilutions of samples in 96-well plates containing HEK293 cells (ATCC CRL1573™) as described in the manufacturer’s instructions (Adeno-X™ Expression System User Manual, Clontech Laboratories Inc.). Prior to infection, cells were washed with PBS and then the infected cells were incubated at 37°C in an atmosphere of 5% CO2 for 7 days. Virus titer was determined by counting the number of plaques. To minimize error, only plates containing between 10 and 100 plaques were counted (ie, depending on the size of the cell culture plate). According to statistical analysis, when 100 plaques are counted, the sample titer will vary by ± 10%.
Western blotting
Each fraction was solubilized in an equal volume of 2× sodium dodecyl sulfate (SDS) gel-loading buffer (90 mM Tris–HCl [pH 6.8], 10% mercaptoethanol, 2% SDS, 0.02% bromophenol blue, and 20% glycerol), boiled for 5 minutes, and then resolved by SDS–polyacrylamide gel electrophoresis (PAGE) using an 8% gel. The bands were then electroblotted onto a polyvinylidene difluoride (PVDF) membrane (Hybond-P; Amersham-Pharmacia Biotech, Piscataway, NJ, USA) for 60 minutes at 15 V. The PVDF membrane was blocked with 5% skimmed milk for 1 hour at room temperature and then incubated with goat anti-adenovirus polyclonal antibody (AP00664PU-N, Acris) in PBS containing 0.1% Tween 20 (PBS-T) and 0.5% skimmed milk for 1 hour at room temperature. After three washes with PBS-T, the membrane was incubated in PBS-T and 0.5% skimmed milk supplemented with anti-goat IgG conjugated to horseradish peroxidase (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA) for 1 hour at room temperature with gentle shaking. The membrane was then washed three times with PBS-T, and protein bands that reacted with the antibodies were visualized using an enhanced chemiluminescence detection kit (Amersham-Pharmacia Biotech). Chemiluminescence was detected using EzCaptureMG (ATTO Corp., Tokyo, Japan). Band intensity was analyzed by densitometric analysis with ImageJ software (National Institutes of Health).
X-gal (5-bromo-3-indoyl-β-D-galactopyranoside) staining
For X-gal staining, X-Gal Staining™ solution (Genlantis and Gene Therapy Systems, Inc, San Diego, CA, USA) was used. Briefly, cells were fixed with formaldehyde–glutaraldehyde buffer and washed with PBS. Samples were then incubated in X-gal staining solution for 18 hours at 37°C in a CO2 incubator. Blue cells, indicating infection with adenovirus, were visualized by optical microscopy.
Viral DNA extraction and PCR amplification
Adenovirus DNA was extracted using a QIAamp Viral RNA mini kit (Qiagen, Hilden, Germany). Viral nucleic acid derived from either the beads or an aliquot of each sample was extracted according to the manufacturer’s instructions. DNA was extracted from the magnetic beads by adding lysis buffer prior to removing the beads. The DNA was then eluted into 60 μL of nuclease-free water and amplified in a reaction mixture containing primers, Ex Taq (Takara bio Inc.) and 1× Ex Taq buffer. Amplification comprised 30 cycles of 94°C for 30 seconds, 50°C for 30 seconds, and 72°C for 2 minutes. PCR was carried out using the following primers with specificity for the adenovirus hexon gene:
Hexon-F: 5′-TGGGTGATAACCGTGTGCTA-3′,
Hexon-R: 5′-TTAATGCTAGCCCCGTCAAC-3′.
The amplified DNA products were analyzed by agarose gel electrophoresis using a 1.2% gel.
Real-time PCR
Extracted viral DNA was also analyzed by real-time PCR using SYBR Premix Ex TaqII (Tli RNase H plus; Takara Bio Inc.) according to the manufacturer’s instructions (Stratagene, La Jolla, CA, USA). Briefly, the real-time PCR components included SYBR Premix Ex Taq II and the forward and reverse target gene primers: realAdenoHexon-F, 5′-GACATGACTTTCGAGGTCGATCCCATGGA-3′; realAdenoHexon-R, 5′-CCGGCTGAGAAGGGTGTGCGCAGGTA-3′. Real-time PCR was performed using a Thermal Cycler Dice Real Time System (Takara Bio Inc.). The cycling program included initial denaturation at 95°C for 30 seconds, followed by 40 cycles of 95°C for 30 seconds and 60°C for 30 seconds. Each reaction was carried out in quadruplicate, and the results were analyzed using Thermal Cycler Dice Real Time System Single software (Takara Bio Inc.). The relative expression ratio of each sample was calculated using a mathematical model based on the amplification efficiency. PCR specificity was verified by dissociation curve analysis of the amplified DNA fragments of step 1 (95°C/15 seconds), step 2 (60°C/30 seconds), and step 3 (95°C/15 seconds).
