Back to Journals » Drug Design, Development and Therapy » Volume 13
Caffeic acid phenethyl ester suppressed growth and metastasis of nasopharyngeal carcinoma cells by inactivating the NF-κB pathway
Authors Liang Y, Feng G, Wu L, Zhong S, Gao X, Tong Y, Cui W , Qin Y, Xu WQ, Xiao X, Zhang Z, Huang G, Zhou X
Received 21 December 2018
Accepted for publication 23 March 2019
Published 26 April 2019 Volume 2019:13 Pages 1335—1345
DOI https://doi.org/10.2147/DDDT.S199182
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Cristiana Tanase
Yushan Liang,1,2 Guofei Feng,1,2 Liang Wu,3 Suhua Zhong,1,2 Xiaoyu Gao,1,2 Yan Tong,1,2 Wanmeng Cui,1 Yongying Qin,1 WenQing Xu,1 Xue Xiao,1,2 Zhe Zhang,1,2 Guangwu Huang,1,2 Xiaoying Zhou1,4
1Key laboratory of High-Incidence-Tumor Prevention & Treatment, Ministry of Education, Guangxi Medical University, Nanning, People’s Republic of China; 2Department of Otolaryngology Head & Neck Surgery, First Affiliated Hospital of Guangxi Medical University, Nanning, People’s Republic of China; 3Jiangsu Key Laboratory of Drug Screening, China Pharmaceutical University, Nanjing, People’s Republic of China; 4Life Science Institute, Guangxi Medical University, Nanning, People’s Republic of China
Purpose: Caffeic acid phenethyl ester (CAPE) is the main polyphenol extracted from honeybee propolis, which inhibits the growth of several kinds of tumor. This study aimed to assess the inhibitory effect of CAPE in nasopharyngeal carcinoma (NPC), evaluate the synergistic action of CAPE in radiotherapy sensitivity of NPC cell lines and further elucidate the possible molecular mechanism involved.
Materials and methods: CCK-8 assay was used to analyze cell proliferation ability. Colony formation assay was used to evaluate the clonogenic ability and radio-sensitiveness of NPC cells by CAPE treatment. Wound-healing and transwell assay were used to assess the motility of cells. The expression of key molecules of the epithelial–mesenchymal transition (EMT) was determined by western blot analysis and changes in radiation sensitivity were measured by colony-formation assay. cDNA microarray analysis was used to determine differentially expressed genes with and without CAPE treatment, with Gene Ontology enrichment of gene function and KEGG pathways determined. Cell cycle and apoptosis were detected by flow cytometry and western blot analysis.
Results: CAPE suppressed the viability of NPC cell lines time- and dose-dependently. It induced apoptosis in NPC cells along with decreased expression of Bcl-XL and increased cleavage of PARP and expression of Bax. G1 phase arrest was induced by CAPE with ower expression of CDK4, CDK6, Rb and p-Rb. The migratory and invasive ability of NPC cells was decreased by the EMT pathway. The irradiation sensitivity of NPC cells was enhanced with CAPE treatment. CAPE specifically inhibited nuclear factor κB (NF-κB) signaling pathway by suppressing p65 subunit translocation from cytoplasm to nucleus. CAPE treatment was synergistic with chemotherapy and radiotherapy.
Conclusion: CAPE may inhibit the proliferation and metastasis of NPC cells but enhance radiosensitivity in NPC therapy by inhibiting the NF-κB pathway. CAPE could be a potential therapeutic compound for NPC therapy.
Keywords: nasopharyngeal carcinoma, CAPE, proliferation, metastasis, NF-κB pathway
Introduction
Nasopharyngeal carcinoma (NPC) is a malignancy derived frequently from the epithelium of Rosenmüller fossa. The racial/ethnic and geographic distribution of NPC is distinctive, with high incidence in some countries including southern China, Southeast Asia, the Arctic and the Middle East/North Africa.1 The interaction of genetic susceptibility, environmental factors and Epstein–Barr virus (EBV) infection contributes to the pathogenesis and development of NPC.2
Radiotherapy is the conventional treatment for NPC because of the cancer’s high sensitivity to radiotherapy. With the development of intensity-modulated radiation, 5-year survival in early diagnosed NPC (stage I and II) is more than 80% but remains less than 10% at stage IV.3 Distant metastasis is the major cause of treatment failure in NPC. Novel therapeutic approaches are needed to improve the outcome with advanced NPC.
