Back to Journals » Clinical Ophthalmology » Volume 16
Calprotectin as a Biomarker for Diagnosis and Severity of Acute Noninfectious Anterior Uveitis in Egyptian Patients
Authors Abd El Meged Nage S , Esmail A
Received 13 September 2022
Accepted for publication 18 November 2022
Published 13 December 2022 Volume 2022:16 Pages 4109—4120
DOI https://doi.org/10.2147/OPTH.S389780
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Sara Abd El Meged Nage, Ahmed Esmail
Ophthalmology Department, Faculty of Medicine, Menoufia University, Menoufia, Egypt
Correspondence: Sara Abd El Meged Nage, Email [email protected]
Purpose: To study the relation between serum calprotectin level and acute noninfectious anterior uveitis in Egyptian patients.
Methods: An observational prospective study carried out at Menoufia University Hospital during the period from March 2021 till June 2022, after informed consent from all studied patients. This study included 20 eyes of patients with Acute Anterior Uveitis (AAU) and 20 eyes healthy individuals matched sex and age as the control group. Full history taking, ophthalmological examination and serum calprotectin levels were performed for both patients and controls.
Results: Serum calprotectin levels were substantially higher in patients’ eyes with acute anterior uveitis than in healthy eyes (61.45± 7.89 vs 32.50± 11.64; 95% CI: 22.58– 35.32; P < 0.001). ROC curve analysis showed that the cut-off point of serum calprotectin in severity detection of AAU was ≥ 58.0, with sensitivity of 95%, specificity of 43% at AUC of 0.986, with reached to significant level (p < 0.001).
Conclusion: Serum calprotectin levels were significantly elevated with positive previous uveitis and marked grade indicating a possible role of calprotectin in the pathogenesis of non-infectious AAU. The serum calprotectin cut-off points for severity detection of AAU were 58.0, with sensitivity of 95% and specificity of 43%. Finally, we identified serum calprotectin as a potential biomarker for detection of anterior uveitis severity and patients’ morbidity risk. Further investigation with large sample size is needed to assess the relationship between calprotectin and uveitis activity.
Keywords: anterior uveitis, biomarkers, serum calprotectin, Egyptian patients, uveitis grading scales
Introduction
Uveitis is regarded as a serious condition that accounts for between 5% and 20% of cases of legal blindness and up to 10% of the visual impairments linked to occupational disabilities.1 The most common kind of uveitis in our environment, NIU has been linked to high expenses for both diagnosis and treatment.2
A variety of illnesses fall under the umbrella term “uveitis.” Although most diseases presumably share the same underlying mechanisms that cause ocular inflammation, different treatment responses show that there must also be disease-specific mediators.3 It is challenging to employ aqueous humour as a daily strategy for assessing disease activity and treatment response in ordinary clinical practice. Unfortunately, there is a lack of serum marker for detecting the degree of inflammation in uveitis.3
To our knowledge, no multicenter investigation has been carried out in Egypt to ascertain the clinical pattern of uveitis. Uveitis has just recently been recognized in Egypt as an independent ophthalmic subspeciality.4
The proteins S100A8 (myeloid-related protein 8) and S100A9 (myeloid-related protein 14) combine to make calprotectin, which is primarily produced by activated granulocytes and monocytes in the blood and inflamed tissues.5 As it binds toll-like receptor 4 (TLR4)-like damage-associated molecular patterns (DAMPs), it is regarded as an endogenous activator of innate immune response, boosting the release of proinflammatory cytokines.6 Different inflammatory disorders, such as bacterial infections, certain rheumatic diseases, malignancies, and cystic fibrosis, result in elevated serum calprotectin levels. This protein, which shows innate immune activity, is secreted during the infiltration process of inflamed tissues.7
Patients with spondyloarthritis (SpA) treated with TNF-blockers and patients with rheumatoid arthritis (RA) treated with both conventional and biologic disease-modifying anti-rheumatic drugs may benefit from using circulating calprotectin as a good indicator of clinical response because these treatments cause a rapid drop in the levels of this molecule.8,9 In addition to correlating with inflammatory indicators and the generation of autoantibodies, joint and vascular damage in RA, it has also been linked to the advancement of spinal structural deterioration in axial SpA.10
Since patients with endogenous posterior uveitis had much higher levels of the protein calprotectin than those with resolving or inactive uveitis or healthy controls, it has been suggested that this protein may be a sensitive predictor of disease activity in these patients.11,12 Calprotectin levels were found to be considerably greater in circulating blood than in non-uveitic controls in recent investigations evaluating the relationship between calprotectin and uveitis activity. Additionally, there is a strong association between macular thickness, anterior uveitis activity, and calprotectin levels.12 So, the aim of work is to study the relation between serum calprotectin level in blood and anterior uveitis in Egyptian patients.
