Back to Journals » Drug Design, Development and Therapy » Volume 14
Cannabis Extract CT-921 Has a High Efficacy–Adverse Effect Profile in a Neuropathic Pain Model
Authors Rouhollahi E, MacLeod BA, Barr AM, Puil E
Received 1 February 2020
Accepted for publication 29 July 2020
Published 17 August 2020 Volume 2020:14 Pages 3351—3361
DOI https://doi.org/10.2147/DDDT.S247584
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Tin Wui Wong
Elham Rouhollahi, Bernard A MacLeod, Alasdair M Barr, Ernest Puil
Department of Anesthesiology, Pharmacology & Therapeutics, University of British Columbia, Vancouver, British Columbia, Canada
Correspondence: Ernest Puil Tel +1 604 822 5080
Fax +1 604 822 6012
Email [email protected]
Background: Legalization of cannabis encourages the development of specific cultivars to treat disease such as neuropathic pain. Because of the large number of cultivars, it is necessary to prioritize extracts before proceeding to clinical trials.
Purpose: To compare extracts of two unique cannabis cultivars (CT-921, CT-928) for treatment of neuropathic pain induced by constriction of sciatic nerve in mice and to illustrate the use of this animal model to set priority for future trials.
Methods: Pain severity was measured by threshold force causing paw withdrawal. Dose–response relationships and time course were determined for intravenously injected extracts of cultivars and vehicle. The doses for allodynia relief were correlated with decreased respiratory rate, temperature and behavioral changes.
Results: Effective analgesic dose for 50 and 95% (ED50An and ED95An) was 15, and 29 mg/kg for CT-921 and 0.9 and 4.7 for CT-928. At ED50An, for both extracts, the duration was 120 min. At ED95An, administration of CT-928 significantly decreased respiratory rate while CT-921 did not. CT-928 decreased temperature more than CT-921. CT-928 produced frantic hyperactivity not seen with CT-921. At equivalent analgesic doses, THC was much less in CT-921 than in CT-928 suggesting interactions with components other than THC influenced the analgesia. At equivalent analgesic doses, efficacy-to-adverse effect profile for CT-928 was worse than for CT-921.
Conclusion: Both extracts relieved neuropathic pain; however, CT-921 had a better efficacy-to-adverse effect profile, a rational basis for prioritizing cultivars for future clinical evaluation.
Keywords: cannabinoid analgesia, neuropathic pain, medical marijuana
Introduction
Cannabis preparations relieve distress in many people. Acceptance of cannabis as an option for medical disorders remains controversial, partly because of limited research in many countries. Although cannabis is readily available in Canada, physicians are reluctant to prescribe cannabis without evidence of efficacy and safety. The therapeutic benefit of a cannabis compound cannot be established by consideration of anecdotal reports or a priori reasoning but requires empirical research.
Certain cannabis extracts may have value in relieving chronic pain in humans.1 Neuropathic pain, a debilitating form of chronic pain often caused by disease and nerve injury,2 is particularly difficult to treat clinically.3 Conventional medications provide limited relief from chronic neuropathic pain with disabling side effects. One cannabis extract containing high cannabidiol (CBD) and low Δ9-tetrahydrocannabinol (THC) concentrations markedly reduces the thermal hyperalgesia and mechanical allodynia that develops after chronic constriction injury of sciatic nerve (CCI).4 This attenuation by the cannabis extract is greater than THC alone4 and is similar in magnitude to the CBD-reduced reduction of hypersensitivity to mechanical stimuli in this animal model.5
Determining which specific cannabis cultivar should be considered for the treatment of a medical condition requires assessing the efficacy and safety, including adverse effects. In industrial drug development, preclinical evaluation of the effectiveness to adverse effects profile is used to focus the scope of clinical trials on specific compounds. This approach is rarely used in evaluating dispensary cannabis preparations.6 Rational analgesic use requires determining which of the thousands of available cannabis cultivars are safe and effective. For specific cannabis preparations, evidence is first needed to establish the efficacy-to-adverse effect relationship.
In the present study, we examined this relationship using the CCI model, a gold standard assay for neuropathic pain.7,8 We compared the effects of the extracts of two specific cultivars, CT-921 and CT-928, chosen from pilot studies for their potential therapeutic efficacy.9 The extract of CT-921 contained a concentration ratio of THC:CBD10,11 of 1:20 while the extract of CT-928 had a ratio of 1:0.003.6 However, cannabis cultivars contain over 120 cannabinoids, 120 terpenoids 26 flavonoids, and 11 steroids.12,13 Interactions of the components can influence the effects of a cultivar, as demonstrated by an exaggeration or attenuation of therapeutic benefits and adverse effects of THC and CBD.10,11
Several adverse effects were measured in this study. Many commercial cannabis preparations contain high concentrations of THC and have CNS side effects such as sedation, confusion, and psychosis that limit clinical usefulness.14 The adverse effects accompanying cannabis analgesia in mice generally relate to the THC content.4 CBD is not without untoward effects but it is not psychoactive and may offset some deleterious effects of THC.4,15,16 In these experiments, we measured several behavioral and well-recognized temperature effects.17 Respiratory rate also was measured18 since THC-induced death in laboratory animals is attributable to respiratory failure.19,20 Death rarely occurs clinically from cannabis alone but the combination with an opioid may result in an increased risk of respiratory depression.21,22
Our experiments demonstrate which of two efficacious cultivars has a better relationship of efficacy-to-adverse effects for the treatment of neuropathic pain. They also illustrate the application of a drug development strategy to determine which of a group of dispensary cannabis cultivars help to establish the priority for a clinical trial.
