Back to Journals » Neuropsychiatric Disease and Treatment » Volume 18
Can Fluoxetine Combined with Cognitive Behavioral Therapy Reduce the Suicide and Non-Suicidal Self-Injury Incidence and Recurrence Rate in Depressed Adolescents Compared with Fluoxetine Alone? A Meta-Analysis
Authors Liu W , Li G, Wang C, Yu M, Zhu M, Yang L
Received 28 March 2022
Accepted for publication 22 October 2022
Published 2 November 2022 Volume 2022:18 Pages 2543—2557
DOI https://doi.org/10.2147/NDT.S367931
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Yuping Ning
Wenliang Liu, Gongying Li, Congjie Wang, Mingchao Yu, MengYa Zhu, Lin Yang
Department of Psychological, Huai’an No.3 People’s Hospital, Huaian, People’s Republic of China
Correspondence: Gongying Li, Department of Psychological, Huai’an No.3 People’s Hospital, No. 272, Huaihai West Road, Huai an, 223001, People’s Republic of China, Tel +86-517-83285124, Email [email protected]
Objective: The efficacy of medication and psychotherapy for adolescent depression is controversial, so we conducted a meta-analysis to evaluate the efficacy of combination therapy.
Methods: We followed the PRISMA checklist in completing the meta-analysis. Relevant literature was searched in PubMed, Web of Science and Embase, Chinese databases CNKI and WanFang Data. We included the literature on the comparison of the fluoxetine plus psychotherapy or cognitive-behavioral therapy (CBT) and each treatment alone for adolescent depression published in 1980– 2021. All statistical analyses were performed using Stata software.
Results: After careful review, a total of 489 relevant articles were retrieved, and 13 studies were finally included. In comparison with the control group (fluoxetine alone), fluoxetine plus CBT achieved higher response rate (RR=1.12, 95% CI: 1.04, 1.21), lower incidence of adverse Reactions (RR=0.62,95% CI:0.40,0.96), lower proportion of suicide or self-injury (RR=0.94,95% CI:0.74,1.20), and lower one-year recurrence rate (RR=0.27, 95% CI: 0.16, 0.45). Before treatment, there were no significant differences in Hamilton Depression Scale score (HAMD), Children’s Depression Rating Scale Revised (CDRS-R) score, and Clinical Global Impression (CGI) Severity score. After treatment, HAMD score (SMD=− 1.01, 95% CI:-1.39,-0.63), CDRS-R score (SMD= − 0.10,95% CI:-0.26,-0.07), and CGI score (SMD = − 0.22, 95% CI: − 0.54, − 0.10) were significantly lower in the combined treatment group than in the control group.
Conclusion: Adolescents simultaneously treated with fluoxetine and CBT had significantly reduced incidence of depressive symptoms, suicide or NSSI, adverse reactions, and one-year recurrence of symptoms, than adolescents treated with fluoxetine alone. This indicates fluoxetine plus CBT may be superior to fluoxetine alone for the clinical treatment of adolescent depression.
Keywords: adolescent depression, fluoxetine, psychotherapy, cognitive behavioral therapy, CBT, meta-analysis
Introduction
Major depressive disorder (MDD) is a common disease burden in people aged between 10 to 24 years.1 In the United States, about 11% to 14% of adolescents between 15 and 18 years are diagnosed with depression, and 25% have sub-threshold depressive symptoms that lead to difficulties in daily functioning.2 Depression in adolescents often leads to impairments in social functioning and poor academic performance. Furthermore, individuals who suffer from depression during adolescence are at an increased risk of developing depression and other psychopathology in adulthood.3 Furthermore, depressed adolescents often engage in self-harming behaviors, including smoking, substance misuse, unhealthy eating, non-suicidal self-injury (NSSI), and suicide.4 NSSI is a widespread problem among depressed adolescents. Studies have shown that about 48% of adolescents with depression have attempted suicide or NSSI.5,6 Because of the harmful effects of depression on adolescents, there is a need to develop effective treatments to manage depression in adolescents.
The standard treatment for depression includes antidepressant medications, psychotherapy, and a combination of medication and psychotherapy. Acute phase treatment interventions for adolescent depression have demonstrated efficacy in achieving response compared to placebo or usual care.