Caffeic acid phenethyl ester (CAPE) is the main polyphenol extracted from honeybee propolis and polyphenol and can directly bind to the signaling molecules involved in carcinogenesis and regulate their activity.4 It has anti-bacterial and viral infection, anti-oxidant, anti-inflammatory and anti-tumor properties.5–8 In several kinds of human cancer, including prostate cancer, breast cancer, ovarian cancer, oral cancer, CAPE inhibited angiogenesis and metastasis and increased radiation sensitivity.9–11 Recently, CAPE was found to attenuate NPC cell (TW04) proliferation and invasion by upregulating N-Myc downstream regulated 1 via the mitogen-activated protein kinase pathway and by inhibiting the phosphorylation of signal transducer and activator of transcription 3.12 However, the effect of CAPE in EBV-positive NPC cells remains unclear, as does the synergistic effect with radiotherapy and chemotherapy.
Pro-inflammatory stimuli in the tumor microenvironment activate the NF-κB pathway. Constitutive activation of nuclear factor κB (NF-κB) signals mediates critical events in the initiation and progression of human cancers by regulating cell proliferation, apoptosis, cell transformation and immunosuppression.13,14 Latent-membrane protein 1 (LMP1), as an EBV oncoprotein, is a potent activator of NF-κB signaling.15 LMP1 can phosphorylate the IKK complex and induce IκBα degradation, thus resulting in constitutive NF-κB activation. Whole-exome sequencing data detected mutation of several NF-κB pathway negative regulators, including CYLD lysine 63 deubiquitinase (CYLD) and TNF receptor-associated factor 3 (TRAF3) in NPC primary tumors.16,17 Constitutive activation of NF-κB was detected in NPC cell lines and primary tumors,18 so targeting inhibitors may be a promising therapeutic for NPC.
CAPE can specifically inhibit NF-κB cell signaling.19 In oral cancer, CAPE remarkably suppressed the proliferation of TW2.6 cells. The half-maximal inhibitory concentration (IC50) was 2–4 times higher for normal human oral fibroblast cells and buccal mucosal fibroblast cells than oral cancer cells.8 Therefore, the toxic effects of CAPE may be lower for normal cells than tumor cells, so CAPE could be a potential agent for cancer therapy.
The aim of this study was to evaluate the effect of CAPE on the biological behavior of NPC cell lines, including proliferation, apoptosis, cell cycle arrest and metastasis, and further elucidate the underlying mechanisms. The synergistic effect of CAPE with radio- and chemotherapy was also investigated.
Materials and methods
Cell culture and chemicals
This study was approved by the ethics committee of Guangxi Medical University. CNE2 and HK1 cell line were maintained in our laboratory. CNE2-EBV was kindly provided by Prof Musheng Zeng’s group (Sun Yat-sen University Cancer Center, China), and HK1-EBV was kindly gifted by Prof Sai-Wah Tsao (Hong Kong University, China). CNE2-EBV and HK1-EBV cells were established as described previously.20 These cell lines were cultured in high glucose DMEM supplemented with penicillin G (100 U/mL), streptomycin (100 µg/mL) and 10% fetal bovine serum (FBS). Cells were grown at 37°C in a humidified atmosphere of 5% CO2 and were routinely sub-cultured by using 0.25% (w/v) trypsin-EDTA solution. CAPE was purchased from Sigma (St. Louis, MO, USA).
CCK-8 proliferation assay
An amount of 2×103 cells/well were seeded in 96-well plates. Cell viability was assessed by CCK8 assay. All experiments were repeated three times. The amount of yellow-color formazan dye is directly proportional to the number of living cells and was determined by measuring the absorbance at 450 nm with a multi-mode plate reader (SYNERGY-HTX, BioTek Instruments, USA). IC50 values were calculated by probit regression with GraphPad software.
cDNA microarray assay
HK1 and HK1-EBV cells were treated with 0.1% DMSO or 100 µM CAPE for 24 hrs. cDNA microarray was performed at Shanghai Genminix Informatics. The genes with differential expression were screened, and further GO and KEGG pathway analysis were performed by using the GCBI online platform. The Gene Expression Omnibus (GEO) accession number for the data sets reported in this paper is GSE126608. The following link has been created to allow review of record while it remains in private status:
Cell apoptosis assay and measurement of caspase3/7 activity
Cells were seeded at 3×105 cells/well in 6-well plates for 24 hrs. After CAPE treatment for 24 hrs, cells were harvested. Cell apoptosis was measured by using the APC-Annexin V apoptosis detection kit (BD Biosciences, San Diego, CA) according to the manufacturer’s instructions and analyzed by flow cytometry (Becton Dickinson, San Jose, CA, USA). Caspase-3/7 activity of NPC cells was measured by using the Caspase-Glo 3/7 Assay Kit (Promega Biotech, USA) according to the manufacturer’s instructions. Luminescence of cell lysis was determined by using a multi-mode reader (BioTek SYNERGY-HTX Instruments, USA). Three independent experiments were performed for each assay.