Materials and Methods
Animal Surgery
Procedures were approved by the University of British Columbia’s Animal Care Committee (Approval No. A15-0115). The guidelines of the Animal Care Committee were established for all procedures; animals were treated in accordance with the Canadian Tri-Council and NIH Guidelines for the Care and Use of Laboratory Animals. A total of 252 Albino-Swiss female CD-1 mice (8 weeks old), weighing 25–30 g, were group housed under a 12 h light/12 h dark cycle with free access to food and water. Surgery was performed on mice under isoflurane anesthesia. A 0.5 cm skin incision was made below right hip, parallel to the femur. A 2 mm polyethylene tubing (PE-20) cuff was placed around the sciatic nerve, and the wound sutured using 6–0 polypropylene.7,8 We initially determined the effects of a cultivar extract in mice of both sexes, seeking to confirm that cannabinoids are more potent in producing antinociception in female rats.23 Therefore, we compared the cultivar effects in female to male mice at 1-week after surgery.
Treatment of Cultivar Extracts
Two seed head and leaf specimens of Cannabis sativa cultivars were purchased from CanniMed Therapeutics Inc. (Saskatoon, Sask., Canada) [see Supplementary data for details]. The extractions were performed by Cannevert Therapeutics Ltd (Vancouver, Canada) and extracts were designated CT-921 and CT-928. Each 5 g sample of vacuum-dried seed heads of Cannabis sativa as supplied by CanniMed was ground by hand using a mortar and pestle in 100 mL ethanol at room temperature. A filtrate of the extract was concentrated at 65°C by rotary evaporation, decarboxylated by heating to >100°C and stored under a nitrogen atmosphere at −20°C until use. For an experiment, a sample was dissolved in a vehicle solution containing 1:1:18 of absolute ethanol: ALKAMULS-620 castor oil surfactant: 0.9% saline. Two preparations (extracts CT-921 and CT-928) were made suitable for intravenous (iv) administration by dissolution with vehicle solution. Injection volume was 30 µL for both extracts except for the highest dose of CT-928 which was 40 µL. A blinded experimenter administered the extracts into the mouse tail vein. Evaluations of analgesia were performed by a separate, blinded experimenter. For chemical assay, a sample was dissolved in a small volume of ethanol and analyzed using Shimadzu Prominence high-performance liquid chromatography. These data are presented in mg/kg of dried extract to mouse body weight (Table 1).
 |
Table 1 Cannabinoid Composition of Cannabis Extracts CT-921 and CT-928* |
Nociceptive Assessment
Von Frey filaments (North Coast Medical, Morgan Hill, CA) were used for testing by applying pressure on hind paw ipsilateral to the surgical cuff and recording the response.24 Filaments were applied in ascending order through a mesh floor to the plantar surface. Responses included flinching, licking or biting of the paw, and hind paw withdrawal. The filament was applied 5 times, each application for ~3 s, with a 5 s interval separating each trial. The endpoint was three responses out of five trials. If less than 3 responses, the next higher filament value was used. If the filament on its own lifted the paw the next lower value was used. Allodynia was measured as a threshold response to withdrawal from stimulation applied to the hind paw on the same side as the implanted cuff. A threshold response that was lower than the responses on the un-operated side or in control animals demonstrated allodynia.
Assessment of Chronic Constriction Injury Model
Tests for allodynia were performed by a blinded experimenter on ipsilateral (operated) and contralateral (un-operated) hind paws 1 day prior to surgery and 3, 7, 14, 21, 28, 35 and 42 days after surgery. Twenty naive mice (10 operated and 10 unoperated) were used for assessment of the CCI model. Each mouse was marked on tail with a code for the measurement of the baseline response before surgery. After surgery, the animals were recorded in blind order by another colleague. The allodynia tests were performed at 3, 7, 14, 21, 28, 35 and 42 days after surgery.
Effects of Extract Treatment
Dose–response relationships and time courses were determined after iv injections of the extracts. For the initial studies on sex differences of the responses to CT-921, there was sufficient material for only four doses. In all other experiments, five doses each of CT-921 and CT-928 were used. The mice were randomized to receive different concentrations of extract (website: www.random.org). Forty mice received CT-921 at 5.6, 11.3, 16.9, 22.6 and 45.1 mg/kg and 40 mice received CT-928 at 0.6, 1.2, 2.3, 6.9 and 20.7 mg/kg (8 mice/dose). Vehicle injection was used in eight mice (controls). The dose–response relationships and time courses for the effects of CT-921 and CT-928 provided the ED50 for analgesia (ie, the most accurately determined point on dose–response curve). The effects of the extracts on the responses to von Frey filaments were determined at 10, 20, 40, 60, 120, and 240 min.
