Back to Journals » Journal of Inflammation Research » Volume 15
Cannabidiol Inhibits Inflammation Induced by Cutibacterium acnes-Derived Extracellular Vesicles via Activation of CB2 Receptor in Keratinocytes
Authors Jiang Z , Jin S, Fan X , Cao K, Liu Y, Wang X , Ma Y, Xiang L
Received 14 May 2022
Accepted for publication 26 July 2022
Published 11 August 2022 Volume 2022:15 Pages 4573—4583
DOI https://doi.org/10.2147/JIR.S374692
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Ziqi Jiang,* Shanglin Jin,* Xiaoyao Fan, Ke Cao, Ye Liu, Xuan Wang, Ying Ma, Leihong Xiang
Department of Dermatology, Huashan Hospital, Fudan University, Shanghai, 200040, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Leihong Xiang; Ying Ma, Department of Dermatology, Huashan Hospital, Fudan University, No. 12 Wulumuqizhong Road, Shanghai, 200040, People’s Republic of China, Tel: +86 21 52889999, Fax: +86 21 62489191, Email [email protected]; [email protected]
Background: Acne is a common inflammatory skin disease, while cannabidiol (CBD) is a representative non-psychoactive phytocannabinoid which has been proved to exert universal anti-inflammatory properties. This study aimed to explore the effect of CBD on acne inflammation induced by Cutibacterium acnes-derived extracellular vesicles (CEVs) in keratinocytes and reveal the underlying mechanisms.
Methods: Normal human epidermal keratinocytes (NHEKs) were stimulated by CEVs in the presence of CBD or vehicle. Interleukin (IL)-6, IL-8, and tumor necrosis factor (TNF)-α levels were examined by RT-PCR and ELISA. The expression of cannabinoid type-2 (CB2) receptor and transient receptor potential vanilloid type-1 (TRPV1) was detected by Western blotting. TNF-α levels in the presence of CB2 receptor antagonist (AM630) or TRPV1 antagonist (Capsazepine) were detected by RT-PCR. The activation of MAPK and NF-κB signaling pathways and the nuclear translocation of NF-κB p65 upon CBD treatment were analyzed by Western blotting and immunofluorescence assay, respectively.
Results: The expression of inflammatory cytokines (IL-6, IL-8 and TNF-α) in CEVs-stimulated NHEKs was suppressed by CBD. CB2 receptor expression was upregulated by CBD, whereas CEVs-promoted TRPV1 expression was downregulated by CBD. AM630 reversed TNF-α levels inhibited by CBD. Capsazepine exerted an inhibitory effect on CEVs-induced inflammation and had synergistic effect with CBD. The phosphorylation of ERK1/2 and NF-κB p65 and nuclear translocation of NF-κB p65 were induced by CEVs but reduced by CBD.
Conclusion: The results indicated that CBD could inhibit inflammation induced by CEVs in NHEKs, which was mediated by activation of CB2 receptor and enhanced by the TRPV1 antagonist, through inactivation of the MAPK and NF-κB signaling pathways. CBD might be a potential novel strategy for acne treatment in the future.
Keywords: cannabidiol, acne, inflammation, Cutibacterium acnes, keratinocytes
Introduction
Acne vulgaris, a chronic inflammatory skin disease involving the pilosebaceous unit, is one of the most common diseases in dermatology that affects 80–85% of individuals globally.1 Some acne lesions may evolve into permanent scarring, which has huge impact on patients’ physical and psychological health.2 Four main factors implicated in the pathogenesis of acne are: excess sebum production, follicular hyperkeratinization, skin microorganisms such as Cutibacterium acnes (C. acnes) colonization of the follicle, inflammation and immune response.3 C. acnes is a gram-positive anaerobic bacteria found as a part of normal skin flora and plays a crucial role in acne progression.4 C. acnes can increase the expression of inflammatory cytokines such as interleukin (IL)-6, IL-8, tumor necrosis factor (TNF)-α, and promote inflammation through activating the mitogen-activated protein kinase (MAPK) and nuclear factor-κB (NF-κB) signaling pathways, resulting in the innate immune response.5 Furthermore, recent studies have found that C. acnes produced extracellular vesicles (EVs), which could increase the IL-8 secretion of keratinocytes, thus contributing to the pathogenesis of acne.6
Many medications are available for acne, but there are limitations due to the increasing antibiotic resistance and various side effects such as abnormal dryness of the skin and mucous membranes caused by isotretinoin, an irritating effect of topical medicines.7 Therefore, several alternative treatments especially botanical extracts like Polyphyllin I, Kaempferia parviflora Extract, Punica granatum Linne, also cannabidiol (CBD), have been investigated and proved to be potential anti-acne agents.8–10 CBD, extracted from Cannabis Sativa, is one of the most studied phytocannabinoids without psychoactivity or addiction.11 A plethora of different receptors such as cannabinoid type-1/2 (CB1/2) receptor, transient receptor potential vanilloid type-1 (TRPV1) and peroxisome proliferator-activated receptor γ (PPARγ) can be activated or antagonized by CBD.12 Accordingly, CBD possesses extensive properties and has already been applied to the treatment of epilepsy and multiple sclerosis.13,14 Besides, studies in skin diseases suggested the inhibition of inflammation in keratinocytes by CBD, which might be mediated by CB2 receptor and TRPV1.15,16 Intriguingly, recent studies highlighted the potential use of cannabidiol (CBD) in acne for its anti-inflammatory properties.17 An in vitro study demonstrated the sebostatic and anti-inflammatory effects of CBD on sebocytes, while a subsequent clinical trial using BTX 1503 (a 5% CBD topical solution) preliminarily confirmed the therapeutic effect and safety of CBD in acne patients.18,19 However, further clinical efficacy and related mechanisms of CBD in acne treatment still remain to be resolved.