Patients and Methods
Study Design and Patient Enrolment
An observational prospective study carried out at Menoufia University Hospital during the period from March 2021 till June 2022, after informed consent from all studied patients. This study included 20 eyes with Acute Noninfectious Anterior Uveitis (AAU) and 20 healthy individuals’ eye matched sex and age as the control group.
Ethical Consideration
After a brief and concise description of the study’s goals, either the patient or the patient’s legal guardian provided a written informed consent. The consent form was created in compliance with the Helsinki Declaration and Egyptian Ministry of Health Quality and Improvement System standards. The Menoufia Faculty of Medicine’s Local Ethical Scientific Committee granted approval for the study plan.
Patients’ Criteria
All patients were selected according to inclusion and exclusion criteria as follows:
Inclusion criteria: Both sex patients with acute noninfectious anterior uveitis (granulomatous and non-granulomatous) and non-uveitis controls.
Exclusion criteria: diabetic people, patients who had previous eye surgery, concurrent infectious disorders (syphilis, tuberculosis, viral infection which was diagnosed by laboratory investigation and consultation), systemic inflammatory conditions unrelated to uveitis (hyperthyroidism), or recent ocular trauma and uveitis associated with Behcet syndrome and sarcoidosis.
Measurements Examination
Full history taking, ophthalmological examination and serum calprotectin levels were performed for both patients and controls as follows:
Full history taking included age, sex, family history, medical history, previous uveitis, and uveitis diagnosis are all factors.
The best-corrected visual acuity (BCVA) by Snellen VA chart and fundus examination were both parts of the ophthalmological examination.
Grading of anterior uveitis: According to the suggestions of the Standardization of Uveitis Nomenclature (SUN) Working Group, uveitis was identified and categorized.13,14 All our patients had active anterior uveitis.
Serum calprotectin levels (CALP): According to the manufacturer’s instructions, the manufacturer’s commercial ELISA kits (SUNLONG BIOTECH CO., LTD, Gongshu District, Hangzhou city, Zhejiang province, Hangzhou, Zhejiang, China) were used to measure the serum calprotectin levels.
Principal of calprotectin ELISA kits: Sandwich-ELISA is the technique used in this ELISA kit. A CALP-specific antibody has been pre-coated on the Microelisa stripplate included in this kit. The appropriate Microelisa stripplate wells are filled with standards or samples, which are then mixed with the designated antibody. Then, each Microelisa stripplate well is filled with a Horseradish Peroxidase (HRP)-conjugated antibody that is specific for CALP, and the plate is incubated. Free parts are removed during washing. To each well, the TMB substrate solution is applied. Only the wells containing CALP and the HRP-conjugated CALP antibody will initially appear blue before changing to yellow after the stop solution is added. At a wavelength of 450 nm, the optical density (OD) is measured spectrophotometrically. The relationship between the OD value and CALP concentration is linear.
Blood Sampling
Blood samples were taken from uveitis patients either on the day of the ophthalmologic examination or in the days that followed, but no later than seven days after the flare-up started. Venipuncture was used to obtain blood samples for tri-potassium EDTA tubes. Plasma was extracted from centrifuged blood and kept at −20°C. Using a Fluorescent Enzyme Sandwich Immunoassay, the amount of plasma calprotectin was measured. In this assay, the calprotectin from patient samples binds to the monoclonal antibody coating the wells, and then, after washing away the unbound components, antibodies against human calprotectin are added to form a complex with them.