Cell cycle assay
Cells with and without CAPE treatment (with IC50 concentration of each cell line) for 24 hrs were harvested, washed with cold phosphate buffered saline (PBS) once, then fixed with 75% cold ethanol for 2 hrs or overnight at 4°C (samples can be stored at −20°C for 1 month). Before staining, fixed cells were harvested and washed in cold PBS. An amount of 100 μL RNase A per sample was added to resuspend fixed cells precipitated in 37°C water bath for 30 mins, and then PI solution was added at 4°C for 30 mins. The percentage distribution of cells in the phases of the cell cycle was determined by flow cytometry with the Cell Cycle Detection Kit (Kaiji, Nanjing, China). Three independent experiments were performed for each assay.
Western blot analysis
Cells were lysed on ice for 30 mins in RIPA with protease/phosphatase inhibitor cocktail. The protein concentration was measured with a BCA assay kit (Beyotime, Shanghai). Protein samples were separated by SDS-PAGE gel and transferred to NC membrane. The membrane was blocked with 5% bovine serum albumin and incubated with primary antibodies at 4°C overnight. Proteins of interest were detected with appropriate IRDyeTM 800/700CW secondary antibodies and detected by using an infrared imaging system (LI-COR Odyssey) (Thermo Scientific, Rockford, IL, USA). Protein levels were normalized to that of GAPDH and quantified by using Image J (US National Institutes of Health).
Colony-formation assay
Colony-formation assay was used to assess the growth inhibitory effects of CAPE on cells in six-well tissue culture plates. Appropriate numbers of NPC cells were seeded into culture plates, then treated with 20 and 40 µM CAPE for 24 hrs. Plates were maintained in fresh culture medium containing 10% FBS for 10–14 days. Finally, colonies were fixed with 4% paraformaldehyde solution and stained with 1% crystal violet dye; colonies containing more than 50 cells were counted. Experiments were repeated at least three times.
Clonogenic survival assay
Cells in log phase were counted, and appropriate numbers of NPC cells were seeded into six-well plates in triplicate. The number of cells plated was increased depending on the radiation dose for obtaining a countable number of colonies. After 24 hrs, to calculate radiosensitivity effects, cells were pre-incubated with CAPE for 24 hrs, then exposed to graded doses of X-ray radiation. Irradiation involved use of a 6-MeV X-ray photon produced by a linear accelerator (Elketa, Sweden) at 200 cGy/min. Colony staining was performed as described previously. The surviving cell fraction was calculated as number of colonies/(number of cells × control plating efficiency). All experiments were performed three times.
Transwell assay and wound-healing assay
The invasiveness of NPC cells was evaluated in 24-well transfected chambers (Costar, Corning, MA, USA) according to the manufacturer’s instructions. Briefly, transwell chambers with an 8-µm diameter pore membrane were coated with 200 µL Matrigel at 200 µg/mL and incubated overnight. Cells (7×104) in 200 µL serum-free medium were seeded into the upper transwell chamber and the lower chamber was filled with 800 µL DMEM containing 10% FBS to induce chemotaxis. After 24–48 hrs of incubation at 37°C in a humidified 5% CO2 atmosphere, cells were fixed in paraformaldehyde and stained with crystal violet, and cells that invaded the pores to the lower surface of the filter were counted under a microscope. Three invasion chambers were used per condition. The values obtained were calculated by averaging the total number of cells from three filters. Wound healing assay was performed with iBidi Culture–Inserts (iBidi, Germany). NPC cells treated with or without CAPE were seeded in the space of Culture–Inserts; after 12-hr attachment, the insert was removed, then cell patches were overlayed with culture medium. A single wound was created in the plate and wound areas were visualized under an optical microscope at magnification ×100. Cell migration capability was expressed by gap closure.
Statistical analysis
Statistical analysis was performed with SPSS 22.0 (SPSS Inc., Chicago, IL, USA). All data are presented as mean±SD of at least three independent experiments. Comparisons between two groups involved Independent samples t test. p<0.05 was considered statistically significant.