DNA sequencing
The products of conventional PCR and real-time PCR were purified and cloned into pT7Blue T-vector (Novagen, Madison, WI, USA). The identity of the PCR products was then verified by DNA sequencing on an ABI PRISM3100 Genetic Analyzer (Applied Biosystems, Foster City, CA, USA) using R-20mer primer and U-19mer primer (Novagen).
Results
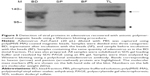
Initially, we performed immunochromatography to verify whether the poly(MVE-MA)-coated anionic magnetic beads could efficiently capture adenovirus. For these experiments, recombinant adenovirus Type 6 (Tonsil99) was used as sample solution. Immunochromatography, employing an antibody against adenovirus hexon protein, was used to detect adenovirus in the bead fraction (BD; Figure 2). Adenovirus was also detected in samples containing the same quantity of adenovirus as in the BD (total fraction, TL). However, the corresponding band was absent in the supernatant after separation from the beads (SP; Figure 2). Compared with the BD fraction, a band of lower intensity was detected in the sample before incubation with the beads (BF fraction). To further examine whether this magnetic capture method can be applied to different serotypes of adenovirus, Adenoid75 (Adenovirus Type 5), Tonsil99 (Adenovirus Type 6), Slobitski (Adenovirus Type 11), AV-587[3911] (Adenovirus Type 19), and Tak(73-3544) (Adenovirus Type 41) were subjected to the capture procedure. Our results showed that all types of adenoviruses could be efficiently captured by the anionic magnetic beads (Figure 3). The band intensities obtained by immunochromatography (Figure 3) were then compared using ImageJ software. The band intensity corresponding to TL for each type of adenovirus was taken as 1. Band intensities for BD, BF, and SP are listed in Table 1. The data indicate that the beads had concentrated the adenovirus 3.7-fold for Adenoid75, 2.1-fold for Tonsil99, 29.0-fold for Slobitski, 3.2-fold for AV-587[3911], and 3.2-fold for Tak(73-3544). The capture efficiencies were 84.2% for Adenoid75, 85.9% for Tonsil99, 100% for Slobitski, 93.2% for AV-587[3911], and 90.0% for Tak(73-3544).
We also carried out similar capture experiments using clinical nasal aspirates (Figure 4). Here, a band corresponding to adenovirus hexon protein was detected in BD and TL but not in SP and BF samples, demonstrating the successful capture of adenovirus from nasal aspirates containing a low quantity of virus particles.
Next, we investigated whether the bead-captured adenovirus could be detected using a Western blotting procedure (Figure 5). Two bands with a molecular mass of ~120 and 80 kDa were detected, corresponding to hexon protein (116 kDa) and penton protein (80 kDa), respectively.25 ImageJ software was used to compare band intensities in the hexon region between the BD and TL fractions from eight independent experiments. These results showed the value for BD represents 73%±5.7% compared to TL (100%).
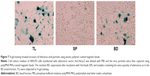
Next, we investigated whether the captured adenovirus retained infectivity after bead separation. Fractions from the cell medium of HEK293 cells transfected with recombinant adenovirus vector AxCAwt2 were subjected to viral titration assays using HEK293 cells (Figure 6). The results showed a similar viral titer (PFU/mL) for the BD and TL fractions. By contrast, the BF fraction had a viral titer of one-tenth that of the BD and TL fractions. Moreover, the viral titer of the SP fraction was below the detection limit for our assay procedure. These observations were further supported by X-gal staining of HEK293 cells transfected with AxCAiLacZ (Figure 7) and enzyme-linked immunosorbent assay (ELISA) using an antiadenovirus hexon antibody (Figure 8). Specifically, X-gal was found to stain cells treated with samples of BD and TL, but not SP (Figure 7). After incubation of HEK293 cells with the TL and BD fractions, adenovirus infected cells were identified by ELISA. However, no adenovirus infected cells were found after incubation with the SP fraction (Figure 8). These results suggest that the vast majority of infectious adenovirus was efficiently captured from solution by the anionic magnetic beads.
