Back to Journals » Drug Design, Development and Therapy » Volume 14
Calycosin Influences the Metabolism of Five Probe Drugs in Rats
Authors Wu M, Lin Y, Wei Y, Du H, Ying X, Tan W, Tang B
Received 26 October 2019
Accepted for publication 17 December 2019
Published 29 January 2020 Volume 2020:14 Pages 429—434
DOI https://doi.org/10.2147/DDDT.S236221
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Anastasios Lymperopoulos
Mei-ling Wu, Yi-ping Lin, Yan-li Wei, Hong-jian Du, Xiao-qian Ying, Wen-zhuang Tan, Bi-e Tang
Faculty of Medicine, Jinhua Polytechnic, Zhejiang, People’s Republic of China
Correspondence: Bi-e Tang
Faculty of Medicine, Jinhua Polytechnic, No. 1188 Wuzhou Street, Jinhua City, Zhejiang Provinc, People’s Republic of China
Email [email protected]
Background: Calycosin (CAL), a type of O-methylated isoflavone extracted from the herb Astralagusmembranaceus (AM), is a bioactive chemical with antioxidative, antiphlogistic and antineoplastic activities commonly used in traditional alternative Chinese medicine. AM has been shown to confer health benefits as an adjuvant in the treatment of a variety of diseases.
Aim: The main objective of this study was to determine whether CAL influences the cytochrome P450 (CYP450) system involved in drug metabolism.
Methods: Midazolam, tolbutamide, omeprazole, metoprolol and phenacetin were selected as probe drugs. Rats were randomly divided into three groups, specifically, 5% Carboxymethyl cellulose (CMC) for 8 days (Control), 5% CMC for 7 days + CAL for 1 day (single CAL) and CAL for 8 days (conc CAL), and metabolism of the five probe drugs evaluated using ultra-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS).
Results: No significant differences were observed for omeprazole and midazolam, compared to the control group. Tmax and t1/2 values of only one probe drug, phenacetin, in the conc CAL group were significantly different from those of the control group (Tmax h: 0.50± 0.00 vs 0.23± 0.15; control vs conc CAL). Cmax of tolbutamide was decreased about two-fold in the conc CAL treatment group (conc vs control: 219.48 vs 429.56, P< 0.001).
Conclusion: Calycosin inhibits the catalytic activities of CYP1A2, CYP2D6 and CYP2C9. Accordingly, we recommend caution, particularly when combining CAL as a modality therapy with drugs metabolized by CYP1A2, CYP2D6 and CYP2C9, to reduce the potential risks of drug accumulation or ineffective treatment.
Keywords: calycosin, herb-drug interactions, UPLC-MS/MS, cocktail, CYP450
Introduction
In the USA alone, 2.66 million patients are diagnosed with breast cancer, resulting in around 40,000 deaths every year.1 Encouragingly, from 1999 to 2007, the incidence of breast cancer has shown a decrease by 18%2 Another type of tumor, osteosarcoma, is a common malignancy in adolescents and young adults3 with a prevalence of 5.8–12.9%.4 Complex mechanisms underlie the clinical progression of breast cancer and osteosarcoma. To achieve more effective relief of disease burden, traditional Chinese medicine is often combined with conventional treatments.
Astragalus has been a component of traditional Chinese medicine (TCM) for centuries and its integration with western medicine for improving healthcare options is a considerable focus of research interest.5 In China, Astragalus is mainly cultivated in Nemeng and Shanxi regions. Triterpenoids are the most abundant compounds in Astragalus. Isoflavones, including genistein, daidzein, and calycosin (CAL), are a class of secondary plant metabolites that have been characterized as active medical ingredients.6 CAL has additionally been isolated from the perennial red cover plant.
CAL displays anti-oxidative, anti-inflammation and anti-tumor activities and is reported to inhibit breast cancer progression through regulation of estrogen receptor signaling.7 EWSAT1-mediated expression regulates the TRAF6 pathway and CAL inhibits nasopharyngeal carcinoma by modulating EWSAT1 activity.8 Moreover, CAL exhibits therapeutic activity against osteosarcoma through suppressing the neoplastic miR-223-IκBα pathway.9 Another newly discovered role of CAL is hepatic anti-fibrosis.10
While CAL may be effectively combined with other clinical treatments, the mechanisms underlying its metabolism in vivo are yet to be established. The cytochrome P450 (CYP450) enzyme family and drugs exert a two-way effect in that: (1) CYP450 participates in drug metabolism, and (2) the drug itself or its metabolite could inhibit or induce enzyme activity.11
The utility of the cocktail approach for researching drug–drug interactions in the human body has been documented. An earlier study12 showed that continuous administration of 600 mg/kg Digeda-4 decoction induced inhibition of four CYP450 subtypes (CYP1A2, CYP2C9, CYP2C19, and CYP3A4). More recently, Corydalis decumbens, an auxiliary herbal medicine for hypertension and rheumatoid arthritis, was shown to induce CYP2C19 while inhibiting CYP1A2 and CYP3A4.13 Based on experimental evidence, a probe cocktail solution was selected in the current study for determining the effects of CAL on the established CYP450 system and consequent metabolism of the drugs affected by these enzymes.