Results
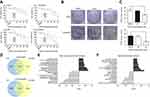
CAPE suppressed the proliferation and colony-formation ability of NPC cells
To investigate the effect of CAPE on growth of NPC cells, we used CCK8 assay with CNE2, CNE2-EBV, HK1 and HK1-EBV cell lines treated or not with CAPE at different concentrations. As compared with the DMSO control, with increasing CAPE concentration and time of treatment, the viability of these cells remarkably decreased (Figure 1A). The IC50 values for CAPE with 24-hr treatment were calculated for the cells: CNE2 (110 μM), CNE2-EBV (80 μM), HK1 (110 μM) and HK1-EBV (110 μM). As compared with DMSO treatment, with 40 μM CAPE treatment for 24 hrs, lower than the IC50, CNE2 and CNE2-EBV cells produced fewer colonies (Figure 1B and C), which suggests that the colony-formation ability of NPC cells was significantly suppressed by CAPE. With 20 µM CAPE, the colony number of NPC cells was reduced but not significantly. Non-malignant nasopharyngeal epithelial cell lines NP69 and NP460 were more resistant to CAPE treatment (data not shown).
Differentially expressed genes regulated by CAPE in NPC cells were mainly involved in apoptosis and cell cycle
To understand the suppressive effect of CAPE on NPC cell lines, we used cDNA microarray assay to screen NPC cell gene profiles regulated by CAPE. We found 4844 and 4919 differentially expressed genes (DEGs) in HK1 and HK1-EBV cell lines, respectively: 1244 genes were downregulated and 1181 were upregulated more than 1.2-fold in both cell lines (Figure 1D). The top 10 significantly upregulated and downregulated GO categories were detected by the negative logarithm of the p-value (Figure 1E). Downregulated DEGs were mainly involved in the regulation of cell proliferation, such as the mitotic cell cycle, M phase of mitotic cell cycle and mitosis. Upregulated DEGs were involved in tumor cell physiological processes including endoplasmic reticulum unfolded protein response, activation of signaling protein activity involved in unfolded protein response, cellular protein metabolic process, protein transport, small molecule metabolic process, protein N-linked glycosylation via asparagine, post-translational protein modification, signal transduction, type I interferon-mediated signaling pathway and apoptotic process. To identify the key pathways that DEGs were involved in, we used KEGG pathway analysis. The cell cycle pathway was included in the top 10 significant pathways of downregulated DEGs (Figure 1F). Therefore, the cell cycle pathway plays an important role in CAPE treatment. Next, we further analyzed CAPE-induced change of cell cycle and cell apoptosis.
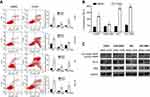
CAPE-induced NPC cell apoptosis
From the microarray analysis, to explore whether the decreased cell viability with CAPE treatment resulted from inducing apoptosis, we evaluated the apoptosis of NPC cells with or without CAPE treatment by flow cytometry. As compared with the DMSO control, after treatment with IC50 doses of CAPE for 24 hrs, the proportion of total apoptotic cells was increased: CNE2, 23.99±9.17%, p=0.068; CNE2-EBV, 9.98±1.24%, p=0.007; HK1, 41.05±13.33%, p=0.037; HK1-EBV, 15.05±1.60%, p=0.001. CAPE treatment mainly increased later-stage apoptosis (Figure 2A). With CAPE treatment, caspase 3/7 activity was markedly increased (Figure 2B), which suggests that caspase3/7 are involved in CAPE-induced apoptosis in NPC cells. To investigate the mechanism by which CAPE causes the increase in apoptosis, we analyzed the expression of related proteins that may be involved in the apoptotic pathway, such as cleaved poly ADP ribose polymerase (PARP), Bax and Bcl-xl (Figure 2C). The protein level of cleaved PARP was upregulated in NPC cells exposed to CAPE. The expression of Bcl-xl protein was downregulated and that of Bax protein was upregulated (Figure 2C).
