Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 12
Calcipotriol Plus Betamethasone Dipropionate Aerosol Foam For Scalp Psoriasis
Authors Anderko M, Navarro Triviño FJ , Sharples CL
Received 27 June 2019
Accepted for publication 4 September 2019
Published 17 September 2019 Volume 2019:12 Pages 699—705
DOI https://doi.org/10.2147/CCID.S221078
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Marian Anderko,1 Francisco José Navarro Triviño,2 Charlotte L Sharples3
1Dermatologikum, Hamburg 20354, Germany; 2Dermatology and Venereology, Hospital Universitario San Cecilio, Granada 18016, Spain; 3LEO Pharma UK, Hurley, Berks, UK
Correspondence: Marian Anderko
Dermatologikum, Stephansplatz 5, Hamburg 20354, Germany
Tel +49 40 3510750
Fax +49 40 35107510
Email [email protected]
Abstract: This article presents real-world experience of the effectiveness of calcipotriol (50 μg/g)/betamethasone dipropionate (0.5 mg/g) (Cal/BD) aerosol foam formulation in three cases of scalp psoriasis, and briefly reviews the literature relating to Cal/BD topical therapy in adults with scalp psoriasis. Patients had long histories of scalp psoriasis and reported negative impacts on their lives (e.g. clothing choices, psychological well-being, employment status). Previous treatments had provided inadequate or only temporary relief. Cal/BD aerosol foam relieved itching in the first few days and was associated with visible improvement of flaky patches on the scalp at the end of the recommended 4-week treatment period. Controlled clinical trials in patients with scalp psoriasis are rare. There have been several trials in adults with scalp psoriasis involving Cal/BD gel or suspension scalp formulations, which have proven more effective and well tolerated compared with the individual components or vehicle alone. The Cal/BD aerosol formulation has enhanced skin penetration and higher bioavailability compared with the older formulations; studies show improved efficacy with Cal/BD aerosol foam, compared with older formulations, in patients with plaque psoriasis. The present cases confirm the benefits of Cal/BD aerosol foam in adults with scalp psoriasis, treated in real-world settings.
Keywords: aerosol, betamethasone dipropionate, calcipotriol, psoriasis, scalp psoriasis
Introduction
Psoriasis is a chronic inflammatory disease which affects the skin, nails and joints and is associated with numerous comorbidities.1 The reported prevalence of psoriasis varies widely between countries, affecting up to 11.4% of individuals.1 Overall, the prevalence has been estimated to be about 2% of the population.2–4 Psoriasis has a significant influence on both physical and psychological conditions and is known to affect quality of life (QoL) and general well-being. Consequently, there are economic impacts through days off work and health care costs.5
Up to 80% of patients with psoriasis experience scalp psoriasis at some time6,7 and, in the majority of these patients, the scalp psoriasis has a negative impact on QoL.7,8 Itch is the most significant and problematic symptom associated with psoriasis in general, as assessed by both clinicians and patients.9 In patients with scalp psoriasis there can also be pain, bleeding, embarrassment and frustrating practical consequences such as limitations on the choice of clothing.10
There are numerous therapeutic options for psoriasis, including topical treatment, phototherapy, conventional systemic medications and biologics. As knowledge of the pathophysiology of the disease has progressed, targeted therapy with biological agents – such as tumor necrosis factor (TNF)-alpha inhibitors and inhibitors of various interleukins (IL) – has become an option. However, these biologics are expensive.11 Topical agents and phototherapy remain an important part of treatment for most patients,12 and topical vitamin D analogs (targeting the vitamin-D receptor and alternative receptors in the skin13,14) and corticosteroids (targeting the glucocorticoid receptor) are still considered first-line therapy.15,16 Combinations of these compounds have been developed to produce a single-product fixed-dose formulation of calcipotriol plus betamethasone dipropionate (Cal/BD).17 These are generally well tolerated and more effective than the individual components.6
Greasy or sticky formulations are less acceptable to patients, particularly for scalp psoriasis, and this may reduce compliance.18 Also, conventional topical treatments are less effective on the scalp than on other parts of the body.8 Thus, fixed-combination, alcohol-free gel formulations of Cal/BD have been developed17 and, more recently, an alcohol-free, aerosol foam formulation. Different preparations can help cater to patient preference.
