Back to Journals » Cancer Management and Research » Volume 11
Cancer-associated fibroblasts endow stem-like qualities to liver cancer cells by modulating autophagy
Authors Zhao Z, Bai S, Wang R, Xiong S, Li Y, Wang X, Chen W , Cheng B
Received 12 December 2018
Accepted for publication 29 May 2019
Published 24 June 2019 Volume 2019:11 Pages 5737—5744
DOI https://doi.org/10.2147/CMAR.S197634
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Rituraj Purohit
Zhenxiong Zhao, Shuya Bai, Ronghua Wang, Si Xiong, Yawen Li, Xiju Wang, Wei Chen, Bin Cheng
Department of Gastroenterology and Hepatology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430030, People’s Republic of China
Purpose: Both cancer-associated fibroblasts (CAFs) and liver cancer stem cells (LCSCs) play an important part in the tumorigenesis, development and metastasis of hepatocellular carcinoma (HCC). Moreover, the stem-like properties in HCC cells could be promoted by CAFs. However, the mechanism remains largely unknown.
Patients and methods: We used conditioned medium (CM) of CAFs to culture Huh7 cells. Stemness of the cells was then examined mainly by sphere formation assay while stemness-associated genes including Nanog, Sox2 and Oct4 were measured by Western blotting. Immunofluorescence staining, Transmission Electron Microscope as well as Western blotting were performed to detect the level of autophagy in Huh7 cells.
Results: Increased level of stemness and autophagy was observed in HCC cells cultured in CAFs-CM compared to the control group. Activation of CAFs-induced autophagic flux could be inhibited by Chloroquine (CQ), which can accumulate LC3-II protein and increase punctate distribution of LC3 localization. Treatment of HCC cells with CQ effectively reversed the CAF-induced stemness, invasion, and metastasis ability in these cells. In vivo, Huh7 cells inoculated together with CAFs developed significantly larger tumors than Huh7 cells injected alone. Moreover, blockage of autophagy in Huh7 cells by CQ greatly reduced the growth of xenografted tumors of Huh7 cells combined with CAFs.
Conclusion: These results reveal that CAFs are capable of promoting stemness and metastasis of HCC cells and blocking autophagy could markedly attenuate the stemness enhanced by CAFs, suggesting that targeting autophagy in HCC could be an effective strategy in HCC treatment.
Keywords: cancer-associated fibroblasts, stemness, liver cancer, autophagy
Introduction
Human hepatocellular carcinoma (HCC) is currently the fourth most frequent cause of cancer death worldwide, claiming about 782,000 deaths annually.1 There is increasing evidence supporting that malignant properties of HCC are, at least in part, caused by a subpopulation of cancer cells defined as cancer stem cells (CSCs). CSCs possess stem-like qualities such as capability for extensive proliferation, self-renewal as well as high tumorigenicity, which are responsible for cancer initiation and development.2,3 There is a close correlation between CSCs and poor prognosis in patients with HCC as well.4,5
Previous studies have demonstrated that Cancer-associated fibroblasts (CAFs), one of the major components in tumor microenvironment,6 play a critical role in driving and maintaining the stem-like properties of CSCs in HCC and other cancers.7–9 While CSCs have the ability to differentiate into non-tumorigenic cancer cells, the latter may acquire the stem-like quality and re-enter a stem-cell state by CAFs.10 However, the role of CAFs on stemness in HCC cells has not been totally understood.
Autophagy is an evolutionarily conserved catabolic pathway that regulates the turnover of long-lived or damaged proteins and organelles through lysosomes.11 It also serves as a pro-survival mechanism and is involved in the maintaining of stemness in cancer stem cells. Increasing evidence shows that autophagy is enhanced in LCSCs and contributes to sustaining their stem-like qualities.12,13
In this study, we have examined the role of autophagy in CAFs’ effect of promoting stem-like qualities in HCC cells and suggest that targeting autophagy could be an effective approach to HCC treatment.
