Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 12
Canagliflozin review – safety and efficacy profile in patients with T2DM
Authors Jakher H, Chang TI, Tan M, Mahaffey KW
Received 18 August 2018
Accepted for publication 2 October 2018
Published 1 February 2019 Volume 2019:12 Pages 209—215
DOI https://doi.org/10.2147/DMSO.S184437
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ming-Hui Zou
Haroon Jakher,1 Tara I Chang,2 Marilyn Tan,3 Kenneth W Mahaffey4
1Department of Medicine, Stanford University School of Medicine, Palo Alto, CA, USA; 2Division of Nephrology, Department of Medicine, Stanford University School of Medicine, Palo Alto, CA, USA; 3Division of Endocrinology, Department of Medicine, Stanford University School of Medicine, Palo Alto, CA, USA; 4Stanford Center for Clinical Research, Department of Medicine, Stanford University School of Medicine, Palo Alto, CA, USA
Abstract: Canagliflozin is a sodium glucose-cotransporter (SGLT) receptor inhibitor approved for the treatment of type 2 diabetes mellitus (T2DM). This article reviews the mechanism of action of SGLT-2 receptor inhibitors and the efficacy of canagliflozin as an antidiabetic agent, its cardiovascular and renal benefits, and safety profile. During the development of canagliflozin, Phase II trials showed an improvement in cardiac and renal biomarkers such as blood pressure, body weight, and albuminuria. The large CANVAS program showed that canagliflozin reduced the composite cardiovascular outcome of cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke. The CANVAS program also showed a possible benefit of canagliflozin on a renal composite of sustained 40% reduction in estimated glomerular filtration rate, the need for renal replacement therapy, or death from renal causes. The safety profile of canagliflozin has been well characterized, and known side effects such as mycotic genital infections were confirmed in CANVAS. However, an increased risk of amputations was observed in CANVAS that requires further study. Overall, canagliflozin is an effective antidiabetic medication with cardiovascular and likely renal benefits, and with a generally well-tolerated safety profile. Results from the CREDENCE trial will further evaluate the safety and potential renal benefits of canagliflozin in patients with established diabetic nephropathy.
Keywords: canagliflozin, sodium glucose-cotransporter, diabetes, CANVAS, Invokana®, renal, cardiac
Introduction
Canagliflozin is a sodium glucose-cotransporter (SGLT) receptor inhibitor approved for the treatment of type 2 diabetes mellitus (T2DM) in the US, Japan, Australia, and the European Union. In addition to its glucose-lowering effects, it exhibits beneficial effects on the heart and potentially the kidney. This article will review the evidence from completed clinical trials about the benefits and adverse side effects of canagliflozin.
Mechanism of action
Canagliflozin acts on two types of SGLT receptors: SGLT-1 and SGLT-2. SGLT-2 is expressed in the proximal renal tubules and is responsible for the reabsorption of about 90% of the glucose filtered by the kidneys.1,2 By inhibiting SGLT-2, canagliflozin increases the urinary excretion of glucose and decreases blood glucose levels. There is also evidence showing that the reabsorption of glucose by SGLT-2 is increased in patients with T2DM compared to patients without T2DM, which increased its attractiveness as a potential drug target.3 SGLT-2 inhibition has little risk of hypoglycemia, since the urinary excretion of glucose decreases as blood glucose levels decrease. SGLT-1 is also expressed in the proximal renal tubules, but the majority of its expression occurs in the distal brush-border membrane surface of the villi in the lumen of the small intestine where it allows for the reabsorption of glucose and galactose.3 SGLT-1 expression also occurs in cardiac myocytes, which may play a role in the underlying mechanism for the cardiovascular benefits that have been described with some SGLT-2 inhibitors.4 Canagliflozin has weaker selectivity for the SGLT-2 receptor vs the SGLT-1 receptor when compared to other drugs of the same class, such as dapagliflozin, empagliflozin, and tofogliflozin.5 The differential SGLT-1/SGLT-2 receptor selectivity and the potential role in the glucose-lowering effects, cardiovascular benefits, and the safety profile of each drug will be discussed in further detail in the following sections. The SGLT-2 inhibitors currently approved by the US Food and Drug Administration (FDA) are given in Table 1.