Herein, we established an in vitro acne model in normal human epidermal keratinocytes (NHEKs) irritated by C. acnes-derived extracellular vesicles (CEVs). We evaluated the effect of CBD on inflammation and revealed the underlying molecular mechanisms in the context of NHEKs under the acne-like conditions.
Materials and Methods
Ethics
The research was approved by the Ethics Committee of Huashan Hospital Fudan University (Shanghai, China) and conducted according to the principles of the Declaration of Helsinki. Written informed consent was signed by all participants (acne patients and NHEK donors).
Isolation and Identification of CEVs
The clinical strains of C. acnes were isolated from the lesions of acne patients in the dermatology department of Huashan Hospital Fudan University and cultured according to the methods in our previous study.20 CEVs were isolated according to literature methods with some modifications.6 The cell-free culture supernatant of C. acnes was collected and sequentially centrifuged at 2000×g for 10 min and 10,000×g for 30 min at 4°C. The supernatant was filtered with a 0.22-μm membrane filter (Merck Millipore, Darmstadt, Germany) and further ultracentrifuged at 100,000×g for 70 min at 4°C (Beckman Coulter, Fullerton, CA). The pellet was washed with phosphate buffered saline (PBS), harvested by ultracentrifugation at 100,000×g for 70 min at 4°C, finally resuspended in PBS and stored at −80°C. The structure and size of CEVs were analyzed by transmission electron microscopy (TEM) (JEM 2100, Tokyo, Japan) as previously described.21
Cell Culture and Intervention
NHEKs were isolated from human foreskin tissues according to the method described previously and were cultured in Keratinocyte Growth Medium 2 (KGM2) (PromoCell, #C-20011, Heidelberg, Germany) under standard conditions.22 Cells were seeded into 6-well culture plates at a cell density of 1×105 cells per well. CBD solution (Sorrento Therapeutics, CA, USA) were dissolved in KGM2. CB2 receptor antagonist AM630 (Sigma, #SML0327, MO, USA) and TRPV1 antagonist Capsazepine (Sigma, #C191, MO, USA) were dissolved in dimethyl sulfoxide (DMSO). For the CEV group and (CEV+CBD) group, cells were treated with either the vehicle or CBD for 18 h, and then stimulated with CEVs. For the (CEV+AM630/Capsazepine) group, cells were treated with AM630 or Capsazepine for 20 h, and then stimulated with CEVs. For the (CEV+CBD+AM630/Capsazepine) group, cells were sequentially treated with AM630 or Capsazepine for 2 h, CBD for 18 h, and CEVs for 12 h.