CAPE-induced G1 cell cycle arrest in NPC cells
To understand the effect of CAPE in the cell cycle of NPC cells, we analyzed the distribution of cells in the phases of the cell cycle by flow cytometry. We found reduced number of cells in the G2/M phase and increased number in the G1 phase in CNE2 and CNE2-EBV cells under CAPE treatment (Figure 3A). In HK1 cells, CAPE treatment led to an increase in the G1-phase population and a decrease in the S-phase population (Figure 3A), with no significant effect in HK1-EBV cells. Our data suggest that CAPE induced G1 cell cycle arrest in CNE2, CNE2-EBV and HK1 cells. CDK6 is an important regulatory factor for cell-cycle G1 phase progression and G1/S transition,21 and CDK4 has been shown to phosphorylate and thus regulate the activity of the tumor suppressor protein Rb. In the early phase of the cell cycle, G1 transition is mainly regulated by cyclin D1 complexed with CDK4 and/or CDK6.21 Our NPC cells showed downregulated Rb, p-Rb, CDK4, and CDK6 with CAPE treatment (Figure 3B and C).
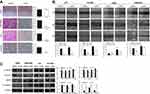
CAPE inhibits the invasion and migration of NPC cells in vitro
To further determine the effect of CAPE on NPC cell migration and invasive abilities, we used transwell and wound-healing assay. CAPE treatment markedly attenuated the migratory ability in CNE2-EBV, HK1 and HK1-EBV but not CNE2 cells (Figure 4A). Similarly, on wound-healing assay, cellular motility was significantly slower with CAPE than DMSO treatment (Figure 4B). Thus, CAPE could inhibit NPC cell migration and invasion in vitro. Western blot analysis revealed that the expression of E-cadherin was elevated in CNE2-EBV (p=0.008), HK1 (p=0.017) and HK1-EBV (p=0.001), vimentin was remarkably downregulated in all of four cell lines, β-catenin was decreased in CNE2 (p=0.009) and CNE2EBV (p=0.018), while MMP9 was decreased in HK1 (p=0.035) and HK1-EBV (P=0.033) upon the treatment of CAPE (Figure 4C). Our data suggest that CAPE hinders migratory and invasive ability of NPC cells by reversing the epithelial–mesenchymal transition (EMT) pathway.
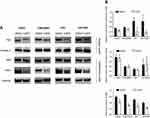
CAPE impeded nuclear translocation of p65 in NPC cells
Because CAPE has been reported as an NF-κB inhibitor, we thus analyzed the expression of p65 in both cytoplasm and nucleus in NPC cell lines after CAPE treatment. The expression of IκB-α was decreased significantly (Figure 5A and B), which indicated that more p65 should be free to transfer to the nucleus and act as a transcriptional factor. However, we found a marked reduction of p65 level in the nuclear fraction but not cytoplasm (Figure 5), so CAPE mainly hindered the nuclear translocation of p65, thereby suppressing the NF-κB signaling pathway. The underlying mechanism remains to be further elucidated.
CAPE enhanced the sensitivity of radiation and cisplatin in NPC cell lines rendering NPC cells more sensitive to cisplatin
To determine the effect of CAPE on the radiosensitivity of NPC cells, CNE2 and CNE2-EBV cells were pretreated with CAPE (20 and 40 μM) for 24 hrs, then exposed to ionizing radiation (IR). Treatment with CAPE (40 μM) led to significant radiosensitization in both CNE2 and CNE2-EBV cells (Figure 6A). The sensitizing enhancement ratios (SERs) at a surviving fraction of 40 were 1.228 for CNE2 and 1.915 for CNE2-EBV cells. Clonogenic analysis showed that CAPE 20 μM treatment significantly decreased the clonogenic survival of CNE2EBV cells combined with 6 Gy X-ray irradiation in a synergistic manner. In contrast, CNE2 cells showed no radiosensitivity enhancement with CAPE 20 μM. CAPE also conferred sensitivity to cisplatin in NPC cells (CNE2, CNE2-EBV, HK1 and HK1-EBV). As compared with cisplatin treatment, CAPE combination therapy increased cisplatin sensitivity and decreased the cell viability of NPC cells (Figure 6B).
Discussion
The anti-cancer effect of CAPE has been reported in several cancers in vivo and in vitro but not in NPC. In this study, we observed dose-dependent growth inhibition of CAPE in NPC cell lines. CAPE reduced the proliferation of ovarian cancer cells via apoptosis induction, with activation of pro-apoptotic genes (BAD, CASP8, FAS, FADD, p53).22 By activating oxidative stress, CAPE promotes apoptosis in human multiple myeloma cells.23 Colorectal cancer cells also showed significant proliferation inhibition and induced cell cycle arrest and apoptosis by decreasing the expression of beta-catenin and the associated signaling pathway target genes.24 CAPE also induced cell cycle arrest in LNCaP human prostate cancer cell lines and affected the abundance and phosphorylation of proteins involved in PI3K-Akt signaling pathways. In vivo, CAPE retarded the growth of LNCaP 104-R1 xenografts in a nude mouse model without notable toxicity.25 In addition, CAPE significantly decreased the cell viability of many other cancer cells, including lung carcinoma, fibrosarcoma, melanoma, osteosarcoma, breast carcinoma and oral cancer.26 In line with other studies, we found that CAPE significantly induced cell cycle arrest and apoptosis.