  | Table 1 FDA-approved SGLT-2 inhibitors Abbreviation: FDA, Food and Drug Administration; SLGT-2, sodium-glucose cotransporter-2. |
Efficacy in T2DM
The CANagliflozin Treatment and Trial Analysis (CANTATA) studies showed that canagliflozin when used as monotherapy (CANTATA-M), as a second-line agent (CANTATA-SU, CANTATA-D), or third-line agent (CANTATA-D2, CANTATA-MSU) reduces hemoglobin A1C, and these trial results supported its approval by the US FDA.6–9 These studies showed a statistically significant (P<0.001) reduction in A1C of –0.77% and –1.03% at 26 weeks for both 100 mg and 300 mg dosages, respectively, with canagliflozin compared with placebo10 (Table 2). A significantly higher proportion of patients also achieved a target hemoglobin A1C of <7.0% with canagliflozin compared with placebo.10
Cardiovascular benefits
In addition to lowering glucose levels, Phase II trials of canagliflozin showed reductions in blood pressure and body weight. In the CANTATA trials, canagliflozin decreased body weight when compared to placebo, from 1.1 to 2.5 kg with 100 mg per day and from 1.7 to 2.5 kg with 300 mg daily dosing (P<0.001 compared with placebo).2 When compared to glimepiride, canagliflozin decreased weight by 4.4 kg and 4.7 kg at 100 mg and 300 mg doses, respectively (P<0.001)6 (Table 2).
A systematic review in 2013 showed a reduction in mean systolic blood pressure with SGLT-2 inhibitors of ~4.5 mm Hg compared with other antidiabetic agents.11 Another meta-analysis in 2014 also showed that canagliflozin was the only SGLT-2 inhibitor with a dose–response decrease in systolic blood pressure of 0.87 mm Hg for each additional 100 mg of canagliflozin studied (P=0.008 vs placebo).12 A limitation of CANTATA trials was the relatively short duration of the trial, ie, 52 weeks. Longer treatment would be needed to assess whether the effects on blood pressure and weight would translate into improved cardiovascular outcomes.
To examine the potential cardiovascular benefits of canagliflozin, the Canagliflozin Cardiovascular Assessment Study (CANVAS) program was designed.13 The CANVAS program included two similarly designed and conducted trials (CANVAS and CANVAS-R). Each study was a multicenter, international, randomized, double-blind, placebo-controlled trial enrolling over 10,000 patients, and there was a prespecified plan to integrate both studies. The CANVAS primary composite end point was death from cardiovascular causes, nonfatal myocardial infarction, and nonfatal stroke. The primary composite end point was significantly reduced with canagliflozin compared with placebo (26.9 per 1,000 patient years in the canagliflozin group vs 31.5 in the placebo group; HR 0.86, 95% CI 0.75–0.97, P<0.001 for non-inferiority, P=0.02 for superiority).14 CANVAS also showed a reduction in hospitalizations for heart failure, an effect seen within the first few months of the trial, suggesting the glucose lowering effects of canagliflozin were unlikely to be driving the reduction in hospitalizations for heart failure (Table 3). This finding was corroborated by a retrospective observational study that also showed a reduction in hospitalizations in patients treated with canagliflozin compared to non-SGLT-2 inhibitors (HR 0.39, 95% CI 0.26–0.60).15 The individual components of the primary composite end point and all-cause mortality were numerically reduced in patients assigned canagliflozin but did not achieve statistical significance.14
  | Table 3 CV and renal effects of canagliflozin from integrated CANVAS data Abbreviations: CV, cardiovascular; eGFR, estimated glomerular filtration rate; MI, myocardial infarction. |
Renal benefits
In Phase II trials, canagliflozin reduced progression of albuminuria with a HR of 0.73 compared to placebo (95% CI 0.67–6.79).14 A study by Heerspink et al showed that compared to glimepiride, canagliflozin slowed the rate of estimated glomerular filtration rate (eGFR) decline. Patients receiving glimepiride had an average reduction of 3.3 mL/min/1.73 m2 per year compared to a reduction of 0.5 mL/min/1.73 m2 per year for patients receiving canagliflozin 100 mg daily and a reduction of 0.9 mL/min/1.73 m2 per year for patients taking canagliflozin 300 mg daily (P<0.01 for each canagliflozin group compared to glimepiride).16 In the CANVAS integrated analyses, composite outcome of sustained reduction of eGFR, the need for renal replacement therapy, and death from renal causes occurred less frequently in patients assigned canagliflozin compared with placebo (HR 0.6, 95% CI 0.47–0.77), although due to the hierarchical testing procedures the findings were not considered statistically significant14 (Table 3). A limitation of CANVAS was the small proportion of participants with chronic kidney disease at baseline and the relatively modest number of renal outcomes. The ongoing Canagliflozin and Renal Endpoints in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE) study will enroll ~4,500 patients with diabetes and established diabetic nephropathy, characterized by moderate to severe albuminuria, and compared the effect of canagliflozin vs placebo on the composite end point of end-stage kidney disease, a twofold increase in serum creatinine, and death due to renal or cardiovascular causes.17
Safety
Common side effects
The most commonly reported adverse events in Phase II trials of canagliflozin were female genital mycotic infections, urinary tract infections, osmotic diuresis-related adverse events, and volume depletion-related adverse events (Table 4).18 These side effects had similar rates at both commonly studied doses (100 mg daily and 300 mg daily).