Real-Time Quantitative Reverse-Transcription PCR
NHEKs treated as described above were lysed and total RNA was isolated with a quick RNA extraction kit (Zymo Research, #TR205, Beijing, China) according to the manufacturer’s instructions. mRNA was reverse-transcribed into cDNA using the PrimeScript RT Master Mix Kit (Takara, Shiga, Japan). Next, qRT-PCR was conducted in ABI Quantstudio 6 Flex system (ThermoFisher, CA, USA) using the TB Green Premix Ex Taq II Kit (Takara, Shiga, Japan) as following settings: initial denaturation at 95°C for 30s, followed by 40 cycles of 95°C for 5 s, 60°C for 34s. GAPDH was used as the endogenous control. All primers were purchased from BioTNT (Shanghai, China) and their sequence were as follows: GAPDH, 5’-GGG AAG GTG AAG GTC GGA GT-3’ (forward) and 5’-GGG GTC ATT GAT GGC AAC A-3’ (reverse); IL-6, 5’-AAC AAC CTG AAC CTT CCA AAG-3’ (forward) and 5’-CAA ACT CCA AAA GAC CAG TGA-3’ (reverse); IL-8, 5’-CTG TTA AAT CTG GCA ACC CTA-3’ (forward) and 5’-GTG AGG TAA GAT GGT GGC TAA-3’ (reverse); TNF-α, 5’-CAG GAC TTG AGA AGA CCT CAC-3’ (forward) and 5’-GTC TGG AAA CAT CTG GAG AGA-3’ (reverse).
Western Blotting Analysis
Western blotting analysis was performed according to the previous methods with some modifications.23 Briefly, total protein was extracted from NHEKs treated as described above by RIPA lysis buffer (Beyotime, Shanghai, China) and the protein contents were determined by BCA protein assay (Solarbio, Beijing, China). Equal amounts of protein were separated by 10% SDS-PAGE and transferred onto a nitrocellulose membrane (Merck Millipore, Darmstadt, Germany). The membrane was blocked with TBST buffer containing 5% skim milk and then incubated overnight at 4°C with primary antibodies listed as follows: IL-6 rabbit antibody, IL-8 rabbit antibody (Abcam, Massachusetts, UK); TNF-α mouse antibody, CB2 receptor mouse antibody (Santa Cruz Biotechnology, Dallas, USA); p44/42 MAPK (Erk1/2) rabbit antibody, phospho-p44/42 MAPK (Erk1/2) (Thr202/Tyr204) rabbit antibody, NF-κB p65 rabbit antibody, phospho-NF-κB p65 (Ser536) rabbit antibody (Cell Signaling Technology, Danvers, USA); β-actin mouse antibody, β-tubulin mouse antibody (Abmart, Shanghai, China); TRPV1 mouse antibody (Proteintech, Illinois, USA). Next, the membrane was washed with TBST and incubated with the horseradish peroxidase (HRP)-conjugated secondary antibody (Beyotime, Shanghai, China) for 1 h at room temperature. Protein bands were visualized on Tanon-5200 Chemiluminescent Imaging System (Tanon Science & Technology, Shanghai, China) after adding Immobilon Western Chemiluminescent HRP Substrate (Sigma, MO, USA). Intensity of the bands was analyzed and quantified using the ImageJ software.
Enzyme-Linked Immunosorbent Assay
Supernatants of NHEKs treated with CBD or vehicle following CEVs stimulation were harvested through centrifugation at 1000×g for 20 min. Human IL-6, IL-8 and TNF-α protein levels in the cell-free supernatant were determined using the enzyme-linked immunosorbent assay (ELISA) kit (Multi Sciences, Hangzhou, China) following the manufacturer’s instructions. The optical density at 450 nm and 570 nm (the corrected wavelength) were measured using Multiskan GO reader (ThermoFisher, CA, USA).
Immunofluorescence Assay
NHEKs treated with either CBD or vehicle following CEVs stimulation were washed with PBS and fixed with 4% paraformaldehyde for 30 min. Cells were permeabilized with 0.5% Triton X-100 for 30 min and blocked with 5% BSA for 1 h at room temperature. After a PBS wash, cells were incubated overnight at 4°C with NF-κB p65 rabbit antibody (Cell Signaling Technology, Danvers, USA) (diluted with 5% BSA) and then incubated with goat anti-mouse IgG H&L (Alexa Fluor® 488) (Abcam, Massachusetts, UK) for 1 h at room temperature in the dark. Finally, cells were stained with DAPI (Beyotime, Shanghai, China) and observed under fluorescence microscope (NIKON, Tokyo, Japan).
Statistical Analysis
All data are presented as mean ± S.E.M. of at least three independent experiments. Statistical analysis between two groups was performed by Student’s t-test, and statistical analysis among three or more than three groups was performed by ANOVA using GraphPad Prism (version 8.0, GraphPad Software Inc., CA, USA). P<0.05 was considered statistically significant.