The main pharmaceutical treatments for depression include selective serotonin reuptake inhibitors, norepinephrine and dopamine reuptake inhibitors, and tricyclic antidepressants.7 However, although antidepressants are an effective treatment for depression and anxiety in adolescents, they also have several side effects. Therefore the use of antidepressants in children and adolescents must be carefully monitored. The Food and Drug Administration (FDA) issued a black box warning that the use of antidepressants in people under 25 years can increase the risk of suicidal thoughts and behavior. However, there was considerable debate in academic journals and the international media. Causal relationships cannot be established with certainty until there is a vast improvement in post-marketing surveillance.8 Eventually, fluoxetine and escitalopram are 2 FDA-approved antidepressants for the short-term management and maintenance of MDD in young adults. Many studies have shown that antidepressants can reduce depressive symptoms, long-term administration can cause serious side effects, such as headaches, sleep disturbances, weight gain, nausea, and constipation.9 Psychotherapy, in the form of interpersonal psychotherapy, family therapy, and cognitive behavioral therapy (CBT), is often used in the management of depression in children and adolescents.10 Although psychotherapy was found to be an effective treatment for depression, it still has numerous limitations. Psychotherapy requires several treatment cycles, and it takes time to produce the desired results.11 The long treatment duration also increases the costs for the patients. In order to overcome the limitations of psychotherapy, some studies proposed using antidepressants in combination with psychotherapy.12 However, there are currently no comprehensive studies evaluating the therapeutic outcomes of fluoxetine combined with psychotherapy. Therefore we aimed to conduct a meta-analysis of the literature published in recent years to evaluate the therapeutic effect and safety of fluoxetine combined with psychotherapy in the management of depression in adolescents.
Materials and Methods
Search Strategy
The PubMed, Web of Science, Embase, China National Knowledge Infrastructure, and WanFang databases were searched to identify relevant research articles published in either English or Chinese up to October 2021. The keywords used for the search were “adolescent depression” AND “fluoxetine” AND “psychotherapy” OR “cognitive behavioral therapy”.
Inclusion and Exclusion Criteria
We adopted the following inclusion criteria: (1) Study design: randomized controlled trial, cohort study and clinical trial; (2) Study subjects: adolescent patients diagnosed with depression; (3) Intervention: fluoxetine plus CBT (treatment group) in relation to fluoxetine alone (control group); (4) Outcome measures: response rate, incidence of adverse reactions, one-year recurrence rate, Hamilton Depression Scale score (HAMD), Children’s Depression Rating Scale–Revised score (CDRS-R), and Clinical Global Impression Severity score (CGI). Adverse effects included headache, nausea, hyperhidrosis, dizziness, lethargy and incidents of suicide or NSSI.
We excluded studies for the following reasons: (1) literature not related to adolescent depression; (2) duplicate literature: more than one report based on the same study population published, in which case only the most comprehensive publication was included in this meta-analysis; (3) literature in which relevant data were not provided, and the data and original text could not be obtained; (4) literature with poor quality, missing data; (5) case reports, systematic reviews, and animal experiments.
Data Extraction and Assessment of Methodological Quality
Two investigators performed the literature search independently. The titles, and abstracts, were reviewed carefully to identify relevant articles. Any duplicate studies were removed. The full texts were then reviewed, and irrelevant studies and those with insufficient data were removed. The final articles retrieved by the investigator were compared, and any disagreements were resolved through discussion. Subsequently, the 2 investigators extracted the following data from the manuscript; first author, year of publication, study design, sample size, main outcome measures, and risk estimates with the corresponding 95% confidence interval (95% CI). In the process of data extraction, any disagreements were resolved through discussion by both parties or judged by a third party. The methodological quality of the included literature was evaluated using the Jadad scoring system. The Jadad score consisted of 5 items and 3 broad categories, including randomization (2 items), blinding (2 items), and dropouts and withdrawals (1 item). Each item within each category is assigned a score of 1 if it complies and 0 if it does not comply. The final score ranges from 0 to 5 points, and a score of 3 or higher indicates good methodological quality.
Statistical Analyses
All statistical analyses were performed using the Stata software version 16 (StataCorp LLC, College Station, TX, USA). The adjusted odds ratio (OR) for each treatment outcome was extracted for the meta-analysis. If the OR was not provided, the raw data were used to calculate it. All outcome measures were expressed as standardized mean difference (SMD) and risk ratio (RR). The statistical heterogeneity between the studies was evaluated using the Q statistic and the I-square (I2) statistic. An I2 value below 50% and a p-value above 0.1 indicate low heterogeneity, and therefore, the fixed-effect model was used to analyze the data. Conversely, the random-effect model was adopted for studies with high heterogeneity. Sensitivity analysis was performed to assess the impact of individual studies on the overall results. Funnel plots were used to assess the publication bias for outcome measures evaluated in more than 9 studies.