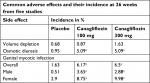  | Table 4 Common adverse effects at 26 weeks Note: aP<0.05. Data from Xiong et al.21 |
Genital and urinary tract infections
Because of the increased glucose content of urine in the genitourinary tract, an increase in genital mycotic infections has been reported with canagliflozin. The incidence of genital mycotic infections in women was 12.73% at 100 mg and 13.78% at 300 mg compared to the placebo incidence of 2.9% at 52 weeks. In men, there was also an increased risk of genital mycotic infections, although the rates were lower than in women. At 26 weeks, the rates were 3.65% at 100 mg and 2.88% at 300 mg compared with 0.51% in placebo. In general, the genital mycotic infections were mild or moderate in intensity and seldom led to discontinuation of canagliflozin.6,14 CANVAS also showed significantly more genital mycotic infections in women (68.8 vs 17.5 events per 1,000 patient years in the canagliflozin groups vs the placebo group; P<0.001) (Table 4). Canagliflozin also appears to increase the incidence of urinary tract infections.19 Although most cases were mild, a review of post-marketing surveillance data by the FDA identified 19 cases of urosepsis or pyelonephritis that resulted from urinary tract infections in patients taking canagliflozin.
Volume depletion- and osmotic diuresis-related adverse events
Adverse events due to volume depletion and osmotic diuresis are more common in patients taking canagliflozin vs placebo.20,21 Osmotic diuresis-related adverse events are commonly described as dry mouth, dry throat, urgency, and nocturia. In CANVAS, the osmotic diuresis event rate per 1,000 patient years was 34.5 in the canagliflozin group and 13.1 in the placebo group (P<0.001).14 Volume depletion-related adverse events included dehydration, orthostatic hypotension, dizziness, pre-syncope, and syncope, and occurred at a rate per 1,000 patient years of 26 vs 18.5 for canagliflozin vs placebo, respectively (P=0.009).14 It has been suggested that select populations such as patients with an eGFR <60 mL/min/1.73 m2, patients >75 years old, and patients on loop diuretics should be monitored more closely for the effects of volume depletion22 (Table 4).
Less common side effects
Renal: In June 2016, the FDA warned about the possibility of acute renal failure (ARF) with canagliflozin based on a review of the data reported from March 2013 to October 2015. As a result, a retrospective study by Perlman et al in 2017 examined the FDA adverse event report system database to explore the relationship between SGLT-2 inhibitors and ARF. They found that there was a significantly increased relative risk of ARF with the use of all SGLT-2 inhibitors (OR 1.68, 95% CI 1.57–1.8, P<0.001) even when accounting for the increased incidence of ARF in patients with DM. They also found that canagliflozin was associated with a greater proportion of ARF compared to other SGLT-2 inhibitors, 7.3% compared to 4.7% and 4.8% with empagliflozin and dapagliflozin, respectively.23 There is also concern about a decrease in eGFR of ~4 mL/min/1.73 m2 (with a range from –2 to –6 mL/min/1.73 m2) in the first 3–6 weeks after initiation of canagliflozin.24,25 However, these decreases in eGFR seem reversible, as evidenced by the return to baseline serum creatinine among patients who discontinued canagliflozin during Phase III trials.