Efficacy-to-Adverse Effects Profile
Respiratory rate, rectal temperature and behavioral or locomotor activity were measured by separate, blinded experimenters and expressed with the anti-allodynia data. The experimental humane endpoints of the UBC Facilities Grading System and Response Guide for Rodents 2016 were followed to assess animal health and welfare (see Supplementary data). All experiments were performed by three individuals who were blind to the chemical analysis, substance injection and analysis, or analgesic assay. The researcher who performed the bio-assays was blinded to the substance and its concentration.
Anti-Allodynic Effects
A dose-ranging study was performed for both extracts. In the subsequent dose–response relationship study, threshold responses to von Frey hair were established in groups of 8 mice at 20 min for vehicle and doses of 5.6, 11.3, 22.6 and 45.1 mg/kg for CT-921, and 0.6, 1.2, 2.3, 6.9, 20.7 mg/kg for CT-928. Effective dose for analgesia in 95% of mice (ED95An obtained from dose–response relationship) was chosen as a clinically significant response at which the adverse effects could be determined.
Respiratory Rate
Immediately after iv injection, a mouse was placed into a Falcon tube (3 cm diameter and 12 cm length) with a darkened tip and multiple small holes. The effects on respiratory rate were video recorded for a 10-min interval, beginning at 20 min after injection. Another video recording of a 10-min interval was made at 40 min after injection. A blinded experimenter analyzed the videos in both CT-921 and CT-928 groups, each of 48 mice.
Body Temperature
Rectal temperature was measured with a thermistor probe (BIOSEB, Pinellas Park, USA) inserted ~2 cm into the rectum at 20 and 40 min after injection. The magnitude of the hypothermic response to THC in mice varies markedly with the ambient temperature.25 Therefore, the mice were acclimatized for 1 h to the experiment room maintained at 22–23°C.
Behavioral Activity
Behavioral activity was observed continuously for 4 h after injection, either singly in a small chamber or in groups of 4 in their home cage. Behavior included locomotor activity (walking, running, digging, cage climbing or hanging), grooming, drinking and eating, standing on hind limbs, sniffing air, occasional biting and remaining totally inactive. Sleeping was considered immobility. Behavior was scored on a Likert scale, from most to least active; categories included hyperactive, normal or hypoactive. We used the following criteria for hyperactive, normal, or hypoactive animals which were scored by a blinded observer. Hyperactive animals moved very fast, jumped, were sensitive to touch, difficult to catch after injection (ie, for return to cage), and if unrestrained, would run off the table. Hypoactive animals often would sit without moving from a cage corner and when placed in a cage with other mice, would not interact with the others. These observations were made by observers blinded to the test substances.
Statistical Analyses
To analyze the changes in allodynia over time following surgery, we conducted a 2-factor repeated measures (RM) ANOVA, with limb treatment (operated, unoperated and control) as the between-subjects factor, and time since surgery (0, 3,7,14,21,28,35 and 42 days) as the within-subjects factor. Anti-nociceptive activity was determined at the time of maximal effect (20 min post-injection) by comparing scores to pre-drug baseline values, analyzed with paired sample t-tests; an independent samples t-test also compared post-drug treatment scores to a separate group (n = 8) of vehicle-treated animals. Dose–response relationships were plotted as dose (mg/kg) against log response (force in grams x 10,000) using GraphPad Prism software (v7.0, GraphPad, San Diego, CA). The number of mice was based on GPower analysis for effect size and variance based on previous experiments and pilot studies.26 The dose–response relationships were fitted with a sigmoidal model.27 A one-way ANOVA was used to assess effects on temperature and respiratory rate as well as the effect of sex on reaction to cannabis. Differences were considered statistically significant at P <0.05. Behavioral data were analyzed using Pearson’s chi-squared test with dose at each time point as the independent variable, and Likert activity score as the outcome measure.
Results
Chemical Profiles of Extracts
Table 1 presents the cannabinoid composition of CT-921 and CT-928 as provided by the company that performed the extraction and analysis (see Methods). A comparison of these data shows that all 10 cannabinoid concentrations differed between the extracts. Notably, CT-921 contained a higher concentration of CBD than THC (9.7 versus 0.02 mg/mL). CT-928 contained high THC (7.54 mg/mL) and low CBD (0.49 mg/mL) concentrations. Other cannabinoids present in low concentrations also may have relevance for antinociceptive action (see Discussion).
Validity of Assay for Allodynia
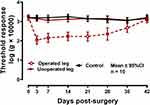
The repeated measures ANOVA indicated a significant main effect of limb treatment [F (2,27) = 94.19; P <0.001] as the operated limb group exhibited significantly reduced paw response thresholds compared to the other two groups. There was also a significant main effect of time [F (7189) = 17.72, P <0.001]. Furthermore, there was a highly significant treatment by time interaction [F(14,189) = 13.67; P <0.001]. Post-hoc analysis indicated that allodynia was present at 3 days after cuff placement and remained approximately constant in magnitude until 28 days (Figure 1). The allodynia diminished to mean control value at 6 weeks. The homologous area on the un-operated leg did not exhibit allodynia. At 7 days after surgery, the responses to von Frey filament testing before and 20 min after vehicle injection were of similar magnitude to the responses evoked in 48 naïve male mice.