Results
Search results and Description of Studies
The initial literature search yielded a total of 489 articles, of which 196 were duplicated articles and were therefore removed. After screening the titles and abstracts, 255 articles were deemed irrelevant to the study, and another 25 studies were excluded after evaluating the full texts due to insufficient data. Finally, 13 studies were included in the meta-analysis.14–26 The literature screening process and results are illustrated in Figure 1. Further details of the included studies are shown in Table 1. Figure 2A illustrates the overall risk of bias, and Figure 2B illustrates the risk of bias for each of the 13 included studies.
 |
Table 1 Characteristics of Studies Included in the Meta-Analysis |
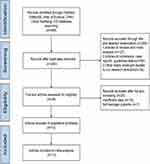 |
Figure 1 Flow diagram illustrating the study selection. |
 |
Figure 2 (A) The risk of bias graph. (B) The risk of bias for each of the included studies. |
Response Rate and Recurrence Rate After Treatment
Seven studies compared the response rate between treatment and control group.13–15,18–20,25 No marked heterogeneity was found among the included studies (I2 = 0.0%, P = 0.598), so we used a fixed-effect model for analysis. The result showed a significantly higher response rate in the fluoxetine plus CBT group compared with the control group (RR = 1.12, 95% CI: 1.04, 1.21, Figure 3A).
 |
Figure 3 (A) Forest plots comparing the response rate between the treatment and control groups. (B) Forest plots comparing the recurrence rate between the treatment and control groups. |
Six included studies compared the recurrence rate between treatment and control group.15,17,19,21–23 The fixed-effect model was also used for analysis since the heterogeneity among these studies was low (I2 = 5.8%, P = 0.379). Compared with the control group, the treatment group had a significantly lower one-year recurrence rate (RR = 0.27, 95% CI: 0.16, 0.45, Figure 3B).
The sensitivity analysis did not reveal any major changes in the pooled results indicating that the results of the response and recurrence rates were robust (Figures 4A and B).
 |
Figure 4 (A) Sensitivity analysis of response rate after treatment. (B) Sensitivity analysis of recurrence rate after treatment. |
Incidence of Adverse Reactions and Proportion of Suicide or NSSI After Treatment
Seven studies compared the incidence rate of adverse reactions between treatment and control group.14,18–20,23–25 Since no marked heterogeneity was found among the included studies (I2 = 0.0%, P = 0.901), the fixed-effect model was used to analyze the data. The incidence rate of adverse events in the fluoxetine plus CBT group was lower when compared with the control group (RR = 0.62, 95% CI: 0.40, 0.96, Figure 5A).
 |
Figure 5 (A) Forest plot comparing the incidence of adverse reactions after treatment. (B) Forest plot comparing the proportion of suicide or NSSI after treatment. |
Three of the included studies compared the proportion of suicide or NSSI between the two groups.23–25 The fixed-effect model was used for analysis since the heterogeneity was low (I2 = 0.0%, P = 0.442). The results showed that fluoxetine plus CBT resulted in a more significant reduction in the rate of suicide and NSSI when compared with the control group (RR = 0.94, 95% CI: 0.74, 1.20, Figure 5B).
The sensitivity analysis showed low sensitivity, confirming that the incidence of adverse reactions and suicide or NSSI results were robust (Figures 6A and B).
 |
Figure 6 (A) Sensitivity analysis of incidence of adverse reactions after treatment. (B) Sensitivity analysis of proportion of suicide or NSSI after treatment. |
HAMD Score After Treatment
Nine studies compared the HAMD score between the treatment and control groups.14–22 Significant heterogeneity (I2 = 78.5%, P ≤ 0.001) was noted between the studies, and therefore, the random-effects model was used to analyze the data. The patients treated with fluoxetine plus CBT had a significantly lower HAMD score when compared with the control group (SMD = −1.01, 95% CI: −1.39, −0.63, Figure 7).
 |
Figure 7 Forest plot comparing the HAMD score between the treatment and control groups. |
The symmetrically shaped funnel plot indicates a low risk of publication bias (Figure 8). Additionally, the sensitivity analysis confirmed that the pooled effect size was still of statistical significance, and the direction of the forest plot did not change significantly before and after the removal of any literature (Figure 9).