Materials and methods
Cell line and culture
Human HCC Huh7 cell line was obtained from American Type of Culture Collection (ATCC) and Cell Bank of Chinese Academy of Sciences (Shanghai, China). The cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM, GIBCO) supplemented with 10% fetal bovine serum (FBS, GIBCO) at 37 °C, 5% CO2 condition.
Isolation of CAFs
Human liver tumor and peri-tumor tissues (3 cm away from the tumor border) were obtained from five patients with HCC who underwent surgical resection in Tongji Hospital, Huazhong University of Science and Technology (Wuhan, China). All human experimentations were approved by the ethics committee of Tongji Hospital. The patients whose tissues were used in this research provided written informed consent, and this was conducted in accordance with the Declaration of Helsinki. Cancer associated fibroblasts (CAFs) were isolated from tumor tissues while normal fibroblasts (NFs) from peri-tumor tissues. The fresh liver tissues were washed in D-Hanks solution containing 100 U/mL penicillin and 100 ug/mL streptomycin and minced into small pieces of 2×2 mm. The minced fragments were incubated in a culture plate at 37 °C and 5% CO2 for twenty minutes to allow attachment to the plate. Then DMEM containing 15% fetal bovine serum was added into the culture plate. Following incubation, the medium was replenished every two days and the unattached cells were removed. One to two weeks later, fibroblasts were observed to grow out of liver fragments. After 2–3 passages, purified CAFs and NFs were harvested. Fibroblasts from passages 3–10 were used later for various experiments.
Preparation of conditioned medium (CM)
CAFs were seeded on six-well plates at 1×105 cells per well density. Culture medium was discarded 24 hrs later and 1 ml serum-free DMEM per well was used to culture CAFs for 36 hrs. Then, the medium was collected from the supernatants after centrifugation and filtration through a 0.45 mm membrane to remove any cells and cell debris. Conditioned medium obtained were stored at −80 °C and Two milliliters of CAFs-CM were added in each well to culture Huh7 cells.
Colony-forming assay
Huh7 cells were planted at a density of 2000 cells per well in six well plates. About a week later, clones were fixed by 4% methanol and dyed with Giemsa (Sigma Aldrich) and clone numbers were counted.
Sphere formation assay
To assay sphere formation efficiency, a single-cell suspension of HCC cells was created in serum-free DMEM/F12 medium (cat# 12400-024; GIBCO) with 20 ng/ml human recombinant epidermal growth factor (EGF; cat# PHG0311; GIBCO), 10 ng/ml human recombinant basic fibroblast growth factor (bFGF; cat# PHG0266; GIBCO), 100 IU/ml penicillin, 100 μg/ml streptomycin, 2% B27 supplement (cat# 17504-044; GIBCO), 1% N-2 supplement (cat# 17502-048; GIBCO), and 1% methyl cellulose (cat# M0262; Sigma-Aldrich, St. Louis, MO, USA) to prevent cell aggregation. Cells were subsequently seeded in ultra-low adherence 24-well plates (Corning, NY, USA; 200 viable cells per well) at a density of 104 cells/ml. Spheres containing over 100 cells were counted.
Wound healing assay
When cell density reached 100%, wounds were created in the central area using a 200 μL pipette tip. Photos of the scrape line were taken at both 0 and 24 hrs. Each experiment was repeated 3 times. Cell migration was expressed as the percentage of wound closure wound area using Image J software.
Transwell assay
Transwell chambers without Matrigel were used to examine cell ability of migration and transwell chambers were coated with 50 μl Matrigel to measure invasion. 5×104 cells were serum-starved for 12 h and resuspended in 100 μL serum-freed medium and were added to the upper compartment of the chamber, while the bottom chamber was filled with medium supplemented with 10% FBS. After incubation at 37 °C in a 5% CO2 humidified atmosphere, the chambers were analyzed 24 hrs later for migration and 48 hrs for invasion. The experiments were repeated independently three times.