Materials and Methods
CAL was obtained from Chengdu Lingher Biotechnology Co. Ltd. Midazolam and Omeprazole were obtained from Jinhua medical college. Tolbutamide, Metoprolol and Phenacetin were obtained from Canspec, China. Carbamazepine was provided by Sigma, Shanghai. Acetonitrile (ACN), Methanol and Isopropanol were provided by Merck, Germany.
Animal Experiments
Rats were randomly divided into three treatment groups: (A) 5% CMC for 8 days (control), (B) 5% CMC for 7 days + CAL for 1-day (single CAL), and (C) CAL for 8 days (conc CAL). All three groups were intragastrically treated for 7 days and force-fed the probe mixture on day 8. 30 min after probe cocktail administration, 25 mg/kg CAL was administered to groups B and C. The time-points for obtaining blood were 0, 0.17, 0.33, 0.75, 1, 1.5, 2, 3, 4, 6, 8, 10, 12, and 24 hrs. Serum samples were centrifuged at a speed of 13,000 rpm for 5 min and stored at −80°C until use.
Ethic
All experimental procedures and protocols were reviewed and approved by the Animal Care and Use Committee of Jinhua Polytechnic and were in accordance with the Guide for the Care and Use of Laboratory Animals.
Sample Processing
Agilent UHPLC unit (Agilent Corporation, USA) with a ZORBAX Eclipse Plus C18 column (1.8 m, 2.1 × 50 mm, USA) was a combination for Chromatographic separation.
Mobile phase was consists of 0.1% formic acid in water (A) and ACN (B). Elution was in a linear gradient, with A and B as follows: 0–0.3 min (30-30% B), 0.3–1.3 min (30–50% B), 1.3–1.8 min (50–95% B), and 1.8–2.8 min (95% B). The flow rate was 0.40 mL/min. The quantitative analysis of target ions was performed with m/z 180.1→109.9 for phenacetin (collision energy: 24 V), m/z 346.12→135.9 for omeprazole (collision energy: 44 V), m/z 268.19→115.9 for metoprolol (collision energy: 17 V), m/z 271.11→91.0 for tolbutamide (collision energy: 36 V), m/z 326.09→ 290.8 for midazolam (collision energy: 44 V), and m/z 237.1→194.0 for the IS (collision energy: 18 V).
Following the thawing of serum at room temperature, samples were treated with 100 μL serum, 200 μL ACN and IS, and centrifuged at a speed of 13,000 rpm for 10 min. 100-µL supernatant was mixed with an equivalent volume of ultra-distilled water and a 5-µL volume of the mixture used for injection into the UPLC-MS/MS system.
Statistical Analysis
Results are presented as means ± SD (n=5–8). All data were processed with SPSS 18.0 statistical software. P values were calculated using the t-test and differences considered significant at P<0.05 (*P<0.05; **P<0.01, ***P<0.005).
Results and Discussion
CAL is a type of O-methylated isoflavone purified from AstragalusmembranaceusBge.14 Genistein, an isoflavone derived from soy products similar to CAL, and its analogs have been shown to non-competitively inhibit CYP450 2C9.15,16
Several hospital admissions related to adverse drug reactions in patients were reported by Pirmohamed and co-workers (2004).17 In 2004, a study was performed on 18,820 patients, of whom 28 subsequently died because of adverse drug effects,17 such as gastrointestinal bleeding, perforated duodenal ulcer, intracranial hemorrhage, renal failure and lithium toxicity. Another recent review provided evidence of detrimental effects of Chinese medicinal herbs.18 The top three documented adverse effects included digestive and nervous system disorders and mental health or behavior problems. A comprehensive literature search revealed four blood abnormality and two liver function abnormality cases.18 These earlier findings highlight the significance of establishing the potential effects of CAL on liver microsomes.