This alcohol-free, aerosol foam formulation of Cal/BD, in which the active ingredients are dissolved in a mixture of volatile propellants (butane and dimethyl ether) to build a stable, supersaturated solution after evaporation of the propellants. This is associated with enhanced skin penetration and increased bioavailability.19 Relative to comparator ointment and gel preparations, the aerosol foam formulation is associated with greater efficacy, improved patient satisfaction and the potential for greater patient adherence.20,21 In one study in patients with psoriasis vulgaris, treatment with Cal/BD aerosol foam (excluding the scalp, which was not treated) improved clinical symptoms and QoL to a greater extent than a Cal/BD gel formulation over a 12-week study period.22 In patients with psoriasis affecting the body (not the scalp), the aerosol foam also has good efficacy and safety, enhanced cosmetic properties,12 is simple and quick to apply, and is pleasant on the skin.23
The clinical effectiveness and safety of Cal/BD aerosol formulation in patients with psoriasis on the body (but not scalp psoriasis) has been reviewed recently,24 with the authors concluding that Cal/BD aerosol foam offers several treatment advantages, including relief of itch, and is an appropriate first-line topical therapy for consideration in patients with psoriasis of any severity. However, more evidence is required to establish the place of improved topical formulations of fixed-combination Cal/BD for scalp psoriasis, particularly real-world experience. This article presents evidence from three real-world cases of the use of fixed-dose combination Cal/BD aerosol foam for scalp psoriasis, and a brief literature review relating to the use of Cal/BD topical therapy in adults with scalp psoriasis (based on a PubMed search of clinical trials using the terms `psoriasis’, `scalp’, calcipotriol’, `betamethasone’). Patients provided written informed consent for the case details and accompanying photographic images to be published. Ethics committee or institutional review board approval was not necessary for the individual cases reported in this series because each case reflects a retrospective description of clinical findings.
Case Study 1
This case describes a 29-year-old female with a history of moderate-to-severe psoriasis vulgaris, particularly affecting the scalp, since the age of 14 years. Scalp psoriasis caused the patient embarrassment and limited her clothing choices to the point that she generally avoided wearing dark clothes and, on particularly bad days, she wore a headscarf.
At presentation, the patient’s Psoriasis Area and Severity Index (PASI) was 11.4. Prior therapy included various topical cortisone keratolytic treatments as well as phototherapy. Her most recent treatment was clobetasol-17-propionate to the scalp. For the past 4 years, she had undertaken annual 3-week stays at a health resort, where she received psoralen and ultraviolet A (PUVA) treatment, resulting in temporary remission.
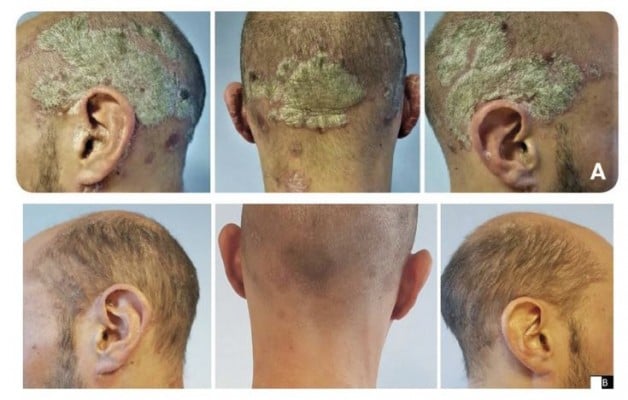
On examination, the patient had marked psoriasis with infiltrating, adherent plaques with deposits of scaly skin. In addition, there was marked psoriasis capitis with plaques of white scales on the hairline and behind the ears (Figure 1A). Methotrexate treatment was contraindicated since the patient was planning to start a family. On 29 June 2018, she began once-daily application of Cal/BD aerosol foam for 4 weeks. For convenience, the foam was applied to the affected area of the scalp in the evening. The patient washed her hair the next day as usual.
 |
Figure 1 Case 1: (A) before and (B) after treatment for 4 weeks with calcipotriol (50 µg/g)/betamethasone dipropionate (0.5 mg/g) aerosol foam. |
The patient reported reduced itching after 4 days of treatment with Cal/BD aerosol foam. After the recommended 4-week treatment period, the flaky patches on her scalp were visibly improved (Figure 1B) and her PASI (scalp) had decreased to 1.6.
Case Study 2
The second case describes a 71-year-old female who had suffered from pronounced psoriasis confined to the scalp for over 30 years. Apart from hypertension and hyperthyroidism there was no other notable medical history.