Western blotting
Western blotting analysis was performed as described previously [21]. Primary antibodies for Nanog (cat#4903), Sox2 (cat#3579), Oct4 (cat#2750), LC3A/B(cat#4108), and SQSTM1/p62 (cat#5114) were purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA), 1:1000 dilution. Monoclonal mouse anti-β-actin (cat#ab8226; Abcam,1:1000 dilution) was used as an internal control.
Immunofluorescence staining
CAFs and NFs were fixed with 4% paraformaldehyde for 20 min, washed with PBS and permeabilized with 0.1% Triton X-100 for 10 min.Then, cells were blocked in PBS with 10% BSA for 1 hr at room temperature. After blocking, samples were incubated with primary antibodies specific for primary antibodies against anti-alpha-smooth muscle actin (α-SMA) (Boster, dilution 1:100), FAP (Boster, dilution 1:100) and Vimentin (Boster, dilution 1:100) at 4 °C overnight. Followed by staining with Fluor-conjugated secondary antibodies (1:1000) for 1 hr 37 °C in a dark room. DAPI was then used for counterstaining the nuclei, The coverslips were blocked with Antifade Mounting Medium.
Electron microscope
Cells were fixed with 2.5% glutaraldehyde acid containing 0.1 mol/L PBS buffer (pH 7.4) for 2 h, incubated in 1% osmium tetroxide in 0.1 M PBS buffer (pH 7.4) for 2–3 h, followed by dehydration with an increasing concentration gradient of ethanol and propylene oxide. Samples were then embedded in Araldite, and finally solidified. Sections (60–80 nm) were cut and stained with 3% uranyl acetate and lead citrate. Images were observed using a transmission electron microscope (HT7700, HITACHI).
Animal experiments
BALB/c- nude mice (male, aged 4 weeks, 20 g) were purchased from Beijing Huafukang Bioscience and bred at pathogen-free conditions. Animal protocols were approved by the Institutional Animal Care and Use Committee of the Huazhong University of Science and Technology. Guidelines for Experimental Animal Ethical Committee of Huazhong University of Science and Technology and Experimental animals administrative regulations of Hubei Province were followed for the welfare of the animals. Nude mice were randomly divided into three groups (n=5). 1×106 Huh7 cells and CAFs (3:1) in serum-free medium were inoculated subcutaneously alone or together into the flanks of the nude mice. The length (L) and width (W) of the tumors were measured externally every two days as from the third week. Tumor volume was calculated with the formula: V = (L x W2)/2. Mice were sacrificed 4 weeks after inoculation.
Statistics
All statistical analyses were carried out using GraphPad Prism Software. Data were expressed as the mean ± SD. Two-group comparisons was performed with unpaired Student’s t-test. In all cases, P<0.05 was considered to be statistically significant. Each experiment was independently repeated at least three times.
Results
Isolation and identification of CAFs in HCC
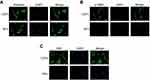
To study the role of CAFs in HCC, we isolated CAFs and NFs successfully from fresh tumor and peri-tumor tissues. Fibroblasts were cultured into passage 4–6 and then were examined by immunofluorescent staining. NFs exhibited a similar expression level of Vimentin to CAFs. However, the expression of the mesenchymal markers alpha-smooth muscle actin (α-SMA) and fibroblast activation protein (FAP) was much higher in CAFs than NFs (Figure 1).
We previously evaluated the expression of α-SMA in tumor and peri-tumor tissues of patients with HCC by immunohistochemistry (IHC) and found that the expression of α-SMA was significantly upregulated in tumors compared to the adjacent non-tumor tissues.10
CAFs endow the stem-like qualities to HCC cells
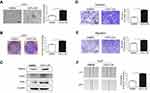
The supernatants of CAFs were collected as CAFs-CM to culture HCC cells. Compared to the control group, Huh7 cells treated with CAFs-CM formed a greater number of spheres and colonies, indicating their effect on HCC self-renewal and proliferation (Figure 2A+B). We further evaluated the expression of stemness-associated genes including Nanog, Sox2 and Oct4 in Huh7 cells incubated with CAFs-CM. As shown in Figure 2C, we observed that CAFs induced higher protein expression of Nanog, Sox2 and Oct4 in Huh7 cells. Additionally, wound healing and transwell assays revealed that Huh7 cells had stronger migratory and invasive abilities when cultured with CAFs-CM (Figure 2D–F). Together, we demonstrated that CAFs induced the stem-cell-like qualities in HCC cells.