The effects of CT-921 were determined in both male and female mice injected with doses of 5.6 to 45.1 mg/kg (n = 8 per group). Females first showed a significant reduction in allodynia at a dose of 16.9 mg/kg (P = 0.006). A significant reduction also was observed at 22.6 and 45.1 mg/kg (P = 0.006). Males showed a significant reduction in allodynia only at the maximum dose of 45.1 mg/kg (see Supplementary Data). The reduced allodynia in both sexes lasted ~2 h. On confirming the previous studies,23 we subsequently used females since the reduction of allodynia in females first occurred at a dose less than in males. Also, the special interest group on Sex, Gender, and Pain, the International Association for the Study of Pain recommends the use of both sexes, or females alone if limited to one sex, to best translate preclinical findings into clinical considerations.28
Dose–Response Relationships of Anti-Allodynic Effects
At 7 days post-surgery, a comparison of pre- to post-drug treatment with CT-921 at doses of 5.6 [t(7) = 1.86, NS] and 11.3 mg/kg [t(7) = 1.33, NS] showed no effect on mechanical allodynia, while doses of 16.9 [t(7) = 4.40, p < 0.005], 22.6 [t(7) = 11.28, p < 0.001] and 45.1 mg/kg [t(7) = 29.24, P < 0.001] decreased it (Figure 2A). Similarly, the post-drug values for the two lower doses did not differ from the vehicle-treated mice [t(14) = 0.94–1.39, NS] whereas the three higher doses significantly increased values compared to controls [t(14) = 4.84–25.14, P <0.001]. The dose–response relationship provided an effective dose at which analgesia was produced in 50% of mice (ED50An). The ED50An for CT-921 was 15.1 mg/kg. CT-921 at 16.9 mg/kg produced a maximum response at 20 min which decayed over 2–4 h (Figure 2B). The ED95An for CT-921 was 29 mg/kg.
At 7 days post-surgery, a comparison of pre to post-drug treatment with CT-928 at the lowest dose of 0.6 mg/kg [t(7) = 2.20, NS] showed a mild but non-significant allodynia. However, allodynia was significant with the 1.2 mg/kg dose [t(7) = 3.49, P = 0.01] and was more robustly significant with the 2.3 mg/kg [t(7) = 11.85, P < 0.001] and 6.9 mg/kg [t(7) = 11.46, P <0.001] doses (Figure 2C). Similarly, the post-drug values for the lowest dose of 0.6 mg was only just significant compared to the vehicle-treated mice [t(14) = 2.58, P <0.05] while notably, the three higher dose values were significantly different compared to controls [t(14) = 3.90–10.7, P <0.005]. The dose–response relationship provided an ED50An of 0.9 mg/kg. Figure 2D shows the time course for the effect of CT-928 at 2.3 mg/kg. At this dose CT-928 produced a large increase in threshold at 10 and 20 min after injection; the response declined from maximum values and lasted 2–4 h. The ED95An for CT-928 was 4.7 mg/kg.
Eight mice administered CT-928 at the highest dose (20.7 mg/kg) exceeded the humane endpoints. Two of the eight mice died spontaneously before euthanization could be performed. The remaining six mice showed a reduced rate of breathing (<95 breaths/min), open mouth breathing and cyanosis. These mice were euthanized with isoflurane and CO2 as per UBC Animal Care Guidelines (UBC Facilities Grading System and Response Guide for Rodents 2016).
Body Temperature
Figure 3A shows that CT-921 at 5.6, 11.3, 22.6 and 45.1 mg/kg, respectively, decreased mean temperature by 4% (P = 0.24), 5% (P = 0.02), 11% (P <0.05) and 14% (P <0.05). Figure 3B shows that CT-928 at 0.6, 1.2, 2.3 and 6.9 mg/kg, respectively, decreased mean temperature by 3% (P = 0.135), 8% (P <0.05), 10% (P <0.05) and 18% (P <0.05). ED95An of CT-928 reduced mean temperature by 6.0°C (P <0.05). CT-921 at ED95An reduced temperature by 4.3°C (P <0.05). CT-928 decreased mean rectal temperature more than CT-921 (P = 0.02).
Respiratory Rate
Both CT-921 and CT-928 depressed mean respiratory rate at 20 min after iv injection in conscious mice (Figure 3C and D). Previous studies showed similar respiratory depression by cannabis extracts or pure THC in anesthetized animals.18 CT-921 at 5.6, 11.3, 22.6 and 45.1 mg/kg, respectively, decreased mean respiratory rate from 208 breaths/min (vehicle administration) to 195 (6%), 171 (18%), 156 (25%) and 100 (52%) breaths/min (P = 0.1, P = 0.4, P = 0.1 and P <0.05). CT-921 at ED95An depressed mean respiratory rate from 208 to 155 breaths/min. However, this depression was not statistically significant while the depression by CT-928 at ED95An was statistically significant. CT-928 at low doses (0.3–0.6 mg/kg) did not change respiratory rate but at higher doses (1.2, 2.3 and 6.9 mg/kg), respectively, decreased mean respiratory rate from 217 breaths/min (vehicle administration) to 136 (37%), 115 (47%) and 85 (61%) breaths/min (P <0.05 and P <0.05; Figure 3D). CT-928 at ED95An depressed mean respiratory rate from 217 to 108 breaths/min. In summary, CT-921 produced less severe respiratory depression than CT-928 when administered in equianalgesic doses, including the ED95An.
