Back to Journals » Clinical Epidemiology » Volume 11
Can diabetic polyneuropathy and foot ulcers in patients with type 2 diabetes be accurately identified based on ICD-10 hospital diagnoses and drug prescriptions?
Authors Christensen DH , Knudsen ST, Nicolaisen SK, Andersen H , Callaghan BC, Finnerup NB , Jensen TS , Thomsen RW
Received 7 December 2018
Accepted for publication 15 February 2019
Published 1 May 2019 Volume 2019:11 Pages 311—321
DOI https://doi.org/10.2147/CLEP.S197474
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Irene Petersen
Diana Hedevang Christensen,1,2 Søren Tang Knudsen,3 Sia Kromann Nicolaisen,1 Henning Andersen,2,4 Brian Christopher Callaghan,2,5 Nanna Brix Finnerup,2,4,6 Troels Staehelin Jensen,2,4,6 Reimar Wernich Thomsen1
1Department of Clinical Epidemiology, Institute of Clinical Medicine, Aarhus University Hospital, Aarhus, Denmark; 2International Diabetic Neuropathy Consortium, Department of Clinical Medicine, Faculty of Health, Aarhus University, Aarhus, Denmark; 3Steno Diabetes Center Aarhus (SDCA), Aarhus University Hospital, Aarhus, Denmark; 4Department of Neurology, Aarhus University Hospital, Aarhus, Denmark; 5Department of Neurology, University of Michigan, Ann Arbor, MI, USA; 6Department of Clinical Medicine, Danish Pain Research Center, Aarhus University, Aarhus, Denmark
Purpose: We examined whether diabetic polyneuropathy (DPN) and diabetic foot ulcers in type 2 diabetes can be accurately identified using International Classification of Diseases, 10th revision discharge diagnosis codes, surgery codes, and drug prescription codes.
Methods: We identified all type 2 diabetes patients in the Central Denmark region, 2009–2016, who had ≥1 primary/secondary diagnosis code of “diabetes with neurological complication” (E10.4-E14.4), “diabetic polyneuropathy” (G63.2), or “polyneuropathy, unspecified” (G62.9). Patients with potential painful DPN and non-painful DPN were identified based on prescription history for serotonin–norepinephrine reuptake inhibitors, tricyclic antidepressants, or gabapentinoids. Likewise, type 2 diabetes patients with potential foot ulcers were identified based on diagnosis or surgery codes. We used medical record review as the reference standard and calculated positive predictive values (PPVs).
Results: Of 53 randomly selected patients with potential painful DPN, 38 were classified as having DPN when validated against medical records; of these, 18 also had neuropathic pain, yielding a PPV of 72% (95% CI: 58–83%) for DPN and 34% (95% CI: 22–48%) for painful DPN. Likewise, among 54 randomly selected patients with potential non-painful DPN, 30 had DPN based on medical record data; of these, 27 had non-painful DPN, yielding PPVs of 56% (95% CI: 41–69%) and 50% (95% CI: 36–64%), respectively. Secondary E-chapter codes often denoted stroke or mononeuropathies, rather than DPN. Excluding secondary E-chapter codes from the algorithm increased the PPV for DPN to 78% (95% CI: 63–89%) for the painful DPN cohort and to 74% (95% CI: 56–87%) for the non-painful DPN cohort. Of 53 randomly selected patients with potential diabetic foot ulcer, only 18 diagnoses were confirmed; PPV=34% (95% CI: 22–48%).
Conclusion: G-chapter and primary E-chapter diagnosis codes can detect type 2 diabetes patients with hospital-diagnosed DPN, and may be useful in epidemiological research. In contrast, our diabetic foot ulcer algorithm did not perform well.
Keywords: positive predictive value, epidemiology, registries, diabetic polyneuropathy, diabetic foot ulcer, type 2 diabetes
Introduction
Diabetic polyneuropathy (DPN) is a common and serious diabetes complication.1 One-fifth of patients with DPN may develop debilitating neuropathic pain.2 Moreover, patients with DPN may suffer from a number of complications including diabetic foot ulcers, lower extremity amputations, and death.1 The etiology and pathogenesis behind painful and non-painful DPN, especially in type 2 diabetes,3 are still not fully understood, which hinders effective prevention and improved treatment of DPN.