The EMT is crucial to the progression and metastasis of various cancers.27,28 NPC is an EBV-associated malignancy typically characterized by early metastasis, and metastasis is also a main reason for relapse and death of NPC patients. CAPE effectively suppressed the invasive ability of NPC cells in vitro, which suggests that CAPE is a novel metastatic suppressor for NPC. In addition, we found that the epithelial marker E-cadherin was upregulated and the mesenchymal marker vimentin was downregulated in NPC cell lines with CAPE treatment. Thus, CAPE inhibited cell invasion and metastasis by reversing EMT-like changes in our NPC cells.
Radio-resistance is a major cause of treatment failure in NPC. Radiotherapy, especially intensity-modulated radiation therapy, is the principle and first choice for non-disseminated NPC because of its anatomical location and radiosensitivity.29 Enhancing the radiosensitivity of NPC may improve the efficacy of radiotherapy in patients with NPC and provide a novel treatment strategy for NPC. We found that CAPE treatment combined with irradiation significantly decreased the number of colonies relative to irradiation alone in CNE2 and CNE2-EBV cells, so CAPE could enhance the radiosensitivity of NPC. The sensitization of cancer cells to irradiation increased by CAPE has focused on lung cancer and colorectal adenocarcinoma cells, by depleting intracellular glutathione and inhibiting NF-κB signaling.10,30 Previous studies have demonstrated that CAPE inhibits clone-forming capacity, maintains radiation-induced DNA damage and acts as a radiosensitizer in breast cancer.11 Cisplatin (CDDP) is the conventional chemotherapeutic for NPC treatment and frequently used for distant metastasis or advanced locoregional recurrence.31 Cisplatin, as a basic chemotherapeutic agent, can enhance the effectiveness of radiotherapy when combined with other drugs. We also found that CAPE could enhance the sensitivity of NPC cells to chemotherapeutics. Further studies are needed to explore the molecular mechanisms of the radiotherapy and chemotherapy sensitivity influenced by CAPE.
Once NF-κB signaling is stimulated, signal transduction activates the IKK complex, which phosphorylates IκBα, leading to its polyubiquitination and subsequent proteasomal degradation. Subsequently, the p65/p50 heterodimer translocates into the nucleus and regulates the downstream NF-κB signaling pathway. This signaling contributes to tumorigenesis of NPC, which is closely associated with EBV infection and massive lymphoid infiltration. Aberrant activation of NF-κB signaling leads to abnormal expression of various genes regulating apoptosis, proliferation, metastasis, inflammation and radiotherapy resistance,32 which is a legitimate target for cancer therapy.33 Different molecule inhibitors, for targeted therapies of the NF-κB pathway, have been discovered and investigated in clinical trials. Bay 11-7082, a highly selective NF-κB inhibitor, mediated blockade of NF-κB by directly inhibiting IKK and enhanced drug sensitivity in therapy-resistant epithelial ovarian cancer cells.34 Bortezomib, a proteasome inhibitor, suppressed NF-κB activity by preventing the degradation of phospho-IκBa. In vitro, bortezomib reduced the viability of EBV-positive and -negative B cells by inducing apoptosis, and in vivo, inhibited the development of EBV B-cell lymphomas in severe combined immunodeficiency mice. We found that the expression of p65 in cytoplasm of NPC cells, both EBV positive and negative, remained under the condition of degradation of IκBα, but the level in the nucleus was reduced by CAPE treatment. Thus, CAPE may inhibit the p65-dependent activation of NF-κB.
CAPE occurs naturally in propolis. The good bioavailability through the oral route and the good historical safety profile of propolis makes CAPE an ideal adjuvant agent for future NPC treatment.
Conclusion
In conclusion, our data support that CAPE suppresses the proliferation, survival, metastasis of human NPC cells by inhibiting the NF-κB signaling pathway. CAPE could be a radiosensitizer for NPC and thus could be combined with radiotherapy for NPC treatment. Thus, we suggest CAPE as a potential adjuvant anti-tumor drug for the treatment of NPC.