Behavioral Activity
Behavioral activity determined from the same mice used in Figure 2 is compiled for CT-921 in Table 2. The chi-squared analysis indicated a significant main effect of the dose of CT-921 on behavior at each of the three different time points [Χ2 (4, N = 40) ≥ 32.48, P = 0.001]. CT-921 at doses (5.6, 11.3 mg/kg) below ED50An did not have an effect on behavior. At 16.9 mg/kg (near ED50An), CT-921 decreased spontaneous locomotion in 5 of 8 mice, measured upon return to the cage. At 22.6 and 45.1 mg/kg, CT-921 reduced locomotor activity in all mice. The effect of CT-921 at 16.9 mg/kg on locomotion lasted for 2 h, and >2 h at the higher doses. CT-921 injection reduced the response to intruder entry into the home cage in 5 of 8 mice at 16.9 mg/kg, and in 8 of 8 mice at 22.6 and 45.1 mg/kg; this effect lasted >2 h (data not compiled in Table 2).
 |
Table 2 Behavior After Intravenous Injection of CT-921 |
Behavioral activity determined from the same mice used in Figure 2 is compiled for CT-928 in Table 3. The chi-squared analysis indicated a significant main effect of the dose of CT-928 on behavior at each of the three different time points [Χ2 (8, N = 40) ≥ 51.28, P = 0.001]. Within a minute after iv injection of CT-928 at doses greater than 0.6 mg/kg, mice exhibited hyperactive behavior. Behavior in 6 of 8 mice at 1.2 mg/kg and all mice at 2.3 and 6.9 mg/kg consisted of aggressively biting the restrainer, fighting, jumping, attempts to escape and hypersensitivity to light touch. The responses were biphasic – hyperactivity for ~20 min gradually followed by hypoactivity lasting >2 h. Administration of CT-928 at ED50An and ED95An resulted in agitation and hyperactivity in contrast to the calmness of mice treated with CT-921.
 |
Table 3 Behavior After Intravenous Injection of CT-928 |
Discussion
In this study, we demonstrate the ability of two specific cannabis cultivars, CT-921 and CT-928, to alleviate pain induced by chronic compression of the sciatic nerve of a mouse, an accepted model of human neuropathic pain. A comparison of the analgesic efficacies and selected side effects of both extracts revealed the best profile consistent with further testing in a clinical trial. While both compounds were effective in relieving pain, one had fewer potentially severe side effects. The efficacy-to-adverse effect profile provides an evidence-based rationale for prioritizing one specific cannabis extract over the other for further development as an analgesic for neuropathic pain. The profile determination provides a means for choosing which of thousands of cultivars has the greatest potential as a clinical analgesic.
The most striking adverse effect was the depression of respiration in conscious mice. CT-928 at a dose that provided analgesia equal to a dose of CT-921, produced greater respiratory depression. At ED95 for analgesia, CT-928 significantly depressed respiratory rate but CT-921 did not. For comparison, there is little literature about the respiratory effects of cannabinoids in conscious animals. Intraperitoneal THC (10 mg/kg) in rats reduces respiratory rate by ≤15% during wakefulness and sleep states.29 Studies in anesthetized cats and rats show that iv administration of Cannabis extract or THC reduces respiratory rate in a dose-dependent manner,18 producing a maximum of >60% depression at 1.5–2.0 mg/kg THC.30 THC at iv doses of ≥10 mg/kg causes terminal depression of respiration in the barbiturate anesthetized cat.31 However, the type of anesthesia would likely have contributed to the THC effects in the previous investigations.32 In the present studies, a high dose of CT-928 resulted in respiratory depression but did not meet the humane endpoints, requiring euthanization. The greater reduction of respiratory rate by CT-928 than CT-921 compromised the safety in conscious animals administered CT-928.
At analgesic doses, both extracts produced the adverse effects on behavior and body temperature. CT-928 caused aggressive behavior followed by long-lasting hypoactivity, similar to other cannabis extracts with a high THC concentration.27 At ED95 for analgesia, temperature decreased much more after administration of CT-928 than CT-921. CT-928 caused a 6°C decrease in core temperature - a remarkable reduction in the conscious animal.
In controlled studies in humans, cannabis and THC produce only mild respiratory depression.33 The effects of THC, morphine and other depressants on respiration are similar in animal experimentation.31 While cannabis side-effects in social use are non-life threatening, the situation in individuals using opioids or with chronic illness is more complex. Minimizing adverse effects is a sensible strategy in choosing which cultivars to investigate. Decreased risk of respiratory depression results in an increased safety aspect for a cannabis analgesic. However, the extent that such adverse effects of cannabis in mice correspond to those in human studies requires further investigation.