There may be a great potential in using large medical registries and administrative databases to study risk and prognosis of DPN in type 2 diabetes, if diagnosis codes of DPN and its complications are valid. A high validity would be expected for codes of well-defined conditions like death and extremity amputations,4–8 whereas this may not be true for DPN and diabetic foot ulcers. Only a few studies have examined the potential of using diagnosis or procedure codes to identify patients with documented painful and non-painful DPN or diabetic foot ulcer. In a US study, an algorithm for painful diabetic peripheral neuropathy consisting of International Classification of Diseases (ICD) version 9 diagnosis codes was developed and validated against medical records in a diabetes registry.9 The authors reported a positive predictive value (PPV) of 79% of the final algorithm.9 Another US study found a PPV >90% of the specific ICD-9 code for “polyneuropathy in diabetes” (357.2) when compared with medical records,10 whereas a third US study validated 5 different ways to identify diabetic foot ulcers using ICD-9 diagnosis codes and Current Procedural Terminology procedure codes and found PPVs between 55% and 88%.11 These results are all from the US exclusively and based on ICD-9 codes. To our knowledge, the potential of using ICD-10 codes together with drug prescription registries to identify patients with painful DPN, non-painful DPN, or diabetic foot ulcer has not previously been studied.
Therefore, we examined whether hospital-diagnosed DPN, including painful DPN and non-painful DPN, and diabetic foot ulcers in patients with type 2 diabetes can be accurately identified using diagnosis codes, surgery codes, and drug prescription codes in Danish registries.
Materials and methods
Design and setting
This cross-sectional validation study is based on data from Danish medical registries and was conducted in the Central Region of Denmark (N≈1.3 million inhabitants), one of the five Danish administrative regions. The Danish National Health Service provides universal tax-supported health care for the entire Danish population including free access to general practitioners and hospitals in Denmark and partial reimbursement for prescribed drugs.12 Since 1968, the Danish Civil Registration System has assigned a unique 10-digit civil personal registration number (the CPR-number) to all Danish residents at birth or immigration.4 The CPR-number is used in all Danish Registries and allows accurate and unambiguous individual-level linkage across the registries.4
Health registries
We used ICD-10 codes to identify type 2 diabetes patients with hospital-diagnosed DPN and diabetic foot ulcers in the Danish National Patient Registry (DNPR).13 The DNPR holds information on all admissions at non-psychiatric hospitals since 1977, on non-psychiatric hospital outpatient and emergency room visits since 1995 and on all psychiatric hospital contacts (inpatient, outpatient, and emergency room) since 1995. From 1994 onwards, all diagnoses have been coded according to the ICD-10, whereas since 1996 all surgery has been coded according to the Nordic Medico-Statistical Committee classification of surgical procedures.13 We used the National Health Service Prescription Database (NHSPD) to obtain complete information on prescriptions on glucose-lowering drugs and neuropathic pain medications in our patients.12 The NHSPD has recorded data on redemption of reimbursed prescriptions from outpatient pharmacies since 2004. The recorded data include the amount and type of drug prescribed according to the Anatomical Therapeutic Chemical (ATC) classification system, and the date on which the drug was dispensed.12
Identification of the type 2 diabetes population
We defined eligible type 2 diabetes patients as those who had at least one in- or outpatient hospital discharge code of “diabetes mellitus” E10-14, “diabetic retinopathy” H36.0, “diabetes mellitus in pregnancy” O24 (excluding “gestational diabetes mellitus” O24.4), or “diabetic polyneuropathy” G63.2 at any hospital in Denmark, or at least one prescription redemption of a glucose-lowering drug, ATC-codes A10 between January 1, 1994, and July 10, 2016, N=436,402. This algorithm has previously been validated; the PPV of diagnosis codes for identifying patients with diabetes is 97% and the sensitivity 64%, whereas the PPV of the glucose-lowering drug prescription codes is 95% and sensitivity 72%.14 To avoid inclusion of patients treated with metformin for polycystic ovary syndrome, we did not include females aged 20–39 treated with metformin monotherapy who did not have a diabetes discharge code. We included other diabetes codes than the type 2 diabetes codes (E11), because type 1 diabetes, type 2 diabetes and other types of diabetes cannot be completely differentiated based on diagnosis codes E10-14 alone.15 In order to minimize misclassification of patients with other types of diabetes than type 2 diabetes, we excluded patients younger than 30 years at diabetes diagnosis treated with insulin monotherapy (Table 1 and Figure 1).