CAL exerted differential effects on the five probes examined, as shown in Figure 1. AUC values of midazolam, omeprazole and tolbutamide were decreased in the single CAL and conc CAL groups while pharmacokinetic alterations of metoprolol displayed an opposite trend, whereby the AUC value of metoprolol was elevated relative to the single CAL and conc CAL groups.
For accurate evaluation of the differences on pharmacokinetic parameters between the control and test groups (conc CAL and sig CAL), SPSS was utilized for statistical analysis, as presented in Table 1. Compared to the control group, omeprazole and midazolam showed no significant any pharmacokinetic differences in the CAL-treated groups. Only one of the probe drugs, phenacetin, in the conc CAL Group, displayed significant differences in Tmax and t1/2 (Tmax h: 0.50±0.00 vs 0.23±0.15; control vs conc CAL). The Cmax value of tolbutamide was decreased about two-fold (conc vs control: 219.48 vs 429.56, P<0.001), indicating that CAL influences the metabolism of this drug.
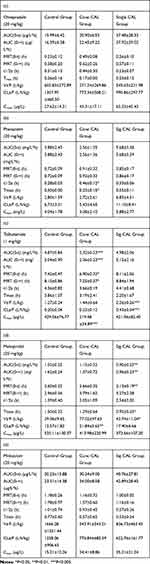 |
Table 1 Main Pharmacokinetic Parameters in Rats |
 |
Table 2 Isoflavones Exert Significant Inhibitory Effects on CYP450 Activity (Kopecna-Zapletalova et al) |
Several studies to date have confirmed the effects of isoflavones, such as genistein, biochanin A, equol and daidzein, on CYP450 enzyme activity. A summary of earlier literature is presented in Table 2.16 Among the five isoflavones studied, genistein showed the most widespread which was influenced CYP3A4, CYP2C9, CYP2E1, CYP2C8 and CYP2C19. Phenacetin displayed marked differences in t1/2 and Tmax (conc CAL vs control) while tolbutamide showed significantly decreased AUC, MRT and Cmax (conc CAL vs control). Metoprolol in the conc CAL group showed a significant difference in CL relative to the control group while decreased Tmax, AUC and MRT of metoprolol were observed in the single CAL group.
Midazolam, tolbutamide, omeprazole, metoprolol and phenacetin are, respectively, metabolized by CYP3A4, CYP2C9, CYP2C19, CYP2D6, and CYP1A2 in humans, which are homologous to rat CYP3A1/2, CYP2C6/2C11, CYP2D1/2, CYP2D2, and CYP1A2/2C11.13,19 CAL inhibited the activity of CYP1A2/2C11 (as observed from its effects on phenacetin) while promoting those of CYP2D2 (specific for metoprolol) and CYP2C6/2C11 (specific for tolbutamide) in rat, as shown in Figure 1 and Table 1. Genistein and Daidzein were both noncompetitively inhibited the ability of CYP2C9 in human liver microsomal cytochromes P450. The specific reasons and competition mechanism for CAL’s inhibition of CYP2C9 need further study.
Conclusion
The cocktail method involves five probe drugs (midazolam, tolbutamide, omeprazole, metoprolol and phenacetin) and can be effectively used to evaluate enzyme kinetics in vivo. Enzymes involved in the drug metabolism of these five probes in humans could be put into a one-to-one correspondence activity for rats. Results on the activities of homologous enzymes in rat involved in the metabolism of these drugs may be extrapolated to humans, supporting the potential significance of this method in clinical research. Our data indicate that CAL inhibits CYP1A2 activity while inducing that of CYP2D6 and CYP2C9. In cases where drugs are metabolized by CYP1A2, CYP2D6 or CYP2C9, combination therapy with CAL may therefore be less effective or more toxic, highlighting the requirement for further research.
Acknowledgment
This work was financially supported by grants from Jinhua City public welfare projects (No.2017-4-049).
Disclosure
The authors have no conflicts of interest to declare.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. doi:10.3322/caac.21442
2. Kohler BA, Ward E, McCarthy BJ, et al. Annual report to the nation on the status of cancer, 1975–2007, featuring tumors of the brain and other nervous system. J Natl Cancer Inst. 2011;103(9):714–736. doi:10.1093/jnci/djr077
3. Stiller CA, Bielack SS, Jundt G, Steliarova-Foucher E. Bone tumours in European children and adolescents, 1978–1997. Report from the automated childhood cancer information system project. Eur J Cancer. 2006;42(13):2124–2135. doi:10.1016/j.ejca.2006.05.015
4. Li C, Balluz LS, Okoro CA, et al. Surveillance of certain health behaviors and conditions among states and selected local areas — behavioral risk factor surveillance system, United States, 2009. MMWR Surveill Summ. 2011;60(9):1–250.