 |
Figure 8 Funnel plot of HAMD score after treatment. |
 |
Figure 9 Sensitivity analysis for the HAMD score after treatment. |
CDRS-R Score and CGI Score After Treatment
Three studies compared the difference in CDRS-R scores between the treatment and control groups.23–25 Since the heterogeneity between the studies was low (I2 = 24.0%, P = 0.268), the fixed-effect model was used to analyze the data. The CDRS-R scores of patients treated with fluoxetine plus CBT were significantly lower when compared with the control group (SMD = −0.10, 95% CI: −0.26, 0.07, Figure 10A).
 |
Figure 10 (A) Forest plot comparing the CDRS-R score between the treatment and control groups. (B) Forest plot comparing the CGI score between the treatment and control groups. |
Three of the included studies compared the CGI scores between the treatment and control groups.23–25 Since the heterogeneity between the studies was high (I2 = 72.1%, P = 0.028), the random-effect model was used to analyze the data. The treatment group had a lower CGI score when compared with the control group (SMD = −0.22, 95% CI: −0.54, 0.10, Figure 10B).
The sensitivity analysis confirmed that the results for the CDRS-R scores and CGI scores were reliable (Figures 11A and B).
 |
Figure 11 (A) Sensitivity analysis of CDRS-R score after treatment. (B) Sensitivity analysis of CGI score after treatment. |
Discussion
Depression in adolescents is a common debilitating condition that leads to poor quality of life and an increased risk of suicide and self-harm. Depressed adolescents are often treated with the antidepressant fluoxetine hydrochloride, a selective serotonin reuptake inhibitor (5-HT). This drug inhibits the absorption of 5-HT in the central nervous system, leading to increased secretion of neurotransmitters that help reduce depressive symptoms. However, the therapeutic effect of this treatment in adolescents is limited, and the incidence of relapse and adverse effects are high.26 Studies have also shown that the prolonged use of antidepressants can also increase suicidal thoughts and behavior in children and adolescents.27 Therefore, more effective intervention methods are required. In order to overcome this problem, antidepressant medications are often used with psychotherapies in routine clinical practice to manage depression. Although numerous meta-analyses have shown that both psychotherapy and antidepressant are effective in reducing depressive symptoms,28 to our knowledge, the comparative efficacy and safety of these interventions are still inconclusive. A network meta-analysis evaluated the treatment outcomes of 16 antidepressants, 7 psychotherapies, and 5 combination therapy regimes to manage acute depressive symptoms in children and adolescents (18 years and younger). The study also found that fluoxetine (either alone or in combination with CBT) provided the best outcomes for managing moderate-to-severe depressive disorders in children and adolescents.7 However, this study still lacked high-quality evidence. CBT is a structured, long, and expensive process designed to replace the patients’ negative thinking with healthier, positive ideas.29 The optimal timing of implementing CBT during the treatment of depressed adolescents is still not well understood. The National Institute for Health and Care Excellence (NICE) guidelines recommend the use of CBT as a first-line treatment for depressed adolescents. However, studies have shown that for severely depressed patients, CBT does not results in better outcomes when compared with treatment using antidepressant medication alone or combined CBT with antidepressants.30 Conversely, other studies have shown that combined therapy can reduce the effectiveness of antidepressive therapy and cause drug resistance in depressed adolescent patients.31
Therefore in this manuscript, we present the first meta-analysis comparing the treatment efficacy and adverse events of fluoxetine treatment with combined fluoxetine and CBT in the management of depression in adolescents. A total of 13 randomized controlled trials were included in this meta-analysis, and 1232 adolescents with depressive disorders were assigned to 2 or 4 controlled treatment conditions.
Our findings indicate that the combination of fluoxetine with CBT effectively reduced the incidence of depressive symptoms and adverse reactions in adolescent patients. Notably, the findings of the meta-analysis indicate that the combined therapy could improve the treatment effectiveness (RR = 1.12, 95% CI: 1.04, 1.21), lower the incidence of adverse reactions (RR = 0.62, 95% CI: 0.40, 0.96), reduce the incidence of suicide or NSSI (RR = 0.94, 95% CI: 0.74, 1.20), and reduce the one-year recurrence rate (RR = 0.27, 95% CI: 0.16, 0.45). The study also found that fluoxetine could reduce the HAMD score, CDRS-R score, and CGI score in depressed adolescents. However, fluoxetine combined with psychotherapy was more effective.