Over the years, the patient had tried various topical treatments, most recently BD plus salicylic acid, as well as overnight application of polydimethylsiloxane for keratolysis.
At the initial consultation, the patient was despondent, saying that she had already tried all possible treatments and did not know what else to try. She refused systemic therapy but was open to the use of topical treatment. On examination, she had pronounced psoriasis capitis with multiple extensive infiltrates of adhering flaky plaques on the scalp as well as the hairline and behind the ears (Figure 2A). She reported severe itching. Her PASI was 4.8.
 |
Figure 2 Case 2: (A) before and (B) after treatment for 4 weeks with calcipotriol (50 µg/g)/betamethasone dipropionate (0.5 mg/g) aerosol foam. |
On 25 June 2018, the patient began once-daily application of Cal/BD aerosol foam for 4 weeks. For convenience, the foam was applied to the affected areas in the evening and was washed out the next day.
The patient reported reduced itching after 4 days of treatment. After 4 weeks, the flaky patches on her scalp had mostly disappeared (Figure 2B) and her PASI had decreased to 0.3. The patient reported being very satisfied with the results of her treatment.
Case Study 3
This case describes a 33-year-old male with a 10-year history of psoriasis. The patient was a smoker and his father and aunt had a history of plaque psoriasis; there was no other personal history of interest. The patient had been working as a hotel receptionist but reported that he had lost his job due to his psoriasis. No current or previous treatment had been received for psoriasis and the patient was referred directly to a dermatology specialist.
At presentation, plaque psoriasis was evident on the trunk, extremities and, notably, the scalp (>50% of the scalp surface was affected, including the external ear canal; Figure 3A). The patient’s PASI was 15, 20% of the body surface area (BSA) was affected and the Dermatology Life Quality Index (DLQI) score was 24. Based on the severity of the psoriasis, the patient was assessed for potential systemic treatment. Meanwhile, the dermatologist recommended the use of once-daily Cal/BD aerosol foam for 4 weeks.
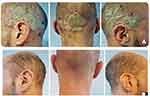 |
Figure 3 Case 3: (A) before and (B) after treatment for 4 weeks with calcipotriol (50 µg/g)/betamethasone dipropionate (0.5 mg/g) aerosol foam. |
After 4 weeks of treatment with Cal/BD foam, partial skin clearance was achieved on the trunk and extremities (PASI 10, BSA 18%, DLQI 14), with almost complete clearance achieved on the scalp (Figure 3B). The patient reported very good overall satisfaction with the therapeutic result obtained and the cosmetic acceptability of the product.
Discussion
The three real-world cases reported herein involved adults with scalp psoriasis, of which two cases were refractory to prior treatment. Cal/BD aerosol foam once daily produced improvement in itching and, after 4 weeks, had markedly improved the signs and severity of scalp psoriasis.
In a recent review of data from several sources, including randomized controlled trials, case reports and real-world evidence, Pinter et al confirmed the efficacy, effectiveness, and favorable safety and tolerability of Cal/BD aerosol foam in patients with psoriasis previously treated with class 3 or 4 topical corticosteroids, those unsatisfied with ongoing phototherapy with topical therapy, and those with moderate-to-severe psoriasis.24 However, that review did not consider patients with scalp psoriasis. Indeed, many clinical trials in patients with psoriasis specifically exclude patients with scalp psoriasis and, where patients with scalp psoriasis are included, they generally also have moderate-to-severe body plaque psoriasis requiring systemic therapy.8
Prospective, controlled clinical trials with any therapies in patients with scalp psoriasis are rare. In an analysis of 1320 clinical studies published in PubMed or Embase including the keywords ‘scalp’, 'psoriasis' and 'treatment', only 27 clinical trials were identified which were specifically in patients with scalp psoriasis.6 Most of those studies involved topical therapies.