To obtain a final fluorescent signal proportionate to the amount of calprotectin complex present, non-bound conjugate is washed away, and the complex is then incubated with a development solution. The higher the response value, the more calprotectin is present in the specimen. The answers are contrasted with the calibrator calibration curve established for concentration calculations.15
Outcome of the Study
Level of serum calprotectin, best-corrected visual acuity (BCVA), Slit-lamp biomicroscopy, grading, were outcomes of the current study.
Statistical Analysis
SPSS V.25 and Microsoft Excel 2019 (Microsoft Corporation, One Microsoft Way Redmond, WA 98052-6399 USA) were used to tabulate and statistically analyze the results (IBM Corporation, 1 Orchard Rd, Armonk, NY 10504, USA), Mean (x), median, and standard deviation (SD) were among the descriptive statistics, while the Chi-square test (X2) to study association between two qualitative variables, Standard Student’s t-test (t) used for comparison of quantitative variables between cases and control groups for normal distributed data, Mann–Whitney test (U-test) used for comparison of quantitative variables between cases and control groups for not normal distributed data, Kruskal Wallis test (K) used for comparison of quantitative variables between more than two groups of not normal distributed data, and ROC curve analysis used to evaluate the performance of classification schemes in which there is one variable of two categories by which subjects are classified. They were constructed by calculating the sensitivities and specificities of the variable. Statistical significance was defined as a P value 0.05 or lower.
Results
A CONSORT flow chart of the study population is shown in Figure 1. Of the 49 eyes of patients with Acute Anterior Uveitis (AAU) and healthy individuals’ eyes matched sex and age included in this study, they attended to Menoufia University Hospital, 9 eyes excluded from our study, 7 of them did not meet the inclusion criteria and 2 declined consent), 40 eyes of patients were willing to participate and consented for participation in the study. Thus, 40 eyes were analysed (20 eyes with AAU and 20 healthy eyes), 6 eyes of patients had Faint grade (+1), 11 eyes had Moderate grade (+2) and 3 eyes had Marked grade (+3). There were no significant differences among the studied groups regarding age and gender (P > 0.05) (Table 1).
 |
Table 1 Socio-Demographic Data Among Eyes of Patients with AAU and Healthy Eyes |
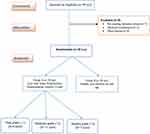 |
Figure 1 Flowchart of the studied patients and healthy groups. |
Results in Table 2 show that, serum calprotectin level significantly increased among eyes of patients with Acute Anterior Uveitis (61.45±7.89) than healthy eyes (32.50±11.64) with (95% CI: 22.58–35.32, P < 0.001) (Table 2, Figure 2). In our study, most eyes of patients with AAU had moderate grade (55%), followed by faint grade (30%), and marked grade (15%), as shown in Figure 3.
 |
Table 2 Serum Calprotectin Levels Among Eyes of Patients with AAU and Healthy Eyes |
 |
Figure 2 Serum calprotectin levels among eyes of patients with AAU and healthy eyes. |
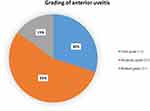 |
Figure 3 Grading of anterior uveitis among eyes of patients with AAU. |
Also, age, gender, uveitis diagnosis, and visual acuity did not all significantly correlate with the severity of anterior uveitis (p > 0.05). However, a significant association was found between previous uveitis (not recurrent or chronic) and severity of anterior uveitis (p = 0.001). Most eyes with moderate grade had positive past uveitis (90.91%), followed by eyes with marked grade by 66.67%, while all the eyes with faint grade had no prior uveitis as shown in Table 3 and Figure 4.
 |
Table 3 Grading of Anterior Uveitis in Relation to Age, Gender, Previous Uveitis, Uveitis Diagnosis and Visual Acuity |
 |
Figure 4 Grading of anterior uveitis in relation to previous uveitis. |
Data represented in Table 4 reveals that, both previous uveitis, and anterior uveitis grades were significantly related with calprotectin serum levels with (p < 0.05). Serum calprotectin significantly higher with positive previous uveitis (64.75± 4.65) and marked grade (67.00± 2.65) as compared negative previous uveitis and faint and moderate anterior uveitis (Figures 5 and Figure 6). While, gender, uveitis diagnosis and visual acuity did not show any relation with serum calprotectin level (P > 0.05) (Table 4).