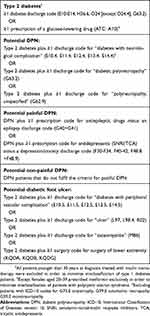 | Table 1 Algorithms of in- and outpatient discharge codes and prescription codes used to identify patients with painful and non-painful DPN and diabetic foot ulcer |
Identification of DPN
Any DPN population
From the population of type 2 diabetes patients, we identified those who had an in- or outpatient hospital diagnosis code that was indicative of DPN: the potential DPN population, N=35,490. Codes indicative of DPN were “polyneuropathy, unspecified” G62.9, “diabetic polyneuropathy” G63.2, or “diabetes with neurological complication” E10.4, E11.4, E12.4, E13.4, E14.4 (excluding among the latter patients who also had a diagnosis code of G73.0 “amyotrophy”, G99.0 “autonomic neuropathy”, or G59.0 “mononeuropathy”). We included both primary (first-listed, ie the primary cause for the hospital contact) and secondary diagnosis codes. Only patients with a DPN code given on the same date or later than a first type 2 diabetes registration (diagnosis or prescription) were included in the DPN population.
Painful DPN population
Next, we combined the DPN-algorithm with prescription data on medications used for the treatment of neuropathic pain in order to define an algorithm to identify patients with potential painful DPN: the painful DPN population, N=6,978. A patient was considered to have painful DPN if that patient had a minimum of one prescription redemption of an anti-epileptic medicine; N03AX09, N03AX12, N03AX16, N03AG01, N03F01, N03F02, or a serotonin–norepinephrine reuptake inhibitor (SNRI)/tricyclic antidepressant (TCA); N06AX16, N06AX21, N06AA02, N06AA04, N06AA09, N06AA10, N06AA21. Prescriptions had to be redeemed within 1 year prior to and a half year after a DPN diagnosis and patients had to have no registration of a relevant exclusion diagnosis in DNPR from 1994 onwards. Exclusion diagnoses were epilepsy (G40, G41) for those with anti-epileptic medicine prescription redemption and depression/anxiety (F30-F34, F40-42, F48.8+F48.9) for those with SNRI/TCA prescription redemption. We did not include NSAIDs and opioids in our algorithm since these drugs are prescribed for a wider and more unspecific range of diseases and conditions.
Non-painful DPN population
DPN-patients who did not fulfill the criteria for painful DPN were considered to have potential non-painful DPN, N=30,338. Of these, 1.826 patients fulfilled the criteria for non-painful DPN and at a later point of time fulfilled the criteria for painful DPN. Thus, they were included in both the non-painful DPN and the painful DPN population with two distinct DPN hospital contacts at two distinct time points.
Diabetic foot ulcer population
We identified all patients from the type 2 diabetes cohort who had at least one hospital diagnosis code or surgery code that was suggestive of diabetic foot ulcer. We used the following codes: “diabetes with peripheral vascular complication” E10.5-E14.5, “ulcer at lower extremity” L97, “chronic ulcer” L98.4, “gangrene” R02, “osteomyelitis” M86, “treatment of ulcer at lower extremity” KQDB, “operations for chronic ulcer/fistula at lower extremity” KQDG, “puncture, incisions and local destructions of pathological tissue in the skin at the lower extremity” KQDA, N=59,437.
The painful DPN validation cohort, the non-painful DPN validation cohort, and the diabetic foot ulcer validation cohort
Next, we restricted the painful DPN population, the non-painful DPN population and the diabetic foot ulcer population to those with a diagnosis in the Central Denmark Region between January 1, 2009 and July 10, 2016 (N=814, N=2,159, and N=5,204, respectively). Patients who had been seen at any department of neurology/neurophysiology, mixed internal medicine, endocrinology, dermatology, vascular surgery, plastic surgery, or orthopedic surgery, at one university hospital and four regional hospitals were randomly listed in each population (not taking into account age, gender, calendar year, specific diagnosis code, etc.) and the 60 first-listed individuals in each population constituted the painful DPN validation cohort, the non-painful DPN validation cohort, and the diabetic foot ulcer validation cohort, respectively.