Cannabis cultivar extracts may decrease the opioid dose needed for analgesia34 reducing the possibility of opioid side effects and addiction. An analgesic synergy with an additive side effect on locomotion has been demonstrated with a cannabinoid–opioid combination in an animal model of neuropathic pain.35 THC can increase the analgesic potency of opioids but has varying effects on ventilation in monkeys,22,36 consistent with clinical studies.21,33 Clinical trials would require testing combinations of opioids with selected cultivars. Considering thousands of available cultivars, clinical trials of all candidate cultivars would be a Herculean task. Preclinical elimination of candidates by establishing the best adverse effect/efficacy profile renders efficient clinical trial testing feasible.
CT-921 and CT-928 contained THC and CBD and both cannabinoids likely contributed to the analgesic effects. The amount of THC-related antinociception by CT-928 at ED50An was greater than that if THC was administered without the other components of the cultivar extract. This has been previously shown for iv administration of a cannabis extract versus pure THC in the mouse tail-flick model.37 CBD by itself reduces mechanical allodynia11,38 and in combination with a low dose of THC, enhances the antinociception without THC-like side effects in the CCI model.4 Other constituents of cannabis extracts on their own have antinociceptive effects.39
At equivalent analgesia (ED95An) CT-921 contains less than 20% of the THC present in CT-928. Therefore, a component or components additional to THC in CT-921 must contribute to the equivalent analgesia (cf. Ref. 11). This effect was also evident from the effects of both extracts on temperature. CT-921 would require twice its ED95An to produce the same 6°C decrease caused by CT-928 at ED95An.
Due to many interacting components in an extract, advancement to therapeutic use requires identification of a specific cultivar (cf. Table 1 and Ref. 12). This is extremely complex because of limitations in plant phenotyping, chemotyping and genotyping.40 A practical method for establishing the reproducibility of the therapeutic action of a specific cultivar is the maintenance of an assemblage of specific plant cultivars and utilizing an animal test (ie, a bioassay). A specific cultivar extract shown to be effective in an animal model and subsequently in humans may be altered by cultivation. Preclinical testing of each batch of preparation would provide a guarantee of the stability of the growing process. To ascertain that each batch will be effective one should repeat the original animal testing to see if the desired effect is unchanged. After initial demonstration of feasibility, detailed protocols and rigid procedures must be followed.
Neuropathic pain is very difficult to treat. Both specific cannabis cultivar extracts CT-921 and CT-928 alleviated neuropathic pain in an accepted mouse model. However, CT-921 (low THC, high CBD) had lesser side effects than did CT-928 (high THC, low CBD), resulting in a better efficacy-to-adverse effect profile. Prior to acceptance for clinical therapy, the adverse effects and efficacy must be ascertained in humans, a difficult and expensive process. Animal testing identifies compounds with the greatest chance of clinical success and reduces the developmental costs of clinical trials of >700 classified cultivars. It can also confirm the efficacy of the drug that remains after continuing cultivation. Here, the difference between the efficacy-to-adverse effect profiles of two cultivars in a model of neuropathic pain illustrates the use of animal testing to establish the priority for clinical testing.
Conclusion
Two dissimilar cannabis extracts, CT-921 and CT-928, produced analgesia. At equianalgesic doses, CT-921 had no important side effects, while CT-928 produced a significant depression of respiratory rate and body temperature and produced undesirable behavior. The use of efficacy-to-adverse effect profile is a rational basis for choosing between specific cannabis cultivars for further investigations in the treatment of neuropathic pain. This maximizes the chance of success of an expensive clinical trial.
Acknowledgments
The authors thank Ying Dong for technical assistance and Dr Andrew Hegle for preparing the cultivar extracts and HPLC analysis and Dr Richard Wall for his comments on the analysis.
Disclosure
Dr Elham Rouhollahi had Postdoctoral Fellowship supported by the MITACS Accelerate Program (#IT10243), University of British Columbia, jointly with Cannevert Therapeutics Ltd.
Dr MacLeod reports being a colleague and friend of Dr E Puil who has recorded a conflict of interest. Dr Bernard A. MacLeod is a minor shareholder in Veritas Pharma Inc which is research-based cannabis company.
Dr Barr reports grants and personal fees from Cannevert Therapeutics, grants from Global Cannabis Applications Corp, Emerald Health Therapeutics, and Entourage Biosciences, and personal fees from Medipure Pharmaceuticals and Vitality Biopharma, outside the submitted work; and has been a scientific advisor to Emerald Health Therapeutics, Cannevert Therapeutics, Global Cannabis Applications Corp, Medipure Pharmaceuticals, Vitality Biopharma and Oakum Cannabis Corp. Dr Puil reports personal fees from Cannevert Therapeutics Ltd, during the conduct of the study; has been a consultant with Cannevert Therapeutics Ltd, and is a minor shareholder in Veritas Pharma Inc., a research-based cannabis company. The authors report no other potential conflicts of interest for this work.