Acknowledgments
This work was supported by the grants from the National Natural Science Foundation of China (grant no 81460412 and 81560439); Guangxi Natural Science Foundation (grant no 2015GXNSFBA139143); Promotion of Basic Ability of Young and Middle-aged Teachers in Universities in Guangxi (grant no KY2016YB080) and Guangxi Medical University Students Innovative Project (grant no 2018263)
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Chang ET, Adami HO. The enigmatic epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol Biomarkers Prev. 2006;15(10):1765–1777. doi:10.1158/1055-9965.EPI-06-0353
2. Tao Q, Chan AT. Nasopharyngeal carcinoma: molecular pathogenesis and therapeutic developments. Expert Rev Mol Med. 2007;9(12):1–24. doi:10.1017/S1462399407000312
3. Jin Y, Shi YX, Cai XY, et al. Erratum to: comparison of five cisplatin-based regimens frequently used as the first-line protocols in metastatic nasopharyngeal carcinoma. J Cancer Res Clin Oncol. 2015;141(4):767. doi:10.1007/s00432-015-1926-1
4. Kang NJ, Shin SH, Lee HJ, Lee KW. Polyphenols as small molecular inhibitors of signaling cascades in carcinogenesis. Pharmacol Ther. 2011;130(3):310–324. doi:10.1016/j.pharmthera.2011.02.004
5. Yildirim O, Yilmaz A, Oz O, et al. Effect of caffeic acid phenethyl ester on treatment of experimentally induced methicillin-resistant Staphylococcus epidermidis endophthalmitis in a rabbit model. Cell Biochem Funct. 2007;25(6):693–700. doi:10.1002/cbf.1377
6. Wang P, Liu C, Sanches T, et al. Design and synthesis of novel nitrogen-containing polyhydroxylated aromatics as HIV-1 integrase inhibitors from caffeic acid phenethyl ester. Bioorg Med Chem Lett. 2009;19(16):4574–4578. doi:10.1016/j.bmcl.2009.06.100
7. Hsu TH, Chu CC, Hung MW, Lee HJ, Hsu HJ, Chang TC. Caffeic acid phenethyl ester induces E2F-1-mediated growth inhibition and cell-cycle arrest in human cervical cancer cells. FEBS J. 2013;280(11):2581–2593. doi:10.1111/febs.12242
8. Kuo YY, Jim WT, Su LC, et al. Caffeic acid phenethyl ester is a potential therapeutic agent for oral cancer. Int J Mol Sci. 2015;16(5):10748–10766. doi:10.3390/ijms160510748
9. Liao HF, Chen YY, Liu JJ, et al. Inhibitory effect of caffeic acid phenethyl ester on angiogenesis, tumor invasion, and metastasis. J Agric Food Chem. 2003;51(27):7907–7912. doi:10.1021/jf034729d
10. Chen YJ, Liao HF, Tsai TH, Wang SY, Shiao MS. Caffeic acid phenethyl ester preferentially sensitizes CT26 colorectal adenocarcinoma to ionizing radiation without affecting bone marrow radioresponse. Int J Radiat Oncol Biol Phys. 2005;63(4):1252–1261. doi:10.1016/j.ijrobp.2005.08.001
11. Khoram NM, Bigdeli B, Nikoofar A, Goliaei B. Caffeic acid phenethyl ester increases radiosensitivity of estrogen receptor-positive and -negative breast cancer cells by prolonging radiation-induced DNA damage. J Breast Cancer. 2016;19(1):18–25. doi:10.4048/jbc.2016.19.1.18
12. Chiang KC, Yang SW, Chang KP, et al. Caffeic acid phenethyl ester induces N-myc downstream regulated gene 1 to inhibit cell proliferation and invasion of human nasopharyngeal cancer cells. Int J Mol Sci. 2018;19(5):1397. doi:10.3390/ijms19051397
13. Baud V, Karin M. Is NF-kappaB a good target for cancer therapy? Hopes and pitfalls. Nat Rev Drug Discov. 2009;8(1):33–40. doi:10.1038/nrd2781
14. DiDonato JA, Mercurio F, Karin M. NF-kappaB and the link between inflammation and cancer. Immunol Rev. 2012;246(1):379–400. doi:10.1111/j.1600-065X.2012.01099.x
15. Shair KHY, Reddy A, Cooper VS. New insights from elucidating the role of LMP1 in nasopharyngeal carcinoma. Cancers. 2018;10(4):86. doi:10.3390/cancers10040086