 |
Table 4 Serum Calprotectin Levels in Relation to Gender, Previous Uveitis, Uveitis Diagnosis, Grading of Anterior Uveitis and Visual Acuity |
 |
Figure 5 Serum calprotectin levels in relation to previous uveitis. |
 |
Figure 6 Serum calprotectin levels in relation to grading of anterior uveitis. |
Regarding the detection of AAU severity, ROC curve analysis showed that the cut-off point of serum calprotectin in severity detection of AAU was ≥58.0, with sensitivity of 95%, specificity of 43% at AUC of 0.986, with reached to significant level (p < 0.001) (Table 5, Figure 7).
 |
Table 5 Cut Off Point of Serum Calprotectin in Detection of AAU Severity |
 |
Figure 7 ROC curve of serum calprotectin in detection of anterior uveitis severity. |
Discussion
Worldwide, uveitis and its aftereffects continue to be a leading cause of blindness. Many times, there is no known cause.16 Although uveitis frequently occurs alone, it is not a single disease. Uveitis can be a part of numerous distinct disease processes, much like arthritis can.17 Uveitis develops as a failure of the ocular immune system, and the condition is characterized by inflammation and tissue damage.18 Importantly, leukocyte migration, activation, and retention in inflamed ocular tissue are mediated by chemokines and their receptors, which may represent potential therapeutic targets.19 Calprotectin is a protein that has been extensively researched in inflammatory disease.20 So, the aim of this work is to investigate the correlation between serum calprotectin level and anterior uveitis in Egyptian patients.
In our study, compared to healthy eyes, patients with acute noninfectious anterior uveitis (granulomatous and non-granulomatous) had considerably higher serum calprotectin levels. Calprotectin has previously been investigated in systemic illness of various clinical phenotypes with overall presentation of uveitis. When compared to patients with idiopathic anterior uveitis and healthy individuals, patients with juvenile idiopathic arthritis-associated uveitis had elevated of serum calprotectin levels, and systemic conditions appeared to interfere with the calprotectin. Additionally, Behcet's disease (BD) patients were found to have elevated serum calprotectin levels, and faecal calprotectin may be useful in assessing the intestinal involvement of BD.21 Calprotectin expression in uveitis should be examined as a single entity to ascertain its precise function in the condition. Olson et al22 reported early in 1996 that elevated calprotectin level in endogenous posterior uveitis, but only enrolled 27 patients with no relation between calprotectin and clinical parameters due to the small sample size of patients. In the same line, Pascual et al23 found age and gender did not substantially differ across the studied groups. Also, serum calprotectin levels were significantly higher in patients’ groups than those of the control groups.
Another study by Gazim, et al24 concluded that, the clinician may be able to categorise the causes of anterior uveitis with the aid of faecal calprotectin levels. Spondylarthritis (SpA) associated uveitis is difficult to diagnose in some cases because some rheumatological findings are absent or insufficient.25 SpA may also present itself initially as uveitis. Finding high faecal calprotectin levels could be another piece of information that contributes to this classification. To fully comprehend the significance of faecal calprotectin levels for detection of SpA among individuals presenting with anterior uveitis, more research is required. Even though none of our patients had Behcet’s illness, it is important to note that this condition is similarly linked to intestinal inflammation and high amounts of faecal calprotectin.26 The relevance of calprotectin levels in differential detection of severity for these two diseases may be clarified by further research comparing the calprotectin levels in uveitis patients with SpA and Behcet's disease.