Results
CEVs Induced Inflammatory Response in NHEKs
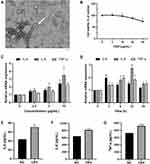
We first established an ideal in vitro acne model in keratinocytes. We extracted CEVs from C. acnes culture supernatants and showed the bilayer spherical morphology of CEVs with TEM (Figure 1A). The size of CEVs ranged from 40 nm to 90 nm. CEVs did not influence the cell viability of NHEKs at concentrations lower than 20 μg/mL (Figure 1B). Next, we verified the immunogenicity of CEVs by monitoring the production of inflammatory cytokines, which exhibited an increase of IL-6, IL-8 and TNF-α in mRNA levels (Figure 1C and D) and in protein levels (Figure 1E–G) in a concentration- and time-dependent manner. In particular, IL-8 expression level was remarkably upregulated. Thus, the optimal stimulation condition for establishing an in vitro acne model was considered to be the incubation of NHEKs with CEVs at a concentration of 10 μg/mL for 12 h.
CBD Suppressed the Inflammatory Reaction Induced by CEVs in NHEKs
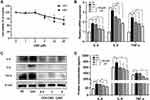
We first confirmed by CCK-8 assay that the appropriate working concentration of CBD was 0–2 μM (Figure 2A). Next, we examined the effect of CBD on the expression of inflammatory cytokines IL-6, IL-8 and TNF-α, by stimulating NHEKs with CBD (0.5, 1 and 2 μM) and CEVs (10 μg/mL) sequentially. Compared with the vehicle, CBD decreased the mRNA levels of IL-6, IL-8 and TNF-α in CEVs-stimulated NHEKs, in a concentration-dependent manner (Figure 2B). Similarly, using Western blotting analysis and ELISA, we also found that CBD could reduce the protein expression and secretion of IL-6, IL-8 and TNF-α in a concentration-dependent manner (Figure 2C and D). These findings suggested that CBD exerted an anti-inflammatory effect in CEVs-stimulated NHEKs.
Anti-Inflammatory Action of CBD Mediated by CB2 Receptor and Enhanced by TRPV1 Antagonist
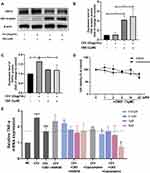
Next, we investigated the anti-inflammatory action of CBD in terms of molecular mechanisms. First, we examined the expression of CB2 receptor and TRPV1 in NHEKs. The protein levels of CB2 receptor in NHEKs, although low at baseline, were upregulated in the presence of CBD (Figure 3A and B). In contrast, the TRPV1 protein levels increased under CEVs stimulation, yet decreased significantly upon CBD treatment (Figure 3A and C).
To further confirm the roles of the two receptors, CB2 receptor antagonist AM630 and TRPV1 antagonist Capsazepine were used in our study. There was no significant difference in cell viability between NHEKs co-stimulated with CBD (1 μM) and different concentrations (0~10 μM) of AM630 or Capsazepine (Figure 3D). We investigated the effects of AM630 and Capsazepine on TNF-α levels in CEVs-stimulated NHEKs in the presence or absence of CBD. Compared with the CEV group, no effect was observed on TNF-α levels when CEVs-stimulated NHEKs were treated with AM630. However, TNF-α levels initially reduced by CBD were increased after co-stimulation with CBD and low concentrations of AM630 (Figure 3E), indicating that the anti-inflammatory effect of CBD on CEVs-stimulated NHEKs was mediated by CB2 receptor.
On the other hand, Capsazepine alone exerted a statistically significant inhibition of TNF-α in CEVs-stimulated NHEKs. Consistent with this result, we found that compared with the group of CEVs-stimulated NHEKs treated with CBD, co-stimulation with CBD and Capsazepine further downregulated TNF-α levels (Figure 3E). It suggested that Capsazepine had synergistic effect with CBD, and CBD might act as a TRPV1 antagonist similar to Capsazepine in the anti-inflammatory process.
Inhibitory Effect of CBD on MAPK and NF-κB Signaling Pathways
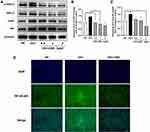
To investigate whether MAPK and NF-κB signaling pathways are involved in the anti-inflammatory effect of CBD, the expression of ERK1/2 MAPK and NF-κB p65 proteins in CEVs-stimulated NHEKs in the presence of CBD or vehicle were detected. In the MAPK signaling pathway, CEVs increased the level of phosphorylated ERK1/2, whereas CBD treatment prior to CEVs stimulation prevented ERK1/2 phosphorylation (Figure 4A and B). However, CBD resulted in only slight changes in total ERK1/2 levels in CEVs-stimulated NHEKs. Similarly in the NF-κB signaling pathway, phosphorylated NF-κB p65 level was significantly increased by CEVs and then decreased concentration-dependently by CBD (Figure 4A and C). Total NF-κB p65 levels were also increased by CEVs, but its inhibition by CBD was not as significant until the concentration reached 2μM. In addition, immunofluorescence staining was performed to examine the location of NF-κB p65 in NHEKs. We found that NF-κB p65 of the negative control group was mainly distributed in cytoplasm and shifted into the nuclei upon CEVs stimulation. While the nuclear translocation was dramatically suppressed by CBD (Figure 4D). These data suggested that the inhibitory effect of CBD on CEVs-induced inflammation might involve inactivation of the MAPK and NF-κB signaling pathways.