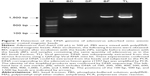
Next, we examined the capacity of the anionic magnetic beads to capture adenovirus using PCR to detect the adenovirus genomic DNA (Figure 9). The amplified PCR product was anticipated to be 1,597 bp in length corresponding to the adenovirus hexon gene. A single band of the expected size was amplified from the BD and from the same quantity of cell culture medium as in the BD fraction (TL) using cell culture medium from AxCAwt2 vector-transfected HEK293 cells. No amplified product was detected in the supernatant after incubation with the beads (SP). The amplified product obtained from both the BD and TL fractions was confirmed to be the hexon gene of adenovirus Type 5 by DNA sequence analysis (ie, identity to Genbank accession number AF542116.1 was 95% using the forward primer and 97% using the reverse primer).
We also measured the amount of adenovirus genomic DNA in the BD, SP, and TL fractions by real-time PCR of the hexon gene, relative to that of a control (BF sample with the highest value was taken as 1; Figure 10). By comparison to TL (100%), the amount of viral DNA detected by real-time PCR in the cell culture medium of HEK293 cells transfected with AxCAiLacZ was determined to be 83.33% for the BD fraction and 5.39% for the BF fraction. The specificity of the PCR was confirmed by dissociation curve analysis of the reaction products. The 140 bp band obtained by real-time PCR was also confirmed to correspond to the hexon gene of adenovirus Type 5 by DNA sequencing (identity to Genbank accession number AF542116.1 was 94% using the forward primer and 96% using the reverse primer). Taken together, these results showed that all of the adenovirus in the cell culture medium could be captured by the magnetic beads. Indeed, all of the analyzed components of adenovirus were identified within the magnetic bead-captured fraction.
Finally, we examined whether our capture method enhances the sensitivity of adenovirus detection by immunochromatography. Thus, a solution containing adenovirus below the detection limit for immunochromatography was subjected to the capture procedure. Serial (×2) dilutions were prepared of an adenovirus-containing solution with a titer varying from 4.0×103 PFU/μL to 2.5×102 PFU/μL. Immunochromatography (Quick Navi-Adeno) successfully detected adenovirus down to a level of 1.0×103 PFU/μL but not 5.0×102 PFU/μL (data not shown). Adenovirus with a titer of 5.0×102 PFU/μL was subjected to the virus capture procedure using the magnetic beads. Immunochromatography was then used to detect adenovirus before and after the bead concentrating procedure (Figure S1). Our results showed that a viral titer of 5.0×102 PFU/μL could be detected by immunochromatography (Quick Navi-Adeno) only after concentration with the beads.
Discussion
The magnetic bead-mediated capture method for adenovirus described in this paper is a simple and quick procedure that significantly enhances the detection of adenoviruses. The overall processing time of this method is approximately 30 minutes, which is considerably quicker than existing methodologies. Moreover, complicated procedures such as column purification are not required. The results from this study suggest that the magnetic bead capture method can identify adenovirus in samples below the current detection limit. In addition, the relatively small number of processing steps reduces problems associated with loss of signal and potential cross-contamination commonly observed with multistep protocols. The magnetic bead-mediated capture method is also fully compatible with conventional detection methods of immunochromatography such as PCR, ELISA, and Western blotting. Indeed, alternative methods for concentrating viruses are often found to be incompatible with conventional detection procedures.13–15 For example, PEG precipitation is sometimes incompatible with PCR due to PEG-mediated inhibition of the DNA polymerase used in the amplification step. In addition, the ultracentrifugation procedure requires expensive specialist equipment and is relatively time consuming compared to the magnetic bead-mediated capture method. Therefore, compared to previous virus concentrating methods, our capture procedure using magnetic beads is a promising approach that is well suited to established detection techniques.