In the present study, serum calprotectin levels were significantly elevated with positive previous uveitis (not recurrent or chronic) and marked grade indicating a possible role of calprotectin in the pathogenesis of IAAU. These findings support Song et al3 who observed that uveitis activity severity grades of idiopathic acute anterior uveitis patients were positively correlated with serum calprotectin levels. However, Pascual et al23 could not find any relation between plasma calprotectin levels and anterior uveitis activity grading as well as calprotectin levels did not show any significant difference when uveitis was cleared. In fact, there is a great deal variation in grades activity assessment could be one explanation for the lack of association with anterior chamber cellularity, which is reflected in the study of Kempen et al in which interobserver agreement in grading intraocular inflammation is found to be moderate.27 In contrast, uveitis grading revealed a favourable correlation with higher levels of calprotectin and ocular inflammation reported by Szepessy et al.28 It was noted that the degree of inflammation in acute anterior uveitis was linked with macular alterations assessed by OCT. In uveitic eyes evaluated by OCT, positive association recorded between level of inflammation and central foveal thickness; the latter can, to some extent, represent uveitis activity.29 Although, macular thickness measuring by OCT also required specific technique, serum detection of calprotectin was more accurate and objective than uveitis grading using anterior chamber cells and anterior chamber flare. All of these contribute to the understanding of serum calprotectin as a novel, practical, quantitative biomarker for uveitis activity evaluation.
Our results showed that, regarding the detection of AAU severity, ROC curve analysis showed that the cut-off point of serum calprotectin for detection of AAU severity was ≥58.0, with sensitivity of 95%, and specificity of 43% at AUC of 0.986, with reached to significant level. According to the results of ROC analysis by Song et al,3 serum calprotectin is more effective at differentiating IAAU compared to healthy controls. The area under curve for serum calprotectin for screening IAAU was 0.935 at the optimal cut-off value of 13.3 ng/mL, with sensitivity and specificity of 88.9% and 87.5%, and facilitating future application of serum calprotectin in clinical practise.
Furthermore, Pato et al7 reported that, an activity index for patients with uveitis (UVEDAI) comprising the pertinent areas was developed relatively recently and needs further validation in addition to the currently available scoring methods for uveitis grading. The uveitis community will need to assess these sensitive and discriminatory tools for objective quantification of anterior chamber inflammation against the current subjective clinical estimates as new forms of quantitative imaging in uveitis are proposed and come to a new understanding on how disease activity in uveitis should be measured.6
In this respect Song et al3 showed that, the data from various organisations and research might be readily compared if there were a uniform set of standards for rating the four features of intraocular inflammation. However, several variables, including semiquantitative parameters like beam height and width, illumination angle, light intensity, and magnification, are likely to have an impact on the accuracy and precision of such scoring schemes. There were differences in opinions among uveitis doctors while determining whether to regularly count the number of cells and utilise laser flare photometry or not.6
Conclusion
Most of the studied patients with AAU had moderate grade. Patients with acute anterior uveitis had higher serum calprotectin levels. Serum calprotectin levels were significantly elevated with positive previous uveitis and marked grade indicating a possible role of calprotectin in the pathogenesis of non-infectious AAU. Serum calprotectin levels were significantly elevated with positive previous uveitis and marked grade indicating a possible role of calprotectin in the pathogenesis of non-infectious AAU. The serum calprotectin cut-off points for severity detection of AAU were 58.0, with sensitivity of 95% and specificity of 43%. Finally, we identified serum calprotectin as a potential biomarker for detection of anterior uveitis severity and patients’ morbidity risk. Further investigation with large sample size is needed to assess the relationship between calprotectin and uveitis activity.
Abbreviations
AAU, Acute Anterior Uveitis; NIU, Noninfectious uveitis; TLR4, Toll-like receptor 4; DAMPs, damage-associated molecular patterns; SpA, spondyloarthritis; BCVA, best-corrected visual acuity; SUN, Standardization of Uveitis Nomenclature; BD, Behcet's disease; HRP, Horseradish Peroxidase; OCT, Optical coherence tomography.
Data Sharing Statement
All data and materials included in this work are available.
Ethics Approval and Consent to Participate
The ethical board committee approval of our institution (Menoufia Faculty of Medicine) was obtained.
Consent for Publication
All patients involved in this study were informed about the aim of the study and signed the informed consent.
Human and Animal Rights
No animals were used for studies that are the basis of this research. All the humans were used in accordance with the Helsinki Declaration of 1975.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare no competing interests in this work.