Endocrine
Although this discussion focuses on the side effects of canagliflozin, it is important to note that the FDA lists a black box warning for all SGLT-2 inhibitors for the risk of diabetic ketoacidosis (DKA). In case reports of DKA with SGLT-2 inhibitors, the serum glucose at presentation has been much lower than would be expected, likely owing to the glycosuric effects of these drugs.26 However, CANVAS found no evidence of increased risk of DKA in patients taking canagliflozin compared to placebo.14
Fractures
Fractures have also been reported in patients assigned to canagliflozin in randomized controlled trials.27 The exact mechanism behind the potential increased risk of fractures is not well understood. One proposed mechanism is that volume depletion events such as syncope may cause increased falls. Additionally, some studies have shown that canagliflozin may be associated with a small, but statistically significant, decrease in bone mineral density at the total hip over 104 weeks, and there is an increase in bone turnover markers such as type 1 beta-carboxytelopeptide and osteocalcin28 (Table 5). The CANVAS trial did find a significantly higher incidence of all fractures in patients taking canagliflozin compared to placebo: 15.4 events per 1,000 patient-years in the canagliflozin group vs 11.9 in the placebo group (P=0.02)14 and low-trauma fractures (11.6 vs 9.2 events per 1,000 patient years for canagliflozin and placebo, respectively, P=0.06). A statistically significant interaction between treatment, fractures, and trials (CANVAS and CANVAS-R) was observed (P interaction 0.005 for all fractures) without a clear reason for the observed potential differences between the two studies.
Amputation
CANVAS showed a significantly higher incidence of amputations with canagliflozin compared with placebo (6.3 vs 3.4 events per 1,000 patient years; P<0.001). Seventy-one percent of patients had their highest level of amputation at the level of toe or metatarsal14 (Table 5). The FDA has issued a warning regarding the increased risk of amputation during the CANVAS trial. The mechanism for the observed increased risk of amputations is not known. More data are expected regarding the amputation risk of canagliflozin from the ongoing CREDENCE trial.
Cancer
There had been concerns that canagliflozin may have carcinogenic effects, as a study by De Jonghe et al in 2014 had shown an increased incidence of renal tubular tumors and Leydig cell tumors in rats when exposed to canagliflozin.20,29,30 However a systematic review of existing randomized control trials found no evidence of a significantly increased risk of cancer in patients taking canagliflozin.31 However, the relatively short duration of the trials limits the ability to determine whether canagliflozin has long-term carcinogenic effects.
Future studies
To date, the studies examining the safety of canagliflozin have done so at a maximum of 104 weeks.32 Canagliflozin was the first SGLT-2 inhibitor to be approved in 2013. As canagliflozin has had more time on the market, there are more opportunities to study it in real-world settings (Table 6). The safety and efficacy on renal and cardiac outcomes of canagliflozin among patients with more advanced diabetic nephropathy is also of particular interest, and the results of CREDENCE trial (NCT02173275) should provide some important insights.17 In addition to CREDENCE, there are several other ongoing studies on the cardiac and renal benefits of SGLT-2 inhibitors in different patient populations and with different agents, including DECLARE (Dapagliflozin Effect on Cardiovascular Events, NCT01730534), EMPA-KIDNEY (Study of Heart and Kidney Protection with Empagliflozin, NCT03594110), DAPA-CKD (Study to Evaluate the Effect of Dapagliflozin on Renal Outcomes and Cardiovascular Mortality in Patients with CKD, NCT03036150), and VERTIS (Evaluation of Ertugliflozin Efficacy and Safety, NCT01986881) trials (DECLARE - NCT01730534, EMPA-KIDNEY - NCT03594110, DAPA-CKD - NCT03036150 and VERTIS - NCT01986881).
  | Table 6 Completed and ongoing trials with at least 500 patients enrolled or planning to enroll Abbreviations: SLGT-2, sodium-glucose cotransporter-2; TZD, thiazolidinedione. |
Conclusion
Canagliflozin is an effective therapy for treatment of patients with T2DM.1 The large integrated CANVAS program demonstrated important reductions in the primary composite of cardiovascular death, nonfatal MI, and nonfatal stroke. There were also important but statistically nominal reductions in hospitalization for heart failure and renal outcomes. Canagliflozin has important adverse events that need to be monitored for and managed appropriately if they occur.