There are several methods for concentrating a virus using magnetic beads coated with an antibody for a specific virus.14,26–30 Polymers such as polyethyleneimine have been used to concentrate simian virus 40,31 herpes simplex virus Type 1,31 Sindbis virus,31 vesicular stomatitis virus,31 amphotropic murine leukemia virus,32 poliovirus,33 hepatitis A virus,33 hepatitis B virus,33 hepatitis C virus,33 and cytomegalovirus (CMV).34 Sulfonated magnetic beads in the presence of divalent cations are known to concentrate CMV,34 Sindbis virus,34 poliovirus,34 and porcine parvovirus.34 Moreover, poly(MVE-MA)-coated magnetic beads can be used for the efficient capture of avian and human influenza virus,17,21 RSV,19 Borna disease virus,20 dengue virus,22 HIV-1,16,18 CMV,16 herpesvirus,35 and vaccinia virus.35
This study clearly shows that poly(MVE-MA)-coated magnetic beads can be used to capture adenovirus in various sample types, including cell culture medium and nasal aspirates. Nonetheless, further work is required to demonstrate the ability of the magnetic beads to specifically concentrate adenovirus from a mixture of viruses present in a biological sample. Indeed, a previous study using RSV showed that the binding of RSV to the magnetic beads is abrogated in a competitive manner by the presence of influenza A and B virus.19 This observation suggests that the binding mechanism is the same for these viruses. Thus, further studies are required to clarify whether this is also the case for adenovirus.
Previous studies using RSV and HIV-1 have shown that preincubation of the beads with neutralizing antibody against Env of the respective virus prevents capture.18,19 These findings suggested Env may be involved in the binding process between the magnetic beads and viral particles. However, this study demonstrates that the nonenveloped adenovirus also binds to the magnetic beads, showing the envelope is not necessarily required for binding.
Given that poly(MVE-MA) is a negatively charged molecule, modification of the spatial organization of this polymer may alter its binding efficiency/capacity for viral particles. Previous studies reported that the surface of adenovirus is also highly negatively charged.36 Thus, an anionic charge on the polymer might not be preferable for adenovirus binding. However, other functions of the polymer besides its charge may contribute to the binding of adenovirus. This idea is supported by the observation that adenovirus binding to the beads was not influenced by different pH conditions (Figure S2). Therefore, it is assumed that the mechanism for adenovirus binding is not dependent on charge. However, our previous studies using RSV have shown that a slightly lower level of virus binding to the magnetic beads is observed under high pH conditions.19 Thus, the contribution of charge to the binding mechanism may vary between viruses. Further analysis of the effect of charge density and steric spatial organization of poly(MVE-MA) on the binding to various types of virus may help elucidate the mechanism of interaction. In addition, information on the exact size and charge values of the polymer on the beads would also be important for understanding binding mechanisms. To test this hypothesis, further studies are required to examine whether changes to the spatial organization and length of poly(MVE-MA) alter the binding to the viruses.
Of the different serotypes of adenovirus analyzed in this study, Type1 Slobitski was most effectively bound by the beads (Table 1). This observation implies that different adenovirus types may have different binding capacities to the beads. A previous study has shown varying pH stabilities among different types of adenovirus.37 Moreover, the stability of adenovirus penton protein is known to be strongly pH dependent.3 Thus, binding analysis using a combination of various adenovirus types under different pH conditions may also help to elucidate the mechanisms by which adenoviruses bind to the beads.
A number of factors that inhibit binding between the magnetic beads and viruses have been reported.17 These inhibitory factors include blood and albumin, which nonspecifically bind to the beads and reduce their binding capacity. Clinical samples are often contaminated with inhibitory factors, especially from blood. Thus, a strategy for reducing the inhibitory effect of these factors would be advantageous. Modification of the charge density and surface organization of the anionic polymers on the magnetic beads may be effective in reducing nonspecific binding.
We have demonstrated a simple and straightforward procedure involving magnetic beads coated with anionic polymers that can efficiently capture and concentrate adenovirus. Viral genomic DNA as well as the major capsid proteins hexon and penton were detected in the captured samples either using PCR, real-time PCR, immunochromatography, or Western blotting. Therefore, our capture method is fully compatible with conventional means of viral detection. Finally, our novel method enables early and sensitive detection of adenovirus, which can potentially improve the therapeutic outcome where currently only palliative treatment is possible.
Acknowledgments
The authors thank Mr Satoru Shigeno and Mr Shinya Iriguchi for technical assistance. This work was supported by a Grant-in-Aid for the Promotion of Basic Research Activities for Innovative Biosciences from Bio-oriented Technology Research Advancement Institution (BRAIN) and the Japan Science and Technology Agency (JST) as well as a Grant-in-Aid for Scientific Research on Innovative Areas from the Japan Society for the Promotion of Science and a Grant-in-Aid from Science and Technology Research Promotion Program for Agriculture, Forestry, Fisheries, and Food Industry.