References
1. de Smet MD, Taylor SRJ, Bodaghi B, et al. Understanding uveitis: the impact of research on visual outcomes. Prog Retin Eye Res. 2011;30(6):452–470. doi:10.1016/j.preteyeres.2011.06.005
2. Adán-Civera AM, Benítez-del-Castillo JM, Blanco-Alonso R, Pato-Cour E, Sellas-Fernández A, Bañares-Cañizares A. Burden and direct costs of non-infectious uveitis in Spain. Reumatol Clínica. 2016;12(4):196–200. doi:10.1016/j.reuma.2015.08.004
3. Song G, Huang J, Deng Y, Liang Z, Lin Y. The expression of calprotectin and factors in TLR4/NF-κB/MyD88 pathway in patients with idiopathic acute anterior uveitis. Ocul Immunol Inflamm. 2019;27(7):1144–1148. doi:10.1080/09273948.2018.1485956
4. Abd El Latif E, Ammar H. Uveitis referral pattern in upper and lower Egypt. Ocul Immunol Inflamm. 2018;27(6):875–882. doi:10.1080/09273948.2017.1410183
5. Groen-Hakan F, Eurelings L, van Laar J, Rothova A. Relevance of erythrocyte sedimentation rate and C-reactive protein in patients with active uveitis. Graefes Arch Clin Exp Ophthalmol. 2019;257(1):175–180. doi:10.1007/s00417-018-4174-7
6. Denniston AK, Keane PA, Srivastava SK. Biomarkers and surrogate endpoints in uveitis: the impact of quantitative imaging. Investig Opthalmol Vis Sci. 2017;58(6):BIO131–BIO140. doi:10.1167/iovs.17-21788
7. Pato E, Martin-Martinez M, Castelló A, et al. Development of an activity disease score in patients with uveitis [UVEDAI]. Rheumatol Int. 2017;37(4):647–656. doi:10.1007/s00296-016-3593-1
8. Nordal HH, Brokstad KA, Solheim M, Halse A-K, Kvien TK, Hammer HB. Calprotectin (S100A8/A9) has the strongest association with ultrasound-detected synovitis and predicts response to biologic treatment: results from a longitudinal study of patients with established rheumatoid arthritis. Arthritis Res Ther. 2017;19(1):3. doi:10.1186/s13075-016-1201-0
9. Inciarte-Mundo J, Hernández MV, Ruiz-Esquide V, et al. Serum calprotectin versus acute-phase reactants in the discrimination of inflammatory disease activity in rheumatoid arthritis patients receiving tumor necrosis factor inhibitors: calprotectin, CRP, and ESR in RA patients receiving TNFi. Arthritis Care Res. 2016;68(7):899–906. doi:10.1002/acr.22795
10. Turina MC, Sieper J, Yeremenko N, et al. Calprotectin serum level is an independent marker for radiographic spinal progression in axial spondyloarthritis. Ann Rheum Dis. 2014;73(9):1746–1748. doi:10.1136/annrheumdis-2014-205506
11. Giudice V, Wu Z, Kajigaya S, et al. Circulating S100A8 and S100A9 protein levels in plasma of patients with acquired aplastic anemia and myelodysplastic syndromes. Cytokine. 2019;113:462–465. doi:10.1016/j.cyto.2018.06.025
12. Mondet J, Chevalier S, Mossuz P. Pathogenic roles of S100A8 and S100A9 proteins in acute myeloid and lymphoid leukemia: clinical and therapeutic impacts. Molecules. 2021;26(5):1323. doi:10.3390/molecules26051323
13. Jabs DA, Nussenblatt RB, Rosenbaum JT; Standardization of Uveitis Nomenclature (SUN) Working Group. Standardization of uveitis nomenclature for reporting clinical data. Results of the first international workshop. Am J Ophthalmol. 2005;140(3):509–516. doi:10.1016/j.ajo.2005.03.057
14. Huang J, Yin Z, Song G, Cui S, Jiang J, Zhang L. Discriminating value of calprotectin in disease activity and progression of nonradiographic axial spondyloarthritis and ankylosing spondylitis. Dis Markers. 2017;2017:7574147. doi:10.1155/2017/7574147