Discussion
Acne vulgaris is a multifactorial inflammatory skin disease. Inflammation is observed in the onset, development and resolution stages of acne.24 C. acnes, by secreting a series of inflammatory stimulators, can trigger the local inflammatory reaction and systemic immune response.25 It has been reported that various conventional C. acnes extracts were used to induce acne-like phenotypes, but their preparing process was complicated.26 While CEV is a new type of C. acnes extract isolated from the culture supernatant of C. acnes. We found that NHEKs displayed a time- and concentration-dependent increase in the levels of inflammatory cytokines IL-6, IL-8 and TNF-α following stimulation with CEVs. Notably, the effect on IL-8 was particularly robust. Our results are in perfect agreement with the changes in gene expression, especially the strong increase in IL-8 gene in inflammatory acne lesions that have been described in a gene array study.27 Previous studies also showed that IL-6, IL-8 and TNF-α were detected in the serum and skin lesions of acne patients, and the expression levels were much higher than those in healthy individuals.28,29 These results verified the immunogenicity of CEV and its role in establishing an in vitro acne model.
CBD is a representative phytocannabinoid which has been proved to exert universal anti-inflammatory effects on several cell types such as microglia and synovial fibroblasts. Consequently, CBD may antagonize the progression of inflammation in Parkinson’s disease, rheumatoid arthritis and diabetes.30–32 Besides, CBD can also inhibit skin inflammation. The inflammatory cytokines levels could be downregulated by CBD in an in vitro allergic contact dermatitis (ACD) model which was established by poly-(I:C)-irritated HaCaT cells (an immortalized keratinocyte cell line).16 IL-8 release induced by TNF-α in HaCaT cells could also be reduced by CBD.15 In this study, we aimed at investigating whether CBD could inhibit acne inflammation in keratinocytes. We found that CBD strongly downregulated both mRNA and protein levels of inflammatory cytokines IL-6, IL-8 and TNF-α induced by CEVs in NHEKs in a concentration-dependent manner, which was consistent with previous studies describing the anti-inflammatory effect of CBD on keratinocytes in other skin disease models. Our results suggested that the inflammatory cascade in acne might be alleviated by CBD through the inhibition of inflammatory cytokines.
Next, we draw attention to the molecular mechanisms of the pharmacological reduction of acne inflammation by CBD. It is well known that CBD exhibits various properties at multiple target receptors including CB1/2 receptor, TRPV1, PPARγ and 5-hydroxytryptamine (5-HT)1A.33 A study in ACD revealed that CBD exerted the anti-inflammatory property through CB2 receptor activation and TRPV1 regulation.16 In a hypoxic ischemic brain injury model, CB2 receptor antagonists reversed the inhibition of IL-1 levels by CBD.34 In a separate study, CBD-induced suppression in liver inflammatory response was found to be dependent on TRPV1.35 On the other hand, studies have shown that TPRV1 activation caused release of inflammatory cytokines, while CB2 receptor activation prevented local and systemic immune response.36,37 These earlier studies prompted us to focus on the roles of CB2 receptor and TRPV1 in the anti-inflammatory action of CBD. In our study, the expression of CB2 receptor and TRPV1 in keratinocytes was confirmed and the effects of antagonists of the two receptors were studied. Our results showed that CBD promoted the expression of CB2 receptor and might act as a CB2 receptor agonist in keratinocytes. AM630 had no effect on CEVs-induced inflammatory response in the absence of CBD. But AM630 significantly reversed the inhibitory effect of CBD on TNF-α expression in CEVs-stimulated NHEKs. On the contrary, we found that CBD downregulated TRPV1 expression induced by CEVs in keratinocytes. The TRPV1 antagonist Capsazepine was able to suppress inflammation in CEVs-stimulated NHEKs in the absence of CBD. As expected, Capsazepine further enhanced the inhibitory effect of CBD on inflammation. These results suggested that (1) the inhibitory effect of CBD on inflammation was mediated by activation of CB2 receptor, since the effect could be abrogated by the CB2 receptor antagonist AM630; (2) the TRPV1 antagonist Capsazepine had synergistic effect with CBD in the anti-inflammatory process; (3) CBD might act as a TRPV1 antagonist and inhibit acne inflammation through the inhibition of TRPV1, which remains to be proved by further experiments.