CAFs promote autophagy activation in HCC cells
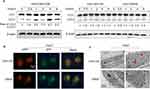
To investigate our hypothesis that autophagy was biologically involved in the process of CAFs promoting stemness in HCC, we first elucidated whether CAFs could induce autophagic flux in HCC cells. As shown in Figure 3A, when Huh7 cells were cultured in CAFs-CM, the level of autophagy was largely upregulated than in the control groups, as presented by the enhanced conversion of LC3-I to LC3-II, a common marker for autophagy activation.
Furthermore, we established Huh7 cells that steadily expressed a mRFP-GFP-LC3 construct. With treatment of CAFs-CM, increased numbers of red dots and yellow dots were observed in HCC cells in the merged picture, which represented enhanced autophagic flux (Figure 3B). Transmission Election microscopy also confirmed that more autolysosomes were presented in Huh7 cells treated with CAFs-CM than DMEM (Figure 3C). Collectively, our data indicated that CAFs activated the autophagy pathway in HCC cells.
Autophagy inhibition attenuates CAFs-induced stem-like properties in HCC cells in vitro and in vivo
To find out whether CAF-induced stem-like properties was mediated by autophagy, we next treated Huh7 cells with Chloroquine (CQ), which is an autophagy lysosomal inhibitor. CQ effectively blocked the autophagic flux in Huh7 cells, even when cells were cultured in CAFs-CM (Figure 4A). Huh7 cells showed decreased sphere and colony forming numbers (Figure 4B+C) as well as migration and invasive abilities (Figure 4E–G) in CQ-added CAFs-CM compared to the control group. Moreover, suppressing autophagy in Huh7 cells attenuated the upregulated expression of stemness-associated genes induced by CAFs-CM (Figure 4D). Therefore, inhibiting autophagy with CQ abolished CAFs-induced stem-like qualities of HCC cells in vitro.
In addition, to confirm if CAFs promote HCC tumorigenesis in vivo, which is an important characteristic of CSCs, we subcutaneously inoculated 106 Huh7 cells into the flanks of BALB/c nude mice with or without CAFs (3:1). Furthermore, to evaluate the role of the autophagy pathway in CAFs’ effects on hepatocarcinogenesis, we pretreated Huh7 cells with CQ for 48 h and then injected the cells along with CAFs into BALB/c nude mice. Three weeks later, all the mice were sacrificed and tumors were shown in Figure 4H. Injection with Huh7 cells and CAFs resulted in much larger tumors than Huh7 cells alone, and this effect was greatly reduced by the addition of CQ. Mice models injected with CAFs alone did not develop tumors (Figure 4H). From those data, we established that CAFs stimulated HCC tumorigenesis and blocking autophagy in HCC cells markedly attenuated the tumorigenic activity enhanced by CAFs.
Collectively, CAFs promoted stem-like properties such as self-renewal, proliferation, tumorigenesis and metastasis in HCC cells via the autophagy pathway.
Discussion
There is increasing evidence that HCC is driven and maintained by LCSCs, which are highly tumorigenic, metastatic, resistant to chemotherapy and radiation, and responsible for tumor recurrence and development. Our research team previously confirmed that LCSCs displayed much stronger abilities to initiate and sustain tumor growth as well as cancer metastasis than unsorted HCC cells. Moreover, Notch and Wnt signaling pathways played an important role in sustaining the stemness characteristics of LCSCs.4,5 However, emerging studies indicate that tumor microenvironment contributes to the maintenance of CSCs activity.