Disclosure
HJ reports no financial disclosures. KWM’s financial disclosures can be viewed at http://med.stanford.edu/profiles/kenneth-mahaffey. TIC serves as the Co-US National Leader of CREDENCE and reports receiving consulting fees from Janssen Research and Development, LLC, and Novo Nordisk. MT reports no financial disclosures. The authors report no other conflicts of interest in this work.
References
Deeks ED, Scheen AJ. Canagliflozin: A Review in Type 2 Diabetes. Drugs. 2017;77(14):1577–1592. | ||
Plosker GL. Canagliflozin: a review of its use in patients with type 2 diabetes mellitus. Drugs. 2014;74(7):807–824. | ||
Takebayashi K, Inukai T. Effect of Sodium Glucose Cotransporter 2 Inhibitors With Low SGLT2/SGLT1 Selectivity on Circulating Glucagon-Like Peptide 1 Levels in Type 2 Diabetes Mellitus. J Clin Med Res. 2017;9(9):745–753. | ||
Szablewski L. Glucose transporters in healthy heart and in cardiac disease. Int J Cardiol. 2017;230:70–75. | ||
Mudaliar S, Polidori D, Zambrowicz B, Henry RR. Sodium-Glucose Cotransporter Inhibitors: Effects on Renal and Intestinal Glucose Transport: From Bench to Bedside. Diabetes Care. 2015;38(12):2344–2353. | ||
Cefalu WT, Leiter LA, Yoon KH, et al. Efficacy and safety of canagliflozin versus glimepiride in patients with type 2 diabetes inadequately controlled with metformin (CANTATA-SU): 52 week results from a randomised, double-blind, phase 3 non-inferiority trial. Lancet. 2013;382(9896):941–950. | ||
Davis SN. Canagliflozin versus glimepiride treatment in patients with type 2 diabetes inadequately controlled with metformin (CANTATA-SU trial. Expert Rev Clin Pharmacol. 2014;7(1):21–23. | ||
Forst T, Guthrie R, Goldenberg R, et al. Efficacy and safety of canagliflozin over 52 weeks in patients with type 2 diabetes on background metformin and pioglitazone. Diabetes Obes Metab. 2014;16(5):467–477. | ||
Wilding JP, Charpentier G, Hollander P, et al. Efficacy and safety of canagliflozin in patients with type 2 diabetes mellitus inadequately controlled with metformin and sulphonylurea: a randomised trial. Int J Clin Pract. 2013;67(12):1267–1282. | ||
Stenlöf K, Cefalu WT, Kim KA, et al. Long-term efficacy and safety of canagliflozin monotherapy in patients with type 2 diabetes inadequately controlled with diet and exercise: findings from the 52-week CANTATA-M study. Curr Med Res Opin. 2014;30(2):163–175. | ||
Vasilakou D, Karagiannis T, Athanasiadou E, et al. Sodium-glucose cotransporter 2 inhibitors for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med. 2013;159(4):262–274. | ||
Baker WL, Smyth LR, Riche DM, Bourret EM, Chamberlin KW, White WB. Effects of sodium-glucose co-transporter 2 inhibitors on blood pressure: a systematic review and meta-analysis. J Am Soc Hypertens. 2014;8(4):e269:262–275. | ||
Neal B, Perkovic V, de Zeeuw D, et al. Rationale, design, and baseline characteristics of the Canagliflozin Cardiovascular Assessment Study (CANVAS) – a randomized placebo-controlled trial. Am Heart J. 2013;166(2):217–223.e11. | ||
Neal B, Perkovic V, Mahaffey KW, et al; CANVAS Program Collaborative Group. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N Engl J Med. 2017;377(7):644–657. | ||
Ryan PB, Buse JB, Schuemie MJ. Comparative effectiveness of canagliflozin, SGLT2 inhibitors and non-SGLT2 inhibitors on the risk of hospitalization for heart failure and amputation in patients with type 2 diabetes mellitus: A real-world meta-analysis of 4 observational databases (OBSERVE-4D). Diabetes Obes Metab. 2018;20(11):2585–2597. | ||