This study has some limitations that have to be acknowledged. It is important to note that the follow-up period of the included studies was short. Therefore, more longitudinal studies are required to evaluate the long-term benefits of combined therapy. Furthermore, only a few studies reported the incidence rate of suicide or self-harm, leading to insufficient analysis and weakening the credibility of the results. Significant heterogeneity was also reported for the CGI and HAMD indicators. The heterogeneity was attributed to the psychotherapy technique and the professional background of the psychologists. Other factors, such as the wide range of outcome measures used to evaluate the severity of depressive symptoms and the difficulty of diagnosing depression, may also have contributed to heterogeneity between studies.
Conclusion
Compared with fluoxetine therapy alone, the combination of fluoxetine with CBT improved the treatment efficacy and safety for depression in adolescents. Most importantly, the combined therapy reduced the risk of suicide or self-injury and the relapse rate in depressed adolescents. However, the quality of this meta-analysis is limited by the small number of published randomized controlled trials, the short-term follow-up, and the heterogeneity in the evaluation of psychotherapy effects. Therefore more high-quality longitudinal studies are recommended to confirm the findings of this meta-analysis.
Data Sharing Statement
All datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Acknowledgments
We would like to thank TopEdit for the English language editing of this manuscript.
Funding
This study was supported by Huai’an natural science research program (HAB202044).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Mokdad AH, Forouzanfar MH, Daoud F. Global burden of diseases, injuries, and risk factors for young people’s health during 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2016;387(10036):2383–2401. doi:10.1016/S0140-6736(16)00648-6
2. Avenevoli S, Swendsen J, He JP, et al. Major depression in the national comorbidity survey–adolescent supplement: prevalence, correlates, and treatment. J Am Acad Child Adolesc Psychiatry. 2015;54(1):37–44. doi:10.1016/j.jaac.2014.10.010
3. Foster S, Mohler-Kuo M. Treating a broader range of depressed adolescents with combined therapy. J Affect Disord. 2018;241:417–424. doi:10.1016/j.jad.2018.08.027
4. Vander VP, Balint A, Drews E, et al. Pain sensitivity and plasma beta-endorphin in adolescent non-suicidal self-injury. J Affect Disord. 2021;278:199–208. doi:10.1016/j.jad.2020.09.036
5. Williams J, Barnhofer T, Crane C, et al. Pre-adult onset and patterns of suicidality in patients with a history of recurrent depression. J Affect Disord. 2012;138(1–2):173–179. doi:10.1016/j.jad.2011.12.011
6. Lim KS, Wong CH, McIntyre RS, et al. Global lifetime and 12-month prevalence of suicidal behavior, deliberate self-harm and non-suicidal self-injury in children and adolescents between 1989 and 2018: a meta-analysis. Int J Environ Res Public Health. 2019;16(22):4581. doi:10.3390/ijerph16224581
7. Zhou X, Teng T, Zhang Y, et al. Comparative efficacy and acceptability of Antidepressants, psychotherapies, and their combination for acute treatment of children and adolescents with depressive disorder: a systematic review and network meta-analysis. Lancet Psychiatry. 2020;7(7):581–601. doi:10.1016/S2215-0366(20)30137-1
8. Whitely M, Raven M, Jureidini J. Antidepressant prescribing and suicide/self-harm by young Australians: regulatory warnings, contradictory advice, and long-term trends. Front Psychiatry. 2020;11:478. doi:10.3389/fpsyt.2020.00478
9. Andrea C, Furukawa TA, Georgia S, et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet. 2018;391(10128):1357–1366. doi:10.1016/S0140-6736(17)32802-7
10. Anthes E. Depression: a change of mind. Nature. 2014;515(7526):185–187. doi:10.1038/515185a
11. Hazell P. Updates in treatment of depression in children and adolescents. Curr Opin Psychiatry. 2021;34(6):593–599. doi:10.1097/YCO.0000000000000749
12. Liu W, Li G, Wang C, et al. Efficacy of sertraline combined with cognitive behavioral therapy for adolescent depression: a systematic review and meta-analysis. Comput Math Methods Med. 2021;2021:5309588. doi:10.1155/2021/5309588
13. Lu ZC, Gan XW, Yuan GC, et al. Clinical effect of drug combined with Psychobehavioral therapy on adolescent depression and its influence on patient compliance. The Northern Pharmaceut. 2021;18(2):37–38. doi:10.3969/j.issn.1672-8351.2021.02.018
14. Guo TY, Ji WD, Zhou JX, et al. A control study on the clinical effect of comprehensive psychotherapy on first-episode adolescent depression. J Clin Psychosom Dis. 2010;16(4):331–333.