Medical chart review
We attained permission to access medical record data on the 180 randomly selected patients from the Danish Health and Medicine Authorities. One physician (DHC) performed the medical record reviews. All cases with an uncertain diagnosis based on the available information were discussed with a specialist physician in diabetology (STK) and diagnoses were made according to consensus among the reviewing and specialist physician. We used a predefined checklist of symptoms, signs, and diagnostic test results described in the medical record as the gold standard (see Table 2 for details). We categorized patients from the painful DPN and non-painful DPN validation cohorts as “having DPN” if they fulfilled one of the following four criteria: 1) positive nerve conduction test supporting DPN; 2) ≥one symptom of polyneuropathy in feet (including neuropathic pain), eg numbness, prickling/tingling, shooting pain, stabbing pain; 3) ≥one sign of polyneuropathy, eg abnormal vibration, abnormal light touch, abnormal pinprick; or 4) physician notes documenting presence of polyneuropathy (eg noted in the medical record: “This T2D patient who has late complications including polyneuropathy, nephropathy…”). Patients, who did not fulfill one of these criteria, were categorized as “not having DPN”. Moreover, if a patient had another more likely and significant cause of polyneuropathy (eg cancer, chemotherapy treatment, sarcoidosis, hereditary, and inflammatory polyneuropathy) the patient was also classified as “not having DPN”. For alcohol overuse and vitamin B12 deficiency, severity and duration were often vaguely described,16 and the diagnosis given by the treating physician was most often DPN despite alcohol overuse/B12 deficiency description in the medical records. Thus, only if it was unequivocally stated in the medical record that polyneuropathy was caused by these conditions, the patient was categorized as “not having DPN”. For all patients, it was noted whether neuropathic pain was described in the medical record.
 | Table 2 Descriptions of symptoms and signs in both feet, and diagnostic test results used to verify DPN in the medical records |
We classified patients with explicitly noted “diabetic foot ulcer” in the medical record or with ≥one ulcer on toes/feet and no other pathogenesis to foot ulcer than diabetes (eg, trauma, gout) as “having diabetic foot ulcer”. All other patients in the diabetic foot ulcer validation cohort were categorized as “not having diabetic foot ulcer”.
Statistical analyses
Our study outcome was the PPV of the three algorithms defined as the proportion of painful DPN, non-painful DPN and diabetic foot ulcer patients identified by the algorithms, which could be classified as having the disease when validated against the medical records. We provide 95% CIs as the exact binomial CI. For the painful DPN algorithm, we calculated both the PPV for having DPN (painful or non-painful) and the PPV for having painful DPN. Likewise, for the non-painful DPN algorithm, we calculated a PPV for having DPN (painful or non-painful) and non-painful DPN.
We stratified the PPVs according to hospital type, department type, admission type, diagnosis type, and diagnosis/surgery code. Moreover, we investigated different combinations of the diagnosis codes, eg, we separately investigated the PPV of the ICD-10 G-codes and the E-codes.
Research ethics and informed consent
This study was approved by the Danish Data Protection Agency (record number KEA-2015-13 and KEA-2015-4). Permission to access information from medical records without individually informed patient consent was granted by the Danish Health and Medicine Authorities (record number 3-3013-1479/1 and 3-3013-1479/2) in accordance with Danish law. Since this study was non-experimental and used only existing registry data, additional ethical committee approval was not required.
Results
Descriptive data
We were able to retrieve medical record data for 53 of 60 (88%) patients in the painful DPN validation cohort, 54 of 60 (90%) patients in the non-painful DPN validation cohort, and 53 of 60 (88%) patients in the diabetic foot ulcer validation cohort. Table 3 shows characteristics of the included patients. In all three cohorts most patients were diagnosed in the hospital outpatient clinic setting (painful DPN: n=44 [83%], non-painful DPN: n=41 [76%], diabetic foot ulcer: n=37 [70%]) versus inpatient setting. For both DPN validation cohorts, most patients were diagnosed in the departments of neurophysiology (painful DPN: n=22 [42%], non-painful DPN: n=18 [33%]), neurology (painful DPN: n=9 [17%], non-painful DPN: n=15 [28%]), or internal medicine (painful DPN: n=13 [25%], non-painful DPN: n=12 [22%]). In the diabetic foot ulcer validation cohort, 49% (n=26) had diagnosis codes only, 34% (n=18) had surgery codes only, and 17% (n=9) had both. The most frequent surgery code was “treatment of ulcer at lower extremity” KQDB accounting for 86% (n=24) of all surgery codes (45% of patients in the diabetic foot ulcer validation cohort), whereas “diabetes with peripheral vascular complication” E10.5-E14.5 were the most used diagnosis codes accounting for 72% (n=26) of all diagnosis codes (49% of patients in the diabetic foot ulcer validation cohort). Most patients in the diabetic foot ulcer validation cohort were diagnosed in the departments of orthopaedic surgery (n=21 [40%]), vascular surgery (n=14 [26%]), or internal medicine (n=12 [23%]).