References
1. Nurmikko TJ, Serpell MG, Hoggart B, Toomey PJ, Morlion BJ, Haines D. Sativex successfully treats neuropathic pain characterised by allodynia: a randomised, double-bind, placebo-controlled clinical trial. Pain. 2007;133(13):210–220. doi:10.1016/j.pain.2007.08.028
2. Treede RD, Jensen TS, Campbell JN, et al. Redefinition of neuropathic pain and a grading system for clinical use: consensus statement on clinical and research diagnostic criteria. Neurology. 2008;70(18):1630–1635. doi:10.1212/01.wnl.0000282763.29778.59
3. Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: systematic review, meta-analysis and updated NeuPSIG recommendations. Lancet Neurol. 2015;14(2):162–173. doi:10.1016/S1474-4422(14)70251-0
4. Casey SL, Atwal N, Vaughan CW. Cannabis constituent synergy in a mouse neuropathic pain model. Pain. 2017;158(12):2452–2460. doi:10.1097/j.pain.0000000000001051
5. Costa B, Trovato AE, Comelli F, Giagnoni G, Colleoni M. The non-psychoactive cannabis constituent cannabidiol is an orally effective therapeutic agent in rat chronic inflammatory and neuropathic pain. Eur J Pharmacol. 2007;556(13):75–83. doi:10.1016/j.ejphar.2006.11.006
6. Häuser W, Petzke F, Fitzcharles MA. Efficacy, tolerability and safety of cannabis-based medicines for chronic pain management an overview of systematic reviews. Eur J Pain. 2018;22(3):455–470. doi:10.1002/ejp.1118
7. Benbouzid M, Pallage V, Rajalu M, et al. Sciatic nerve cuffing in mice: a model of sustained neuropathic pain. Eur J Pain. 2008;12(5):591–599. doi:10.1016/j.ejpain.2007.10.002
8. Yalcin I, Megat S, Barthas F, et al. The sciatic nerve cuffing model of neuropathic pain in mice. J Vis Exp. 2014;89:51608.
9. Rouhollahi E, Kiyota B, Barr A, Puil E, MacLeod BA. Effects of cannabis extract on murine neuropathic pain model. British Pharmacol Society Proc. 2018. http://www.pa2online.org/abstract/abstract.jsp?abid=33490&author=Rouhollahi&cat=-1&period=-1.
10. Goncalves J, Rosado T, Soares S, et al. Cannabis and its secondary metabolites: their use as therapeutic drugs, toxicological aspects, and analytical determination. Medicines. 2019;6(1):31. doi:10.3390/medicines6010031
11. Comelli F, Giagnoni G, Bettoni I, Colleoni M, Costa B. Antihyperalgesic effect of a Cannabis sativa extract in a rat model of neuropathic pain: mechanisms involved. Phytother Res. 2008;22(8):1017–1024. doi:10.1002/ptr.2401
12. ElSohly MA, Radwan MM, Gul W, Chandra S, Galal A. Phytochemistry of Cannabis sativa L. Phytocannabinoids. 2017;103:1–36.
13. Jin D, Jin S, Chen J Cannabis classification systems and growth trends of the North American medical cannabis industry.
14. D’Souza DC, Perry E, MacDougall L, et al. The psychotomimetic effects of intravenous Δ9-tetrahydrocannabinol in healthy individuals: implications for psychosis. Neuropsychopharmacology. 2004;29(8):1558–1572. doi:10.1038/sj.npp.1300496
15. Niesink RJ, van Laar MW. Does cannabidiol protect against adverse psychological effects of THC? Front Psychiatry. 2013;4:130. doi:10.3389/fpsyt.2013.00130
16. Morgan CJA, Gardener C, Schafer G, et al. Sub-chronic impact of cannabinoids in street cannabis on cognition, psychotic-like symptoms and psychological well-being. Psychol Med. 2012;42:391–400. doi:10.1017/S0033291711001322
17. Fitton AG, Pertwee RG. Changes in body temperature and oxygen consumption rate of conscious mice produced by intra-hypothalamic and intra-cerebroventricular injections of Δ9-tetra-hydrocannabinol. Br J Pharmacol. 1982;75:409–414. doi:10.1111/j.1476-5381.1982.tb08802.x
18. Graham JDP, Li DMF. Cardiovascular and respiratory effects of cannabis in cat and rat. Br J Pharmacol. 1973;49(1):1–10. doi:10.1111/j.1476-5381.1973.tb08262.x
19. Rosenkrantz H, Heyman IA, Braude MC. Inhalation, parenteral and oral LD50 values of Δ9-tetrahydrocannabinol in Fischer rats. Toxicol Appl Pharmacol. 1974;28(1):18–27. doi:10.1016/0041-008X(74)90126-4
20. Phillips RN, Turk RF, Forney RB. Acute toxicity of Δ9-tetrahydrocannabinol in rats and mice. Proc Soc Exp Biol Med. 1971;136(1):260–263. doi:10.3181/00379727-136-35241
21. Johnstone RE, Lief PL, Kulp RA, Smith TC. Combination of delta-9-tetrahydrocannabinol with oxymorphone or pentobarbital: effects on ventilatory control and cardiovascular dynamics. Anesthesiology. 1975;42:674–684. doi:10.1097/00000542-197506000-00009
22. Weed PF, Gerak LR, France CP. Ventilatory-depressant effects of opioids alone and in combination with cannabinoids in rhesus monkeys. Eur J Pharmacol. 2018;833:94–99. doi:10.1016/j.ejphar.2018.05.041
23. Craft RM, Marusich JA, Wiley JL. Sex differences in cannabinoid pharmacology: A reflection of differences in the endocannabinoid system? Life Sci. 2013;92:476–481. doi:10.1016/j.lfs.2012.06.009
24. Matthew JG, Bradman F, Salio C, Merighi A. Practical mechanical threshold estimation in rodents using von Frey hairs/Semmes–Weinstein monofilaments: towards a rational method. J Neurosci Meth. 2015;255:92–103. doi:10.1016/j.jneumeth.2015.08.010
25. Haavik CO, Hardman HF. Evaluation of the hypothermic action of tetrahydrocannabinols in mice and squirrel monkeys. J Pharmacol Exp Ther. 1973;187(3):568–574.