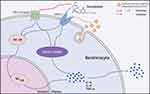
On the other hand, the MAPK and NF-κB signaling cascades are classic pathways involved in acne inflammation. MAPK phosphorylation, activated by C. acnes, enables the translocation of different transcription factors into the nucleus, while the NF-κB protein migrates into the nucleus and acts as a transcription factor.38 These transcription factors lead to the expression of various inflammatory cytokines. We investigated the levels of MAPK- and NF-κB-related proteins in the presence of CBD or vehicle, to determine whether they were related to the anti-inflammatory property of CBD. Indeed, the CEVs-induced phosphorylation of ERK1/2 MAPK and NF-κB p65 were both prevented by CBD pretreatment in a concentration-dependent manner. The decrease of phosphorylated ERK1/2 was more remarkable than that of phosphorylated p65. Meanwhile, we found that CBD prevented the nuclear translocation of NF-κB p65 which was activated by CEVs. The results indicated that CBD inhibited the CEVs-induced inflammation via inactivation of the MAPK and NF-κB signaling pathways. Interestingly, studies have found that CB2 receptor agonists could inactivate both NF-κB and ERK1/2 signaling pathways.39,40 TRPV1 inhibition was also able to decrease capsaicin-induced NF-κB transcriptional activity and ERK1/2 phosphorylation.41,42 Taken together, our data, along with the intriguing literature findings, indicated that CBD might inactivate the MAPK and NF-κB signaling pathways through CB2 receptor or TRPV1. In brief, in the CEVs-stimulated NHEKs, CBD could promote the expression of CB2 receptor and inhibit the expression of TRPV1, and suppress the activation of the MAPK and NF-κB signaling pathways, thus reducing the production of inflammatory cytokines (Figure 5).
Conclusion
In conclusion, our results provided the evidence that CBD exerted an inhibitory effect on acne inflammation in keratinocytes induced by CEVs, which was mediated by CB2 receptor and through inactivating the MAPK and NF-κB signaling pathways. The TRPV1 antagonist Capsazepine had synergistic effect with CBD, but whether TRPV1 directly mediated the anti-inflammatory action of CBD still remains to be confirmed. Besides, the regulatory effect of CB2 receptor and TRPV1 on the MAPK and NF-κB signaling pathways in the acne model should also be further unraveled. Despite several limitations above, our study proposes the anti-acne property of CBD and a potent novel therapeutic approach for acne.
Acknowledgments
The authors gratefully acknowledge the financial support from the National Natural Science Foundation of China (Grant No. 82173444) and the Clinical Research Plan of Shanghai Hospital Development Center (No. SHDC22022302).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Hay RJ, Johns NE, Williams HC., et al. The global burden of skin disease in 2010: an analysis of the prevalence and impact of skin conditions. J Invest Dermatol. 2014;134(6):1527–1534. doi:10.1038/jid.2013.446
2. Suh DH, Kwon HH. What’s new in the physiopathology of acne. Br J Dermatol. 2015;172(1):13–19. doi:10.1111/bjd.13634
3. Harper JC. Acne vulgaris: what’s new in our 40th year. J Am Acad Dermatol. 2020;82(2):526–527. doi:10.1016/j.jaad.2019.01.092
4. Dréno B, Pécastaings S, Corvec S, Veraldi S, Khammari A, Roques C. Cutibacterium acnes (Propionibacterium acnes) and acne vulgaris: a brief look at the latest updates. J Eur Acad Dermatol Venereol. 2018;5–14. doi:10.1111/jdv.15043
5. Dréno B. What is new in the pathophysiology of acne, an overview. J Eur Acad Dermatol Venereol. 2017;31(5):8–12. doi:10.1111/jdv.14374
6. Choi EJ, Lee HG, Bae IH, et al. Propionibacterium acnes-derived extracellular vesicles promote acne-like phenotypes in human epidermis. J Invest Dermatol. 2018;138(6):1371–1379. doi:10.1016/j.jid.2018.01.007