Fixed-dose scalp combination therapies of Cal/BD (not the aerosol foam formulation) applied once daily have proven superior to placebo or the individual components or calcipotriol scalp solution in adults with scalp psoriasis.25–29 In an 8-week, multicenter, randomized, double-blind study in >1500 adults with scalp psoriasis, more patients achieved `absent’ or `very mild’ disease at week 8 with the two-compound scalp formulation (71.2%) compared with BD in the same vehicle (64.0%, P=0.011), Cal in the same vehicle (36.8%, P<0.0001), or the vehicle alone (22.8%, P<0.0001).25 In a double-blind, parallel group study, scalp psoriasis was rated as clear or almost clear in 69.6% of adult patients treated with the two-compound formulation versus 59.9% receiving BD alone (P<0.0001) and 44.7% receiving Cal alone (P=0.0006).26 Among Hispanic/Latino and Black/African American adults with scalp psoriasis, included in a double-blind, parallel group study, 8 weeks of treatment with the two-compound scalp formulation of Cal/BD resulted in clearance or minimal disease in 71.9% of patients versus 40.5% of those who received vehicle only (P<0.001).27 In an investigator-blind, parallel group study, in adults with moderately severe scalp psoriasis, 8 weeks of treatment with the two-compound scalp formulation of Cal/BD resulted in clear or minimal disease in 68.6% of patients versus 31.4% of those receiving Cal alone (P<0.001).28 A study in Chinese adults involved a two-compound Cal/BD gel product; 87.5% of patients receiving the combination achieved disease control after 4 weeks compared with 50.8% of those treated with Cal solution (P<0.0001).29
Fixed-combination Cal/BD aerosol foam has been evaluated in a randomized, double-blind, multicenter study, demonstrating greater overall efficacy (treatment success) than Cal aerosol foam or BD aerosol foam after 4 weeks of treatment in 302 patients with psoriasis (66% of whom had moderate scalp psoriasis).30 Treatment success of the scalp with Cal/BD aerosol foam was significantly greater than with Cal aerosol foam at Week 4 (53.0% vs 35.6%; OR 1.91, 95% CI 1.09–3.35; P=0.021), but not BD aerosol foam (47.5%; OR 1.24, 95% CI 0.71–2.16; P=0.45).30 Cal/BD foam was also associated with significant reductions in scalp lesion severity for erythema, scaliness, and elevation at week 4, with effects observed as early as week 1.31 The frequency of adverse events was low and mostly mild/moderate in intensity in all treatment groups.31
An observational study following patients with psoriasis on the British Association of Dermatologists Biologic Interventions Register (BADBIR) showed that patients ineligible for clinical trials with biologics have a poorer clinical outcome to therapy (lower efficacy and higher incidence of serious adverse events) than those eligible for clinical trials. Thus, among patients with psoriasis, clinical trial populations may not reflect the real-world situation.32 Case reports are of significance, therefore, in presenting real-world experience of clinical activity.
In a previous report of real-world experience with Cal/BD aerosol foam, five patients with psoriasis, varying from mild-to-moderate to moderate-to-severe (PASI from 4.8 to 12.0), achieved effective relief of psoriasis, including itching symptoms, and reported treatment satisfaction.24 The cases reported here extend those findings to patients with scalp psoriasis, two of which were resistant to previous treatment.
Conclusion
Based on the cases reported herein, Cal/BD aerosol foam provided effective therapy for scalp psoriasis in adults, including cases which were resistant to previous treatment. Use of Cal/BD aerosol foam for the recommended 4 weeks was associated with visible improvements in scalp psoriasis, and patients reported marked relief from itching and satisfaction with treatment.
Abbreviations
BADBIR, British Association of Dermatologists Biologic Interventions Register; Cal/BD, calcipotriol/betamethasone dipropionate; IL, interleukin; PASI, Psoriasis Area and Severity Index; PUVA, psoralen and ultraviolet A; TNF, tumor necrosis factor.
Acknowledgments
Medical writing support, under the direction of the authors, was provided by David P Figgitt PhD, ISMPP CMPP™, Content Ed Net (Germany), with funding from LEO Pharma GmbH, Frankfurt, Germany. Funding for the cases described in this article was provided by LEO Pharma GmbH, Frankfurt, Germany.
Disclosure
Dr. Anderko has received funding from LEO Pharma GmbH. Dr. Sharples is an employee of LEO Pharma GmbH. Dr. Navarro reports no conflicts of interest in this work.
References
1. World Health Organization. Global Report On Psoriasis. WHO Press: Geneva; 2016:1–48. Available from: https://apps.who.int/iris/bitstream/handle/10665/204417/9789241565189_eng.pdf?sequence=1&isAllowed=y.
2. Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis. Section 3. Guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60(4):643–659. doi:10.1016/j.jaad.2008.12.032
3. Uva L, Miguel D, Pinheiro C, et al. Mechanisms of action of topical corticosteroids in psoriasis. Int J Endocrinol. 2012;2012:561018. doi:10.1155/2012/561018
4. Polistena B, Calzavara-Pinton P, Altomare G, et al. The impact of biologic therapy in chronic plaque psoriasis from a societal perspective: an analysis based on Italian actual clinical practice. J Eur Acad Dermatol Venereol. 2015;29(12):2411–2416. doi:10.1111/jdv.12669
5. Schadler ED, Ortel B, Mehlis SL. Biologics for the primary care physician: review and treatment of psoriasis. Dis Mon. 2019;65(3):51–90.
6. Wang TS, Tsai TF. Managing scalp psoriasis: an evidence-based review. Am J Clin Dermatol. 2017;18(1):17–43.
7. Guenther L. Current management of scalp psoriasis. Skin Therapy Lett. 2015;20(3):5–7.
8. Bagel J, Duffin KC, Moore A, et al. The effect of secukinumab on moderate-to-severe scalp psoriasis: results of a 24-week, randomized, double-blind, placebo-controlled phase 3b study. J Am Acad Dermatol. 2017;77(4):667–674.
9. Globe D, Bayliss MS, Harrison DJ. The impact of itch symptoms in psoriasis: results from physician interviews and patient focus groups. Health Qual Life Outcomes. 2009;7:62.
10. Merola JF, Qureshi A, Husni ME. Underdiagnosed and undertreated psoriasis: nuances of treating psoriasis affecting the scalp, face, intertriginous areas, genitals, hands, feet, and nails. Dermatol Ther. 2018;31(3):e12589. doi:10.1111/dth.12589
11. Kromer C, Celis D, Sonntag D, Peitsch WK. Biologicals and small molecules in psoriasis: a systematic review of economic evaluations. PLoS One. 2018;13(1):e0189765. doi:10.1371/journal.pone.0189765
12. Frieder J, Kivelevitch D, Menter A. Calcipotriene betamethasone dipropionate aerosol foam in the treatment of plaque psoriasis: a review of the literature. Ther Deliv. 2017;8(9):737–746. doi:10.4155/tde-2017-0058
13. Slominski AT, Kim TK, Takeda Y, et al. RORα and ROR γ are expressed in human skin and serve as receptors for endogenously produced noncalcemic 20-hydroxy- and 20,23-dihydroxyvitamin D. Faseb J. 2014;28(7):2775–2789. doi:10.1096/fj.13-242040
14. Slominski AT, Kim TK, Janjetovic Z, et al. Differential and overlapping effects of 20,23(OH)₂D3 and 1,25(OH)₂D3 on gene expression in human epidermal keratinocytes: identification of ahr as an alternative receptor for 20,23(OH)₂D3. Int J Mol Sci. 2018;19(10):
15. Cohen SN, Baron SE, Archer CB. British association of dermatologists and royal college of general practitioners. Guidance on the diagnosis and clinical management of psoriasis. Clin Exp Dermatol. 2012;37(Suppl 1):13–18. doi:10.1111/j.1365-2230.2012.04337.x
16. Mason A, Mason J, Cork M, Hancock H, Dooley G. Topical treatments for chronic plaque psoriasis: an abridged cochrane systematic review. J Am Acad Dermatol. 2013;69(5):799–807. doi:10.1016/j.jaad.2013.06.027
17. Gooderham M, Debarre JM, Keddy-Grant J, Xu Z, Kurvits M, Goodfield M. Safety and efficacy of calcipotriol plus betamethasone dipropionate gel in the treatment of scalp psoriasis in adolescents 12–17 years of age. Br J Dermatol. 2014;171(6):1470–1477. doi:10.1111/bjd.13235
18. Svendsen MT, Andersen F, Hansen J, Johannessen H, Andersen KE. Medical adherence to topical corticosteroid preparations prescribed for psoriasis: a systematic review. J Dermatolog Treat. 2017;28(1):32–39. doi:10.1080/09546634.2016.1178375
19. Lind M, Nielsen KT, Schefe LH, et al. Supersaturation of calcipotriene and betamethasone dipropionate in a novel aerosol foam formulation for topical treatment of psoriasis provides enhanced bioavailability of the active ingredients. Dermatol Ther (Heidelb). 2016;6(3):413–425. doi:10.1007/s13555-016-0125-6
20. Paul C, Bang B, Lebwohl M. Fixed combination calcipotriol plus betamethasone dipropionate aerosol foam in the treatment of psoriasis vulgaris: rationale for development and clinical profile. Expert Opin Pharmacother. 2017;18(1):115–121. doi:10.1080/14656566.2016.1269749
21. Girolomoni G, Calzavara Pinton P, Cristaudo A, Cicchetti A. Back to the future: a new topical approach for mild-to-moderate psoriasis. G Ital Dermatol Venereol. 2018;153(3):375–382.