16. Li YY, Chung GT, Lui VW, et al. Exome and genome sequencing of nasopharynx cancer identifies NF-kappaB pathway activating mutations. Nat Commun. 2017;8:14121. doi:10.1038/ncomms14121
17. Zheng H, Dai W, Cheung AK, et al. Whole-exome sequencing identifies multiple loss-of-function mutations of NF-kappaB pathway regulators in nasopharyngeal carcinoma. Proc Natl Acad Sci U S A. 2016;113(40):11283–11288. doi:10.1073/pnas.1607606113
18. Chung GT, Lou WP, Chow C, et al. Constitutive activation of distinct NF-kappaB signals in EBV-associated nasopharyngeal carcinoma. J Pathol. 2013;231(3):311–322. doi:10.1002/path.4239
19. Natarajan K, Singh S, Burke TR
20. Lo AK, Lo KW, Tsao SW, et al. Epstein-Barr virus infection alters cellular signal cascades in human nasopharyngeal epithelial cells. Neoplasia. 2006;8(3):173–180. doi:10.1593/neo.05625
21. Kozar K, Sicinski P. Cell cycle progression without cyclin D-CDK4 and cyclin D-CDK6 complexes. Cell Cycle. 2005;4(3):388–391. doi:10.4161/cc.4.3.1551
22. Gherman C, Braicu OL, Zanoaga O, et al. Caffeic acid phenethyl ester activates pro-apoptotic and epithelial-mesenchymal transition-related genes in ovarian cancer cells A2780 and A2780cis. Mol Cell Biochem. 2016;413(1–2):189–198. doi:10.1007/s11010-015-2652-3
23. Marin EH, Paek H, Li M, et al. Caffeic acid phenethyl ester exerts apoptotic and oxidative stress on human multiple myeloma cells. Invest New Drugs. 2018. doi:10.1007/s10637-018-0701-y
24. He YJ, Liu BH, Xiang DB, Qiao ZY, Fu T, He YH. Inhibitory effect of caffeic acid phenethyl ester on the growth of SW480 colorectal tumor cells involves beta-catenin associated signaling pathway down-regulation. World J Gastroenterol. 2006;12(31):4981–4985.
25. Lin HP, Lin CY, Huo C, et al. Caffeic acid phenethyl ester induced cell cycle arrest and growth inhibition in androgen-independent prostate cancer cells via regulation of Skp2, p53, p21Cip1 and p27Kip1. Oncotarget. 2015;6(9):6684–6707. doi:10.18632/oncotarget.3246
26. Wadhwa R, Nigam N, Bhargava P, et al. Molecular characterization and enhancement of anticancer activity of caffeic acid phenethyl ester by gamma cyclodextrin. J Cancer. 2016;7(13):1755–1771. doi:10.7150/jca.15170
27. Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7(2):131–142. doi:10.1038/nrm1835
28. Liang X. EMT: new signals from the invasive front. Oral Oncol. 2011;47(8):686–687. doi:10.1016/j.oraloncology.2011.04.016
29. Xu C, Chen YP, Ma J. Clinical trials in nasopharyngeal carcinoma-past, present and future. Chin Clin Oncol. 2016;5(2):20. doi:10.21037/cco.2016.03.12
30. Chen MF, Wu CT, Chen YJ, Keng PC, Chen WC. Cell killing and radiosensitization by caffeic acid phenethyl ester (CAPE) in lung cancer cells. J Radiat Res. 2004;45(2):253–260.
31. Gu MF, Liu LZ, He LJ, et al. Sequential chemoradiotherapy with gemcitabine and cisplatin for locoregionally advanced nasopharyngeal carcinoma. Int J Cancer. 2013;132(1):215–223. doi:10.1002/ijc.27638
32. Ahn KS, Aggarwal BB. Transcription factor NF-kappaB: a sensor for smoke and stress signals. Ann N Y Acad Sci. 2005;1056:218–233. doi:10.1196/annals.1352.026
33. Arteaga CL. EGF receptor mutations in lung cancer: from humans to mice and maybe back to humans. Cancer Cell. 2006;9(6):421–423. doi:10.1016/j.ccr.2006.05.014
34. Momeny M, Yousefi H, Eyvani H, et al. Blockade of nuclear factor-kappaB (NF-kappaB) pathway inhibits growth and induces apoptosis in chemoresistant ovarian carcinoma cells. Int J Biochem Cell Biol. 2018;99:1–9. doi:10.1016/j.biocel.2018.03.015
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.