Disclosure
The authors report no conflicts of interest in this work.
References
Horwitz MS. Adenoviruses. In: Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus SE, editors. Fields Virology. 4th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2001:2301–2326. | ||
Fong TT, Phanikumar MS, Xagoraraki I, Rose JB. Quantitative detection of human adenoviruses in wastewater and combined sewer overflows influencing a Michigan river. Appl Environ Microbiol. 2010;76(3):715–723. | ||
Russell WC. Adenoviruses: update on structure and function. J Gen Virol. 2009;90(Pt 1):1–20. | ||
Ishiko H, Shimada Y, Konno T, et al. Novel human adenovirus causing nosocomial epidemic keratoconjunctivitis. J Clin Microbiol. 2008;46(6):2002–2008. | ||
Echavarria M. Adenoviruses in immunocompromised hosts. Clin Microbiol Rev. 2008;21(4):704–715. | ||
Echavarria M, Maldonado D, Elbert G, Videla C, Rappaport R, Carballal G. Use of PCR to demonstrate presence of adenovirus species B, C, or F as well as coinfection with two adenovirus species in children with flu-like symptoms. J Clin Microbiol. 2006;44(2):625–627. | ||
Morozumi M, Shimizu H, Matsushima Y, et al. Evaluation of new immunochromatographic assay kit for adenovirus detection in throat swab: comparison with culture and real-time PCR results. J Infect Chemother. 2014;20(5):303–306. | ||
Kim J, Kim HS, Kim JS, et al. Evaluation of an immunochromatographic assay for the rapid and simultaneous detection of rotavirus and adenovirus in stool samples. Ann Lab Med. 2014;34(3):216–222. | ||
Romero-Gómez MP, López López R, González Montes R, et al. Immunochromatographic test for detection of adenovirus from respiratory samples: is it a real solution for pediatric emergency department? J Virol Methods. 2014;195:236–239. | ||
Kittigul L, Khamoun P, Sujirarat D, et al. An improved method for concentrating rotavirus from water samples. Mem Inst Oswaldo Cruz. 2001;96(6):815–821. | ||
Sanyal D, Kudesia G, Corbitt G. Comparison of ultracentrifugation and polyethylene glycol precipitation for concentration of hepatitis B virus (HBV) DNA for molecular hybridisation tests and the relationship of HBV-DNA to HBe antigen and anti-HBe status. J Med Microbiol. 1991;35(5):291–293. | ||
Trépanier P, Payment P, Trudel M. Concentration of human respiratory syncytial virus using ammonium sulfate, polyethylene glycol or hollow fiber ultrafiltration. J Virol Methods. 1981;3(4):201–211. | ||
Roth WK, Weber M, Seifried E. Feasibility and efficacy of routine PCR screening of blood donations for hepatitis C virus, hepatitis B virus, and HIV-1 in a blood-bank setting. Lancet. 1999;353(9150):359–363. | ||
Kobayashi S, Natori K, Takeda N, Sakae K. Immunomagnetic capture rt-PCR for detection of norovirus from foods implicated in a foodborne outbreak. Microbiol Immunol. 2004;48(3):201–204. | ||
Novotný J, Svobodová J, Ransnäs LA, Kubistová K. A method for the preparation of purified antigens of coxsackievirus B3 from a large volume of cell culture supernatant. Acta Virol. 1992;36(5):483–487. | ||
Flavigny E, Gaboyard M, Merel P, Fleury H. Magnetic particle-mediated virus concentration for clinical virology. Poster presented at: 104th General Meeting of the American Society for Microbiology, New Orleans, American Society for Microbiology, May 22–27, 2004, Washington, DC. | ||
Sakudo A, Baba K, Tsukamoto M, et al. Anionic polymer, poly(methyl vinyl ether-maleic anhydride)-coated beads-based capture of human influenza A and B virus. Bioorg Med Chem. 2009;17(2):752–757. | ||
Sakudo A, Ikuta K. A technique for capturing broad subtypes and circulating recombinant forms of HIV-1 based on anionic polymer-coated magnetic beads. Int J Mol Med. 2012;30(2):437–442. | ||