Positive predictive values
Of the 53 patients with potential painful DPN, 38 were classified as having DPN when validated against medical record data; of these, 19 had neuropathic pain, corresponding to a PPV of 72% (95% CI: 58–83) for hospital-diagnosed DPN and 36% (95% CI: 23–50) for painful DPN (Table 4). Among the 54 patients with potential non-painful DPN, 30 had DPN when validated against the medical records; of these, 27 had non-painful DPN, corresponding to PPVs of 56% (95% CI: 41–69) for hospital-diagnosed DPN and 50% (95% CI: 36–64) for non-painful DPN, respectively. E-chapter codes, especially when listed as a secondary diagnosis (
 | Table 4 Numbers and positive predictive values of potential DPN and diabetic foot ulcer |
Among the 53 patients with potential diabetic foot ulcer, only 18 patients had diabetic foot ulcer based on the medical record data corresponding to a PPV of 34% (95% CI: 22–48). The PPVs for E10.5-E14.5 (N=26) and KQDB (N = 24), that constituted the most frequent diagnosis and surgery codes in the diabetic foot ulcer validation cohort were 46% (95% CI: 27–67) and 29% (95% CI: 13–51) (Table S3). Around half of the E10.5-E14.5 codes represented peripheral ischemia rather than ulcer, and the remaining a mixture of conditions like Charcot foot, callosities, and clavus. The KQDB procedure code was often used for ulcers above malleoli level, ulcers in relation to gout, and debridement of callosities.
Discussion
The main finding of this study was that ICD-10 diagnosis codes for “diabetic polyneuropathy” G63.2, “polyneuropathy, unspecified” G62.9, and primary diagnosis codes for “diabetes with neurological complication” E10.4-E14.4 can be used to identify type 2 diabetes patients with hospital-diagnosed DPN in health care registers, whereas the secondary E-chapter codes often represented diseases like stroke or mononeuropathies. Patients with painful versus non-painful DPN could not be accurately distinguished based on prescription redemption of neuropathic pain treatment when validated against medical records. Finally, our algorithm for diabetic foot ulcer did not perform well for identification of diabetic foot ulcer patients.
Validated against medical record data, Hartsfield et al9 reported a PPV of 79% of an ICD-9 diagnosis code-based algorithm to identify patients with painful diabetic peripheral neuropathy (including other types of peripheral neuropathy, eg, mononeuropathies, autonomic peripheral neuropathy). In their initial algorithm, they found – like us – that prescription codes for neuropathic pain treatment did not perform well in identifying patients with painful diabetic neuropathy. There are several explanations for our low PPV for the presence of pain. First, even if a person has true neuropathic pain this may not necessarily be described in the medical record if the main reason for the hospital contact is unrelated to polyneuropathy, thus falsely underestimating the PPV for painful DPN. Second, we did not have data on possibly milder cases of treated depression/anxiety diagnosed by general practitioners. However, half of the painful DPN validation cohort patients with verified DPN and missing pain description in the medical record data were prescribed gabapentinoids. These drugs are primarily used for either hospital specialist diagnosed epilepsy (which we excluded) or neuropathic pain, suggesting that it was missing descriptions of true pain that led to falsely low PPVs.