15. Li XB. Clinical observation of psychological intervention combined with fluoxetine in the treatment of juvenile depression in 78 cases. Chin J Clin Rational Drug Use. 2013;6(3):90–91. doi:10.3969/j.issn.1674-3296.2013.07.072
16. Jia QM, Hu M. Effect of cognitive behavioral therapy combined with fluoxetine in the treatment of depression in children and adolescents. Chin J Mod Drug Appl. 2015;9(2):27–28. doi:10.14164/j.cnki.cn11-5581/r.2015.02.014
17. Zhu JJ, Wan QH, Qian JH. Effect of fluoxetine combined with psychotherapy on adolescent depression. Med J Chin Peoples Health. 2009;21(17):135–136. doi:10.3969/j.issn.1672-0369.2009.17.051
18. Fan Y. Effect of fluoxetine combined with psychological intervention on adolescent depression and its influence on memory. Psychol Monthly Mag. 2021;1:51–52. doi:10.19738/j.cnki.psy.2021.01.020
19. Han XL, Han HL. Efficacy of fluoxetine combined with psychological intervention in the treatment of adolescent depression. Xizang Na Defense Med. 2019;28(2):54–55. doi:10.3969/j.issn.1004-0188.2019.01.020
20. Huang XY, Wang Z. Comparison of fluoxetine combined with psychotherapy on adolescent depression. Chin J Misdiagn. 2009;9(7):1543.
21. Liu XF, Yang ZW, Fan CY. Observation on psychotherapy combined with fluoxetine in treatment of 90 cases of adolescent depression. Chin J Pract Neurol Dis. 2015;18(7):89–90. doi:10.3969/j.issn.1673-5110.2015.07.054
22. Shen QY, Li LJ, Liang W. Effect of fluoxetine combined with psychotherapy on adolescent depression. Rep Xuzhou Med Coll. 2008;28(7):461–463. doi:10.3969/j.issn.1000-2065.2008.07.015
23. Kennard BD, Emslie GJ, Mayes TL, et al. Sequential treatment with fluoxetine and relapse--prevention CBT to improve outcomes in pediatric depression. Am J Psychiatry. 2014;171(10):1083–1090. doi:10.1176/appi.ajp.2014.13111460
24. March J, Silva S, Petrycki S, et al. Fluoxetine, cognitive-behavioral therapy, and their combination for adolescents with depression: Treatment for Adolescents With Depression Study (TADS) randomized controlled trial. JAMA. 2004;292(7):807–820. doi:10.1001/jama.292.7.807
25. Goodyer IM, Dubicka B, Wilkinson P, et al. A randomised controlled trial of cognitive behaviour therapy in adolescents with major depression treated by selective serotonin reuptake inhibitors. The ADAPT trial. Health Technol Assess. 2008;12(14):iii–iv, ix–60. doi:10.3310/hta12140
26. Berlim Marcelo T, Gustavo T. Definition, assessment, and staging of treatment resistant refractory major depression: a review of current concepts and methods. Can J Psychiatry. 2007;52(1):46–54. doi:10.1177/070674370705200108
27. Suicidality in children and adolescents being treated with antidepressant medications. US Food and Drug Administration; Oct 15, 2004.
28. Cuijpers P, Andersson G, Donker T, et al. Psychological treatment of depression: results of a series of meta-analyses. Nord J Psychiatry. 2011;65(6):354. doi:10.3109/08039488.2011.596570
29. Eirini K, Heleen R, Jos T, et al. Efficacy of self-guided internet-based cognitive behavioral therapy in the treatment of depressive symptoms: a meta-analysis of individual participant data. JAMA Psychiatry. 2017;74(4):351–359. doi:10.1001/jamapsychiatry.2017.0044
30. Kamenov K, Twomey C, Cabello M, et al. The efficacy of psychotherapy, pharmacotherapy and their combination on functioning and quality of life in depression: a meta-analysis. Psychol Med. 2017;47(3):414–425. doi:10.1017/S0033291716002774
31. Jobst A, Brakemeier E-L, Buchheim A, et al. European psychiatric association guidance on psychotherapy in chronic depression across Europe. Eur Psychiatry. 2016;33(1):18–36. doi:10.1016/j.eurpsy.2015.12.003
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