Sakudo A, Baba K, Tsukamoto M, Ikuta K. Use of anionic polymer, poly(methyl vinyl ether-maleic anhydride)-coated beads for capture of respiratory syncytial virus. Bioorg Med Chem Lett. 2009;19(15):4488–4491. | ||
Sakudo A, Tanaka Y, Ikuta K. Capture of infectious borna disease virus using anionic polymer-coated magnetic beads. Neurosci Lett. 2011;494(3):237–239. | ||
Sakudo A, Ikuta K. Efficient capture of infectious H5 avian influenza virus utilizing magnetic beads coated with anionic polymer. Biochem Biophys Res Commun. 2008;377(1):85–88. | ||
Sakudo A, Masrinoul P, Tanaka Y, Ikuta K. Capture of dengue virus type 3 using anionic polymer-coated magnetic beads. Int J Mol Med. 2011;28(4):625–628. | ||
Baba K, Inventor; Kochi-kai. Virus collecting instrument. Japan Patent 2008-119552. May 29, 2008. | ||
Mitamura K, Shimizu H, Yamazaki M, et al. Evaluation of new rapid diagnosis kit for adenovirus, Quicknavi™-Adeno. Jpn J Med Pharm Sci. 2008;60:143–150. | ||
Tuteja U, Batra HV. Generation and characterization of monoclonal antibodies to adenovirus. Indian J Exp Biol. 2000;38(12):1259–1262. | ||
Clavet CR, Margolin AB, Regan PM. Herpes simplex virus type-2 specific glycoprotein G-2 immunomagnetically captured from HEp-2 infected tissue culture extracts. J Virol Methods. 2004;119(2):121–128. | ||
Jothikumar N, Cliver DO, Mariam TW. Immunomagnetic capture PCR for rapid concentration and detection of hepatitis A virus from environmental samples. Appl Environ Microbiol. 1998;64(2):504–508. | ||
Sakudo A, Chou H, Nagatsu M. Antibody-integrated and functionalized graphite-encapsulated magnetic beads, produced using ammonia gas plasma technology, for capturing Salmonella. Bioorg Med Chem Lett. 2015;25(5):1012–1016. | ||
Sakudo A, Chou H, Ikuta K, Nagatsu M. Integration of antibody by surface functionalization of graphite-encapsulated magnetic beads using ammonia gas plasma technology for capturing influenza A virus. Bioorg Med Chem Lett. 2015;25(9):1876–1879. | ||
Sakudo A, Viswan A, Chou H, Sasaki T, Ikuta K, Nagatsu M. Capture of dengue viruses using antibody-integrated graphite encapsulated magnetic beads produced by gas plasma technology. Mol Med Rep, in press. | ||
Satoh K, Iwata A, Murata M, Hikata M, Hayakawa T, Yamaguchi T. Virus concentration using polyethyleneimine-conjugated magnetic beads for improving the sensitivity of nucleic acid amplification tests. J Virol Methods. 2003;114(1):11–19. | ||
Uchida E, Sato K, Iwata A, et al. An improved method for detection of replication-competent retrovirus in retrovirus vector products. Biologicals. 2004;32(3):139–146. | ||
Uchida E, Kogi M, Oshizawa T, et al. Optimization of the virus concentration method using polyethyleneimine-conjugated magnetic beads and its application to the detection of human hepatitis A, B and C viruses. J Virol Methods. 2007;143(1):95–103. | ||
Iwata A, Satoh K, Murata M, Hikata M, Hayakawa T, Yamaguchi T. Virus concentration using sulfonated magnetic beads to improve sensitivity in nucleic acid amplification tests. Biol Pharm Bull. 2003;26(8):1065–1069. | ||
Hatano B, Kojima A, Sata T, Katano H. Virus detection using Viro-Adembeads, a rapid capture system for viruses, and plaque assay in intentionally virus-contaminated beverages. Jpn J Infect Dis. 2010;63(1):52–54. | ||
Prazeres DMF, Santos JAL. Production and purification of adenovirus vectors for gene therapy. In: Gad SC, editor. Handbook of Pharmaceutical Biotechnology. Hoboken, NJ: Wiley-Interscience; 2006:1261–1295. | ||
Rafajko RR, Young JC. Thermal and pH stability of adenovirus types 12, 14, AND 18. Proc Soc Exp Biol Med. 1964;116:683–685. |
Supplementary materials
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.