Hoffman et al10 evaluated the validity of different polyneuropathy codes among a general population and reported a PPV for DPN of 91% for the ICD-9 code “polyneuropathy in diabetes” 357.2 (N=105), which is similar to our result for the ICD-10 code ”diabetic polyneuropathy” G63.2 (
Sohn et al11 evaluated one newly developed and four previously used diabetic foot ulcer algorithms against medical records. These algorithms varied in complexity. The algorithm most similar to ours – the Holzer algorithm defining diabetic foot ulcer by the use of at least one diagnosis or one procedure code – had a PPV of 72%, compared to our 34%. The remaining four algorithms had PPVs of 61–82%. Opposite to the algorithms validated by Sohn et al we included the frequently used “DM with peripheral vascular complication” E10.5-E14.5 codes, which also cover “Diabetes with foot ulcer” E10.5B-E14.5B. However, these codes turned out to have a low predictive value for diabetic foot ulcer, and as they had been given to half of the diabetic foot ulcer validation cohort, they diminished the overall PPV of our algorithm. A PPV of 82–89% has been reported for the ICD-9 code “ulcer of lower limbs, except decubitus” 707.1x,6,11 corresponding to the ICD-10 diagnosis code L97 in our algorithm (our PPV: 75%, N=4). The L97 code may be valid in Danish registers as well; however, this needs to be investigated in a larger study.
A number of limitations need to be considered when interpreting our results.
First, we used medical record data as the reference standard, which may falsely lower the PPV due to incomplete information as described above. On contrary, our criteria for verifying polyneuropathy were less stringent than those suggested by the Toronto Consensus Panels on DPN implying a risk of overestimation of the PPV.18 Also, determination of intraepidermal nerve fiber density for the diagnosis of small-fiber polyneuropathy is not part of the everyday clinical examination for polyneuropathy and thus was not included in our criteria used to verify DPN based on the medical record data. However, since neuropathic pain in feet was a DPN verifying criteria in our study, we were also able to verify the DPN diagnosis among patients with small-fiber polyneuropathy. Second, we evaluated only the PPV and no other measures of validity, eg, sensitivity, specificity, and negative predictive value. The importance of different validity measures depends on the study question. A high PPV is important when identifying patient cohorts for studies of the prognosis of a given disease. Moreover, the PPV is a good approximation for the specificity when disease-prevalence is low, and even with low sensitivity, a high specificity will lead to unmeasured relative risks,19 eg, in studies of DPN-risk factors. On contrary, low sensitivity may compromise studies of incidence and surveillance. Since we did not examine the sensitivity, cautious interpretation of DPN incidence and surveillance in studies based on the evaluated codes is necessary. Third, the study was conducted only in the Central Denmark region. However, the Danish health care system is uniform in its structure and practice; thus, our results are most likely generalizable to other parts of our country and countries with similar structure. Fourth, only a single reviewer evaluated most of the medical record data, and reviewers were not blinded to the registered discharge diagnosis codes, since a DPN- or diabetic foot ulcer-indicative diagnosis per definition had been given to all evaluated patients. Moreover, if discharge summaries or surgery descriptions were available (with the specific discharge diagnosis codes listed) they were included in the reviewed data. Finally, our validation sample sizes were small and a compromise between expected statistical power and practical feasibility, because we depended on health professionals at all involved departments to identify medical records for our study.
Conclusion
Our data suggest that G-chapter and primary E-chapter discharge diagnosis codes can detect patients with hospital-diagnosed DPN, and thus may be useful in epidemiological research. Our algorithm for diabetic foot ulcer did not perform well in identifying persons with diabetic foot ulcer, and a larger validation study to determine ways of identifying diabetic foot ulcers in Danish registers is warranted.
Acknowledgments
We sincerely thank the health professionals at all participating hospital departments for enabling access to the medical health records. Research reported in this publication is part of the International Diabetic Neuropathy Consortium (IDNC) research programme, which is supported by a Novo Nordisk Foundation Challenge Programme grant (Grant number NNF14OC0011633). The Department of Clinical Epidemiology, Aarhus University Hospital, receives funding for other studies from companies in the form of research grants to (and administered by) Aarhus University. None of these studies has any relation to the present study.
Disclosure
Dr Brian Christopher Callaghan receives research support from Impeto Medical Inc, outside the submitted work. Professor Nanna Brix Finnerup reports personal fees from Grunenthal, personal fees from Novartis Pharma, Teva Pharmaceuticals, Astellas, Mitshubishi Tanabe Pharma, and Merck, outside the submitted work. The other authors report no conflicts of interest pertaining to this work.