7. Dessinioti C, Katsambas A. Propionibacterium acnes and antimicrobial resistance in acne. Clin Dermatol. 2017;35(2):163–167. doi:10.1016/j.clindermatol.2016.10.008
8. Zhu T, Wu W, Yang S, Li D, Sun D, He L. Polyphyllin I inhibits Propionibacterium acnes-induced inflammation in vitro. Inflammation. 2019;42(1):35–44. doi:10.1007/s10753-018-0870-z
9. Jin S, Lee MY. Kaempferia parviflora extract as a potential anti-acne agent with anti-inflammatory, sebostatic and anti-Propionibacterium acnes activity. Int J Mol Sci. 2018;19(11):e3457. doi:10.3390/ijms19113457
10. Lee CJ, Chen LG, Liang WL, Wang CC. Multiple activities of punica granatum linne against acne vulgaris. Int J Mol Sci. 2017;18(1):141. doi:10.3390/ijms18010141
11. Hartsel JA, Eades J, Hickory B, Makriyannis A. Cannabis sativa and Hemp. In: Ramesh CG, editor. Nutraceuticals. 2016:735–754. doi:10.1016/b978-0-12-802147-7.00053-x
12. Toth KF, Adam D, Biro T, Olah A. Cannabinoid signaling in the skin: therapeutic potential of the “C(ut)annabinoid” system. Molecules. 2019;24(5):918. doi:10.3390/molecules24050918
13. Franco V, Perucca E. Pharmacological and therapeutic properties of Cannabidiol for epilepsy. Drugs. 2019;79(13):1435–1454. doi:10.1007/s40265-019-01171-4
14. Keating GM. Delta-9-Tetrahydrocannabinol/Cannabidiol oromucosal spray (Sativex®): a review in multiple sclerosis-related spasticity. Drugs. 2017;77(5):563–574. doi:10.1007/s40265-017-0720-6
15. Sangiovanni E, Fumagalli M, Pacchetti B, et al. Cannabis sativa L. extract and cannabidiol inhibit in vitro mediators of skin inflammation and wound injury. Phytother Res. 2019;33(8):2083–2093. doi:10.1002/ptr.6400
16. Petrosino S, Verde R, Vaia M, Allara M, Iuvone T, Di Marzo V. Anti-inflammatory properties of Cannabidiol, a nonpsychotropic cannabinoid, in experimental allergic contact dermatitis. J Pharmacol Exp Ther. 2018;365(3):652–663. doi:10.1124/jpet.117.244368
17. Peyravian N, Deo S, Daunert S, Jimenez JJ. The anti-inflammatory effects of Cannabidiol (CBD) on acne. J Inflamm Res. 2022;15:2795–2801. doi:10.2147/JIR.S355489
18. Olah A, Toth BI, Borbiro I, et al. Cannabidiol exerts sebostatic and antiinflammatory effects on human sebocytes. J Clin Invest. 2014;124(9):3713–3724. doi:10.1172/JCI64628
19. Spleman L, Sinclair R, Freeman M, Davis M, Gebaueret K. The safety of topical Cannabidiol (CBD) for the treatment of acne. J Investig Dermatol. 2018;138:180.
20. Ma Y, Zhang N, Wu S, Huang H, Cao Y. Antimicrobial activity of topical agents against Propionibacterium acnes: an in vitro study of clinical isolates from a hospital in Shanghai, China. Front Med. 2016;10(4):517–521. doi:10.1007/s11684-016-0480-9
21. Jiang M, Fan X, Jiang Z, et al. Comparative proteomic analysis of membrane vesicles from clinical C. acnes isolates with differential antibiotic resistance. Clin Cosmet Investig Dermatol. 2022;15:703–712. doi:10.2147/CCID.S363537
22. Johansen C. Generation and culturing of primary human keratinocytes from adult skin. J Vis Exp. 2017;130:e56863. doi:10.3791/56863
23. Jin S, Chen L, Xu Z, Xing X, Zhang C, Xiang L. 585 nm light-emitting diodes inhibit melanogenesis through upregulating H19/miR-675 axis in LEDs-irradiated keratinocytes by paracrine effect. J Dermatol Sci. 2020;98(2):102–108. doi:10.1016/j.jdermsci.2020.03.002
24. Cong TX, Hao D, Wen X, Li XH, He G, Jiang X. From pathogenesis of acne vulgaris to anti-acne agents. Arch Dermatol Res. 2019;311(5):337–349. doi:10.1007/s00403-019-01908-x
25. Beylot C, Auffret N, Poli F, et al. Propionibacterium acnes: an update on its role in the pathogenesis of acne. J Eur Acad Dermatol Venereol. 2014;28(3):271–278. doi:10.1111/jdv.12224