15. Pedersen L, Nybo M, Poulsen MK, Henriksen JE, Dahl J, Rasmussen LM. Plasma calprotectin and its association with cardiovascular disease manifestations, obesity and the metabolic syndrome in type 2 diabetes mellitus patients. BMC Cardiovasc Disord. 2014;14(1):196. doi:10.1186/1471-2261-14-196
16. Halyabar O, Mehta J, Ringold S, Rumsey DG, Horton DB. Treatment withdrawal following remission in juvenile idiopathic arthritis: a systematic review of the literature. Pediatric Drugs. 2019;21(6):469–492. doi:10.1007/s40272-019-00362-6
17. Wang Y-Q, Dai X-D, Ran Y, et al. Circulating S100A8/A9 levels reflect intraocular inflammation in uveitis patients. Ocul Immunol Inflamm. 2020;28(1):133–141. doi:10.1080/09273948.2018.1538461
18. Tallouzi MO, Mathers JM, Moore DJ, et al. Development of a core outcome set for clinical trials in non-infectious uveitis of the posterior segment. Ophthalmology. 2021;128(8):1209–1221. doi:10.1016/j.ophtha.2021.01.022
19. Zhang W, Lin H, Zou M, et al. Nicotine in inflammatory diseases: anti-inflammatory and pro-inflammatory effects. Front Immunol. 2022;2022:376. doi:10.3389/fimmu.2022.826889
20. Walscheid K, Heiligenhaus A, Holzinger D, et al. Elevated S100A8/A9 and S100A12 serum levels reflect intraocular inflammation in juvenile idiopathic arthritis-associated uveitis: results from a pilot study. Invest Ophthalmol Vis Sci. 2015;56(13):7653–7660. doi:10.1167/iovs.15-17066
21. Özşeker B, Şahin C, Özşeker HS, Efe SC, Kav T, Bayraktar Y. The role of fecal calprotectin in evaluating intestinal involvement of Behçet’s disease. Dis Markers. 2016;24:2016. doi:10.1155/2016/5423043
22. Olson JA, Forrester M, Clohessy PA, Golden BE, Herriot R, Forrester JV. Calprotectin is raised in endogenous posterior uveitis. Ocul Immunol Inflamm. 1996;4(2):91–98. doi:10.3109/09273949609079638
23. Pascual EV, Martinez-Costa Perez L, Hernandez Pons A, et al. The role of plasma calprotectin in non-infectious uveitis. Curr Eye Res. 2021;46(8):1184–1192. doi:10.1080/02713683.2020.1867749
24. Gazim CC, Borba AA, Castro GK, et al. Fecal calprotectin levels in acute anterior uveitis in patients with spondyloarthritis. Arq Bras Oftalmol. 2022;2022. doi:10.5935/0004-2749.20230008
25. Sykes MP, Hamilton L, Jones C, Gaffney K. Prevalence of axial spondyloarthritis in patients with acute anterior uveitis: a cross-sectional study utilising MRI. RMD Open. 2018;4(1):e000553. doi:10.1136/rmdopen-2017-000553
26. Kim DH, Park Y, Kim B, et al. Fecal calprotectin as a non‐invasive biomarker for intestinal involvement of Behçet’s disease. J Gastroenterol Hepatol. 2017;32(3):595–601. doi:10.1111/jgh.13530
27. Kempen JH, Ganesh SK, Sangwan VS, Rathinam SR. Interobserver agreement in grading activity and site of inflammation in eyes of patients with uveitis. Am J Ophthalmol. 2008;146(6):813–818. doi:10.1016/j.ajo.2008.06.004
28. Szepessy Z, Barsi Á, Németh J. Macular changes correlate with the degree of acute anterior uveitis in patients with spondyloarthropathy. Ocul Immunol Inflamm. 2016;24(2):153–158. doi:10.3109/09273948.2014.946149
29. Invernizzi A, Marchi S, Aldigeri R, et al. Objective quantification of anterior chamber inflammation: measuring cells and flare by anterior segment optical coherence tomography. Ophthalmology. 2017;124(11):1670–1677. doi:10.1016/j.ophtha.2017.05.013
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