26. Mayr S, Erdfelder E, Buchner A, Faul F. A short tutorial of GPower. Tutor Quant Methods Psychol. 2007;3(2):51–59. doi:10.20982/tqmp.03.2.p051
27. Sañudo-Peña MC, Romero J, Seale GE, Fernandez-Ruiz JJ, Walker JM. Activational role of cannabinoids on movement. Eur J Pharmacol. 2000;391(3):269–274. doi:10.1016/S0014-2999(00)00044-3
28. Greenspan JD, Craft RM, LeResche L, et al. Studying sex and gender differences in pain and analgesia: A consensus report. Pain. 2007;132:26–45. doi:10.1016/j.pain.2007.10.014
29. Carley DW, Paviovic S, Janelidze M, Radulovacki M. Functional role for cannabinoids in respiratory stability during sleep. Sleep. 2002;25:391–398. doi:10.1093/sleep/25.4.388
30. McConnell WR, Dewey WL, Harris LS, Borzelleca JF. A study of the effect of delta 9-tetrahydrocannabinol (delta 9-THC) on mammalian salivary flow. J Pharmacol Exp Ther. 1978;206:567–573.
31. Doherty PA, McCarthy LE, Borison HL. Respiratory and cardiovascular depressant effects of nabilone, N-methyllevonantradol and Δ9-tetrahydrocannabinol in anesthetized cats. J Pharmacol Exp Ther. 1983;227(2):508–516.
32. Jandhyala BS, Hamed AT. Pulmonary and systemic hemodynamic effects of delta 9-tetrahydrocannabinol in conscious and morphine-chloralose-anaesthetised dogs: anesthetic influence on drug action. Eur J Pharmacol. 1978;53:63–68. doi:10.1016/0014-2999(78)90268-6
33. Malit LA, Johnstone RE, Bourke DI, Kulp RA, Klein V, Smith TC. Intravenous delta9-tetrahydrocannabinol: effects on ventilatory control and cardiovascular dynamics. Anesthesiology. 1975;42(6):666–673. doi:10.1097/00000542-197506000-00008
34. Babalonis S, Lofwall MR, Sloan PA, Nuzzo PA, Fanucchi LC, Walsh SL. Cannabinoid modulation of opioid analgesia and subjective drug effects in healthy humans. Psychopharmacology. 2019;236(11):3341–3352. doi:10.1007/s00213-019-05293-1
35. Kazantzis NP, Casey SL, Seow PW, Mitchell VA, Vaughan CW. Opioid and cannabinoid synergy in a mouse neuropathic pain model. Br J Pharmacol. 2016;173(16):2521–2531. doi:10.1111/bph.13534
36. Maguire DR, France CP. Impact of efficacy at the µ-opioid receptor on antinociceptive effects of combinations of µ-opioid receptor agonists and cannabinoid receptor agonists. J Pharmacol Exp Ther. 2014;351:383–389. doi:10.1124/jpet.114.216648
37. Varvel SA, Bridgen DT, Tao Q, Thomas BF, Martin BR, Lichtman AH. Δ9-Tetrahydrocannbinol accounts for the antinociceptive, hypothermic, and cataleptic effects of marijuana in mice. J Pharmacol Exp Ther. 2005;314(1):329–337. doi:10.1124/jpet.104.080739
38. De Gregorio D, McLaughlin RJ, Posa L, et al. Cannabidiol modulates serotonergic transmission and reverses both allodynia and anxiety-like behavior in a model of neuropathic pain. Pain. 2019;160(1):136–150. doi:10.1097/j.pain.0000000000001386
39. Klauke AL, Racz I, Pradier B, et al. The cannabinoid CB2 receptor-selective phytocannabinoid beta-caryophyllene exerts analgesic effects in mouse models of inflammatory and neuropathic pain. Eur Neuropsychopharmacol. 2014;24(4):608–620. doi:10.1016/j.euroneuro.2013.10.008
40. Russo EB. The case for the entourage effect and conventional breeding of clinical cannabis: no “strain,” no gain. Front Plant Sci. 2019;9:1969. doi:10.3389/fpls.2018.01969
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.