CAFs are one of the major components of tumor microenvironment in various cancers. Because many HCC cases develop from fibrotic and cirrhotic livers, CAFs have been paid much attention in HCC research.7,14 Our previous studies showed that liver cancer cells could acquire CSC phenotype and re-entered a stem cell state by the tumor microenvironment.10 In this study, we found that CAFs could induce “cancer stemness” phenotype in HCC cells through the autophagy pathway both in vitro and in vivo.
Autophagy is a lysosomal degradation pathway that mostly mediates between clearance of damaged organelles and production of precursor molecules such as amino acids and fatty acids. It serves as a protection mechanism for cancer cells to survive and adapt to harsh environmental conditions, including nutrient deprivation, hypoxia, drugs toxicity and radiation as well.15 Autophagy plays a crucial role in stemness maintenance of the CSCs cells in pancreatic cancer, colorectal cancer and breast cancer.16–18 In 2017, Liu et al demonstrated that mitophagy, a kind of autophagy that selectively removes mitochondria, was required to maintain hepatic cancer stem cell population by removing mitochondria-associated p53, which suppressed the expression of stemness gene Nanog.13 Song et al found that autophagy protected CD133+ LCSCs from hypoxic and nutrient-deprived tumor microenvironment.12 Other studies showed that autophagy was required for CSCs in hepatocarcinogenesis and drug-resistance.19,20 Thus, a pro-survival autophagic pathway may be critical for LCSCs maintenance. However, the mechanism of autophagy activation in HCC cells is unclear.
Recent studies revealed that CAFs, which are located in the tumor microenvironment, might be involved in the intrinsic metabolic process of hepatoma cells such as autophagy.21,22 CAFs promoted lung cancer and melanoma cells to recover from radiation-induced damage by modulating autophagy.21 In tongue cancer, CAFs conferred cisplatin resistance of tumor cells via autophagy activation.22 Our research focused on whether CAFs could induce autophagy in liver cancer cells and confirmed that autophagy contributed to the effect of CAFs on HCC.
Conclusion
Our study revealed that CAFs were capable of promoting the “cancer stemness” properties in HCC cells via activation of autophagy. Inhibiting autophagy in HCC cells by CQ could largely abolish the stem-like qualities endowed from CAFs and could be an effective strategy for HCC treatment.
Abbreviation list
HCC, hepatocellular carcinoma; CAFs, cancer-associated fibroblasts; NFs, normal fibroblasts; CM, conditioned medium; α-SMA, alpha-smooth muscle actin; FAP, fibroblast activation protein; CSCs, cancer stem cells; LCSCs, liver cancer stem cells; CQ, Chloroquine.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (81172063 and 81802418). We would also like to express our appreciation to Rajluxmee Beejadhursing, who helped to edit this paper.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018.
2. Magee JA, Piskounova E, Morrison SJ. Cancer stem cells impact, heterogeneity, and uncertainty. Cancer Cell. 2012;21(3):283–296. doi:10.1016/j.ccr.2012.03.003
3. Yamashita T, Wang XW. Cancer stem cells in the development of liver cancer. J Clin Invest. 2013;123(5):1911–1918. doi:10.1172/JCI66024
4. Luo J, Wang P, Wang R, et al. The Notch pathway promotes the cancer stem cell characteristics of CD90+ cells in hepatocellular carcinoma. Oncotarget. 2016;7(8):9525–9537. doi:10.18632/oncotarget.6672
5. Wang R, Sun Q, Wang P, et al. Notch and Wnt/β-catenin signaling pathway play important roles in activating liver cancer stem cells. Oncotarget. 2016;7(5):5754–5768. doi:10.18632/oncotarget.6805
6. Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21(3):309–322. doi:10.1016/j.ccr.2012.02.022
7. Lau Eunice Yuen T, Lo J, Cheng Bowie Yik L, et al. Cancer-associated fibroblasts regulate tumor-initiating cell plasticity in hepatocellular carcinoma through c-Met/FRA1/HEY1 signaling. Cell Rep. 2016;15(6):1175–1189. doi:10.1016/j.celrep.2016.04.019
8. Chen WJ, Ho CC, Chang YL, et al. Cancer-associated fibroblasts regulate the plasticity of lung cancer stemness via paracrine signalling. Nat Commun. 2014;5:3472. doi:10.1038/ncomms5972
9. Liao CP, Adisetiyo H, Liang M, Roy-Burman P. Cancer-associated fibroblasts enhance the gland-forming capability of prostate cancer stem cells. Cancer Res. 2010;70(18):7294–7303. doi:10.1158/0008-5472.CAN-09-3982
10. Xiong S, Wang R, Chen Q, et al. Cancer-associated fibroblasts promote stem cell-like properties of hepatocellular carcinoma cells through IL-6/STAT3/Notch signaling. Am J Cancer Res. 2018;8(2):302–316.
11. Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132(1):27–42. doi:10.1016/j.cell.2007.12.018
12. Song YJ, Zhang SS, Guo XL, et al. Autophagy contributes to the survival of CD133+ liver cancer stem cells in the hypoxic and nutrient-deprived tumor microenvironment. Cancer Lett. 2013;339(1):70–81. doi:10.1016/j.canlet.2013.07.021
13. Liu K, Lee J, Kim JY, et al. Mitophagy controls the activities of tumor suppressor p53 to regulate hepatic cancer stem cells. Mol Cell. 2017;68(2):281–292 e285. doi:10.1016/j.molcel.2017.09.022
14. Liu J, Chen S, Wang W, et al. Cancer-associated fibroblasts promote hepatocellular carcinoma metastasis through chemokine-activated hedgehog and TGF-beta pathways. Cancer Lett. 2016;379(1):49–59. doi:10.1016/j.canlet.2016.05.022
15. Kimmelman AC, White E. Autophagy and tumor metabolism. Cell Metab. 2017;25(5):1037–1043. doi:10.1016/j.cmet.2017.04.004
16. Rausch V, Liu L, Apel A, et al. Autophagy mediates survival of pancreatic tumour-initiating cells in a hypoxic microenvironment. J Pathol. 2012;227(3):325–335. doi:10.1002/path.3994
17. Kantara C, O’Connell M, Sarkar S, Moya S, Ullrich R, Singh P. Curcumin promotes autophagic survival of a subset of colon cancer stem cells, which are ablated by DCLK1-siRNA. Cancer Res. 2014;74(9):2487–2498. doi:10.1158/0008-5472.CAN-13-3536
18. Maycotte P, Jones KL, Goodall ML, Thorburn J, Thorburn A. Autophagy supports breast cancer stem cell maintenance by regulating IL6 secretion. Mol Cancer Res. 2015;13(4):651–658.
19. Li J, Hu SB, Wang LY, et al. Autophagy-dependent generation of Axin2+ cancer stem-like cells promotes hepatocarcinogenesis in liver cirrhosis. Oncogene. 2017;36(48):6725–6737. doi:10.1038/onc.2017.272
20. Wu R, Murali R, Kabe Y, et al. Baicalein targets GTPase-mediated autophagy to eliminate liver tumor-initiating stem cell-like cells resistant to mTORC1 inhibition. Hepatology. 2018;68(5):1726–1740. doi:10.1002/hep.30071
21. Wang Y, Gan G, Wang B, et al. Cancer-associated fibroblasts promote irradiated cancer cell recovery through autophagy. EBioMedicine. 2017;17:45–56. doi:10.1016/j.ebiom.2017.02.019
22. Liao JK, Zhou B, Zhuang XM, Zhuang PL, Zhang DM, Chen WL. Cancer-associated fi broblasts confer cisplatin resistance of tongue cancer via autophagy activation. Biomed Pharmacother. 2018;97:1341–1348. doi:10.1016/j.biopha.2017.11.024
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.