26. Dagnelie M-A, Khammari A, Dréno B, Corvec S. Assessment of seven protocols to prepare Cutibacterium acnes bacterial lysates to measure its immunogenic potential and review of the literature. Anaerobe. 2019;57:75–81. doi:10.1016/j.anaerobe.2019.03.019
27. Trivedi N, Gilliland K, Zhao W, Liu W, Thiboutot D. Gene array expression profiling in acne lesions reveals marked upregulation of genes involved in inflammation and matrix remodeling. J Invest Dermatol. 2006;126(5):1071–1079. doi:10.1038/sj.jid.5700213
28. Ozkanli S, Karadag A, Ozlu E, et al. A comparative study of MMP-1, MMP-2, and TNF-α expression in different acne vulgaris lesions. Int J Dermatol. 2016;55(12):1402–1407. doi:10.1111/ijd.13275
29. Kim J. Review of the innate immune response in acne vulgaris: activation of Toll-like receptor 2 in acne triggers inflammatory cytokine responses. Dermatology. 2005;211(3):193–198. doi:10.1159/000087011
30. Vallée A, Vallée J, Lecarpentier Y. Potential role of cannabidiol in Parkinson’s disease by targeting the WNT/β-catenin pathway, oxidative stress and inflammation. Aging. 2021;13(7):10796–10813. doi:10.18632/aging.202951
31. Lowin T, Tingting R, Zurmahr J, Classen T, Schneider M, Pongratz G. Cannabidiol (CBD): a killer for inflammatory rheumatoid arthritis synovial fibroblasts. Cell Death Dis. 2020;11(8):714. doi:10.1038/s41419-020-02892-1
32. Gonzalez-Mariscal I, Pozo-Morales M, Romero-Zerbo SY, et al. Abnormal cannabidiol ameliorates inflammation preserving pancreatic beta cells in mouse models of experimental type 1 diabetes and beta cell damage. Biomed Pharmacother. 2022;145:e112361. doi:10.1016/j.biopha.2021.112361
33. Rong C, Lee Y, Carmona NE, et al. Cannabidiol in medical marijuana: research vistas and potential opportunities. Pharmacol Res. 2017;121:213–218. doi:10.1016/j.phrs.2017.05.005
34. Arruza L, Pazos M, Mohammed N, et al. Cannabidiol reduces lung injury induced by hypoxic-ischemic brain damage in newborn piglets. Pediatr Res. 2017;82(1):79–86. doi:10.1038/pr.2017.104
35. Hegde V, Nagarkatti P, Nagarkatti M. Role of myeloid-derived suppressor cells in amelioration of experimental autoimmune hepatitis following activation of TRPV1 receptors by cannabidiol. PLoS One. 2011;6(4):e18281. doi:10.1371/journal.pone.0018281
36. Ho JC, Lee CH. TRP channels in skin: from physiological implications to clinical significances. Biophysics. 2015;11:17–24. doi:10.2142/biophysics.11.17
37. Hu SJ, Cheng G, Zhou H, et al. Identification of novel cannabinoid CB2 receptor agonists from botanical compounds and preliminary evaluation of their anti-osteoporotic effects. Molecules. 2022;27(3):e702. doi:10.3390/molecules27030702
38. Bharti S, Vadlamudi HC. A strategic review on the involvement of receptors, transcription factors and hormones in acne pathogenesis. J Recept Signal Transduct Res. 2021;41(2):105–116. doi:10.1080/10799893.2020.1805626
39. Ali A, El-Tawil O, Al-Mokaddem A, Abd El-Rahman S. Promoted inhibition of TLR4/miR-155/ NFB p65 signaling by cannabinoid receptor 2 agonist (AM1241), aborts inflammation and progress of hepatic fibrosis induced by thioacetamide. Chem Biol Interact. 2021;336:e109398. doi:10.1016/j.cbi.2021.109398
40. Tang J, Tao Y, Tan L, et al. Cannabinoid receptor 2 attenuates microglial accumulation and brain injury following germinal matrix hemorrhage via ERK dephosphorylation in vivo and in vitro. Neuropharmacology. 2015;95:424–433. doi:10.1016/j.neuropharm.2015.04.028
41. Abbas MA. Modulation of TRPV1 channel function by natural products in the treatment of pain. Chem Biol Interact. 2020;330:e109178. doi:10.1016/j.cbi.2020.109178
42. Huang KF, Ma KH, Chang YJ, et al. Baicalein inhibits matrix metalloproteinase 1 expression via activation of TRPV1-Ca-ERK pathway in ultraviolet B-irradiated human dermal fibroblasts. Exp Dermatol. 2019;28(5):568–575. doi:10.1111/exd.13912
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.