22. Griffiths CE, Stein Gold L, Cambazard F, Kalb RE, Lowson D, Møller A, Paul C. Greater improvement in quality of life outcomes in patients using fixed-combination calcipotriol plus betamethasone dipropionate aerosol foam versus gel: results from the PSO-ABLE study. Eur J Dermatol. 2018;28(3):356–363.
23. Vender R, Gooderham MJ, Guenther LC, et al. Psoriasis patients’ preference for an aerosol foam topical formulation. J Eur Acad Dermatol Venereol. 2018;32(11):e400–401. doi:10.1111/jdv.14282
24. Pinter A, Thormann H, Angeletti F, Jalili A. Calcipotriol/betamethasone dipropionate aerosol foam for the treatment of psoriasis vulgaris: case series and review of the literature. Clin Cosmet Investig Dermatol. 2018;11:451–459. doi:10.2147/CCID.S180698
25. Jemec GB, Ganslandt C, Ortonne JP, et al. A new scalp formulation of calcipotriene plus betamethasone compared with its active ingredients and the vehicle in the treatment of scalp psoriasis: a randomized, double-blind, controlled trial. J Am Acad Dermatol. 2008;59(3):455–463. doi:10.1016/j.jaad.2008.04.027
26. van de Kerkhof PCM, Hoffmann V, Anstey A, et al. A new scalp formulation of calcipotriol plus betamethasone dipropionate compared with each of its active ingredients in the same vehicle for the treatment of scalp psoriasis: a randomized, double-blind, controlled trial. Br J Dermatol. 2009;160(1):170–176. doi:10.1111/j.1365-2133.2008.08927.x
27. Tyring S, Mendoza N, Appell M, et al. A calcipotriene/betamethasone dipropionate two-compound scalp formulation in the treatment of scalp psoriasis in Hispanic/Latino and Black/African American patients: results of the randomized, 8-week, double-blind phase of a clinical trial. Int J Dermatol. 2010;49(11):1328–1333. doi:10.1111/j.1365-4632.2010.04598.x
28. Kragballe K, Hoffmann V, Ortonne JP, Tan J, Nordin P, Segaert S. Efficacy and safety of calcipotriol plus betamethasone dipropionate scalp formulation compared with calcipotriol scalp solution in the treatment of scalp psoriasis: a randomized controlled trial. Br J Dermatol. 2009;161(1):159–166. doi:10.1111/j.1365-2133.2009.09116.x
29. Ma L, Yang Q, Yang H, et al. Calcipotriol plus betamethasone dipropionate gel compared with calcipotriol scalp solution in the treatment of scalp psoriasis: a randomized, controlled trial investigating efficacy and safety in a Chinese population. Int J Dermatol. 2016;55(1):106–113. doi:10.1111/ijd.13145
30. Lebwohl M, Tyring S, Bukhalo M, et al. Fixed combination aerosol foam calcipotriene 0.005% (Cal) plus betamethasone dipropionate 0.064% (BD) is more efficacious than Cal or BD aerosol foam alone for psoriasis vulgaris: a randomized, double-blind, multicenter, three-arm, phase 2 study. J Clin Aesthet Dermatol. 2016;9(2):34–41.
31. Patel DS, Veverka KA, Nyeland ME, Yamauchi PS, Alonso-Llamazares J, Lebwohl M. Improvements In Efficacy And Lesion Quality In Scalp Plaque Psoriasis With Fixed Combination Calcipotriene And Betamethasone Dipropionate (cal/bd) Foam Treatment [abstract/poster No. 9803]. American Academy of Dermatology Annual Meeting: Washington, DC, USA; 2019.
32. Mason KJ, Barker JNWN, Smith CH, et al. Comparison of drug discontinuation, effectiveness, and safety between clinical trial eligible and ineligible patients in BADBIR. JAMA Dermatol. 2018;154(5):581–588. doi:10.1001/jamadermatol.2017.6195
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