References
1. Pop-Busui R, Boulton AJ, Feldman EL, et al. Diabetic neuropathy: a position statement by the American diabetes association. Diabetes Care. 2017;40(1):136–154. doi:10.2337/dc16-2042
2. Sloan G, Shillo P, Selvarajah D, et al. A new look at painful diabetic neuropathy. Diabetes Res Clin Pract. 2018;144:177–191. doi:10.1016/j.diabres.2018.08.020
3. Callaghan BC, Little AA, Feldman EL, Hughes RA. Enhanced glucose control for preventing and treating diabetic neuropathy. Cochrane Database Syst Rev. 2012;6:CD007543.
4. Schmidt M, Pedersen L, Sorensen HT. The danish civil registration system as a tool in epidemiology. Eur J Epidemiol. 2014;29(8):541–549. doi:10.1007/s10654-014-9930-3
5. Buckley CM, Kearney PM, Ali F, et al. Concordance studies between hospital discharge data and medical records for the recording of lower extremity amputation and diabetes in the Republic of Ireland. BMC Res Notes. 2013;6:148. doi:10.1186/1756-0500-6-148
6. Newton KM, Wagner EH, Ramsey SD, et al. The use of automated data to identify complications and comorbidities of diabetes: a validation study. J Clin Epidemiol. 1999;52(3):199–207.
7. Vaccaro O, Lodato S, Mariniello P, De Feo E. Diabetes-related lower extremity amputations in the community: a study based on hospital discharge diagnoses. Nutr Metab Cardiovasc Dis. 2002;12(6):331–336.
8. Bruun C, Siersma V, Guassora AD, Holstein P, de Fine Olivarius N. Amputations and foot ulcers in patients newly diagnosed with type 2 diabetes mellitus and observed for 19 years. The role of age, gender and co-morbidity. Diabet Med. 2013;30(8):964–972. doi:10.1111/dme.12196
9. Hartsfield CL, Korner EJ, Ellis JL, Raebel MA, Merenich J, Brandenburg N. Painful diabetic peripheral neuropathy in a managed care setting: patient identification, prevalence estimates, and pharmacy utilization patterns. Popul Health Manag. 2008;11(6):317–328. doi:10.1089/pop.2008.0015
10. Hoffman EM, Staff NP, Robb JM, St Sauver JL, Dyck PJ, Klein CJ. Impairments and comorbidities of polyneuropathy revealed by population-based analyses. Neurology. 2015;84(16):1644–1651. doi:10.1212/WNL.0000000000001492
11. Sohn MW, Budiman-Mak E, Stuck RM, Siddiqui F, Lee TA. Diagnostic accuracy of existing methods for identifying diabetic foot ulcers from inpatient and outpatient datasets. J Foot Ankle Res. 2010;3:
12. Johannesdottir SA, Horvath-Puho E, Ehrenstein V, Schmidt M, Pedersen L, Sorensen HT. Existing data sources for clinical epidemiology: the danish national database of reimbursed prescriptions. Clin Epidemiol. 2012;4:303–313. doi:10.2147/CLEP.S37587
13. Schmidt M, Schmidt SA, Sandegaard JL, Ehrenstein V, Pedersen L, Sorensen HT. The danish national patient registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015;7:449–490. doi:10.2147/CLEP.S91125
14. Carstensen B, Kristensen JK, Marcussen MM, Borch-Johnsen K. The national diabetes register. Scand J Public Health. 2011;39(7 Suppl):58–61. doi:10.1177/1403494811404278
15. Carstensen B, Kristensen JK, Ottosen P, Borch-Johnsen K. Steering group of the national diabetes R. The danish national diabetes register: trends in incidence, prevalence and mortality. Diabetologia. 2008;51(12):2187–2196. doi:10.1007/s00125-008-1156-z
16. Hunt A, Harrington D, Robinson S. Vitamin B12 deficiency. BMJ. 2014;349:g5226. doi:10.1136/bmj.g5226
17. Callaghan B, Kerber K, Longoria R, Feldman E, Lisabeth L. Capturing cases of distal symmetric polyneuropathy in a community. Muscle Nerve. 2012;46(6):943–947. doi:10.1002/mus.23449
18. Tesfaye S, Boulton AJ, Dyck PJ, et al. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care. 2010;33(10):2285–2293. doi:10.2337/dc10-1303
19. Schneeweiss S, Avorn J. A review of uses of health care utilization databases for epidemiologic research on therapeutics. J Clin Epidemiol. 2005;58(4):323–337. doi:10.1016/j.jclinepi.2004.10.012
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