5. Yin M, Yang M, Chu S, et al. Quality analysis of different specification grades of Astragalus membranaceus var. mongholicus (Huangqi) from Hunyuan, Shanxi. J AOAC Int. 2019;102(3):734–740. doi:10.5740/jaoacint.18-0308
6. Krizova L, Dadakova K, Kasparovska J, Kasparovsky T. Isoflavones. Molecules. 2019;24(6):1076. doi:10.3390/molecules24061076
7. Chen J, Zhao X, Li X, Wu Y. Calycosin induces apoptosis by the regulation of ERbeta/miR-17 signaling pathway in human colorectal cancer cells. Food Funct. 2015;6(9):3091–3097. doi:10.1039/C5FO00374A
8. Kong L, Li X, Wang H, He G, Tang A. Calycosin inhibits nasopharyngeal carcinoma cells by influencing EWSAT1 expression to regulate the TRAF6-related pathways. Biomed Pharmacother. 2011;60:342–348. doi:10.1016/j.biopha.2018.06.143
9. Qiu R, Ma G, Li X, et al. Clinical case report of patients with osteosarcoma and anticancer benefit of calycosin against human osteosarcoma cells. J Cell Biochem. 2019;120(6):10697–10706. doi:10.1002/jcb.v120.6
10. Guo T, Liu ZL, Zhao Q, Zhao ZM, Liu CH. A combination of astragaloside I, levistilide A and calycosin exerts anti-liver fibrosis effects in vitro and in vivo. Acta Pharmacol Sin. 2018;39(9):1483–1492. doi:10.1038/aps.2017.175
11. Dahlinger D, Duechting S, Nuecken D, Sydow K, Fuhr U, Frechen S. Development and validation of an in vitro, seven-in-one human cytochrome P450 assay for evaluation of both direct and time-dependent inhibition. J Pharmacol Toxicol Methods. 2016;77:66–75. doi:10.1016/j.vascn.2015.10.003
12. Zhang D, Wu GD, Zhang YD, Xu JP, Zhen HT, Li MH. Effects of digeda-4 decoction on the CYP450 activities in rats using a cocktail method by HPLC. Biomed Res Int. 2018;2018:1415082. doi:10.1155/2018/1415082
13. Cheng C, Qian J, Wang Z, et al. Influences of corydalis decumbens on the activities of CYP450 enzymes in rats with a cocktail approach. Biomed Res Int. 2019;2019:9614781. doi:10.1155/2019/9614781
14. Ma X, Zhang T, Wei Y, Tu P, Chen Y, Ito Y. Preparative isolation and purification of calycosin from Astragalus membranaceus Bge. var. mongholicus (Bge.) Hsiao by high-speed counter-current chromatography. J Chromatogr A. 2002;962(1–2):243–247. doi:10.1016/S0021-9673(02)00535-6
15. Ronis MJ. Effects of soy containing diet and isoflavones on cytochrome P450 enzyme expression and activity. Drug Metab Rev. 2016;48(3):331–341. doi:10.1080/03602532.2016.1206562
16. Kopecna-Zapletalova M, Krasulova K, Anzenbacher P, Hodek P, Anzenbacherova E. Interaction of isoflavonoids with human liver microsomal cytochromes P450: inhibition of CYP enzyme activities. Xenobiotica. 2017;47(4):324–331.
17. Pirmohamed M, James S, Meakin S, et al. Adverse drug reactions as cause of admission to hospital: prospective analysis of 18 820 patients. BMJ. 2004;329(7456):15–19. doi:10.1136/bmj.329.7456.15
18. Lee JY, Jun SA, Hong SS, Ahn YC, Lee DS, Son CG. Systematic review of adverse effects from herbal drugs reported in randomized controlled trials. Phytother Res. 2016;30(9):1412–1419. doi:10.1002/ptr.v30.9
19. Videau O, Pitarque S, Troncale S, et al. Can a cocktail designed for phenotyping pharmacokinetics and metabolism enzymes in human be used efficiently in rat? Xenobiotica. 2012;42(4):349–354. doi:10.3109/00498254.2011.625453
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