Heerspink HJ, Desai M, Jardine M, Balis D, Meininger G, Perkovic V. Canagliflozin Slows Progression of Renal Function Decline Independently of Glycemic Effects. J Am Soc Nephrol. 2017;28(1):368–375. | ||
Jardine MJ, Mahaffey KW, Neal B, et al; CREDENCE study investigators. The Canagliflozin and Renal Endpoints in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE) Study Rationale, Design, and Baseline Characteristics. Am J Nephrol. 2017;46(6):462–472. | ||
Flores E, Santos-Gallego CG, Diaz-Mejía N, Badimon JJ. Do the SGLT-2 Inhibitors Offer More than Hypoglycemic Activity? Cardiovasc Drugs Ther. 2018;32(2):213–222. | ||
Rizzi M, Trevisan R. Genitourinary infections in diabetic patients in the new era of diabetes therapy with sodium-glucose cotransporter-2 inhibitors. Nutr Metab Cardiovasc Dis. 2016;26(11):963–970. | ||
Singh M, Kumar A. Risks Associated with SGLT2 Inhibitors: An Overview. Curr Drug Saf. 2018;13(2):84–91. | ||
Xiong W, Xiao MY, Zhang M, Chang F. Efficacy and safety of canagliflozin in patients with type 2 diabetes: A meta-analysis of randomized controlled trials. Medicine (Baltimore). 2016;95(48):e5473. | ||
Sinclair AJ, Bode B, Harris S, et al. Efficacy and Safety of Canagliflozin in Individuals Aged 75 and Older with Type 2 Diabetes Mellitus: A Pooled Analysis. J Am Geriatr Soc. 2016;64(3):543–552. | ||
Perlman A, Heyman SN, Matok I, Stokar J, Muszkat M, Szalat A. Acute renal failure with sodium-glucose-cotransporter-2 inhibitors: Analysis of the FDA adverse event report system database. Nutr Metab Cardiovasc Dis. 2017;27(12):1108–1113. | ||
Peene B, Benhalima K. Sodium glucose transporter protein 2 inhibitors: focusing on the kidney to treat type 2 diabetes. Ther Adv Endocrinol Metab. 2014;5(5):124–136. | ||
Perkovic V, Jardine M, Vijapurkar U, Meininger G. Renal effects of canagliflozin in type 2 diabetes mellitus. Curr Med Res Opin. 2015;31(12):2219–2231. | ||
Peters AL, Henry RR, Thakkar P, Tong C, Alba M. Diabetic Ketoacidosis With Canagliflozin, a Sodium-Glucose Cotransporter 2 Inhibitor, in Patients With Type 1 Diabetes. Diabetes Care. 2016;39(4):532–538. | ||
Alba M, Xie J, Fung A, Desai M. The effects of canagliflozin, a sodium glucose co-transporter 2 inhibitor, on mineral metabolism and bone in patients with type 2 diabetes mellitus. Curr Med Res Opin. 2016;32(8):1375–1385. | ||
Watts NB, Bilezikian JP, Usiskin K, et al. Effects of Canagliflozin on Fracture Risk in Patients With Type 2 Diabetes Mellitus. J Clin Endocrinol Metab. 2016;101(1):157–166. | ||
De Jonghe S, Proctor J, Vinken P, et al. Carcinogenicity in rats of the SGLT2 inhibitor canagliflozin. Chem Biol Interact. 2014;224:1–12. | ||
Lin HW, Tseng CH. A Review on the Relationship between SGLT2 Inhibitors and Cancer. Int J Endocrinol. 2014;2014:719578. | ||
Tang H, Dai Q, Shi W, Zhai S, Song Y, Han J. SGLT2 inhibitors and risk of cancer in type 2 diabetes: a systematic review and meta-analysis of randomised controlled trials. Diabetologia. 2017;60(10):1862–1872. | ||
Bode B, Stenlöf K, Harris S, et al. Long-term efficacy and safety of canagliflozin over 104 weeks in patients aged 55-80 years with type 2 diabetes. Diabetes Obes Metab. 2015;17(3):294–303. | ||
Desai M, Yavin Y, Balis D, et al. Renal safety of canagliflozin, a sodium glucose co-transporter 2 inhibitor, in patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2017;19(6):897–900. | ||
Erondu N, Desai M, Ways K, Meininger G. Diabetic Ketoacidosis and Related Events in the Canagliflozin Type 2 Diabetes Clinical Program. Diabetes Care. 2015;38(9):1680–1686. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


