Back to Journals » Cancer Management and Research » Volume 12
CAB39 Promotes the Proliferation of Nasopharyngeal Carcinoma CNE-1 Cells via Up-Regulating p-JNK
Authors Peng L, Yan H, Qi S , Deng L
Received 4 March 2020
Accepted for publication 13 May 2020
Published 4 November 2020 Volume 2020:12 Pages 11203—11209
DOI https://doi.org/10.2147/CMAR.S252476
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Eileen O'Reilly
Lifen Peng,1 Hailan Yan,2 Shuyi Qi,3 Lifei Deng4
1Department of Otolaryngology, Jiangxi Provincial People’s Hospital Affiliated to Nanchang University, Nanchang, People’s Republic of China; 2Department of Clinical Medicine, Xi’an Jiaotong University Health Science Center, Xi’an, People’s Republic of China; 3Department of Pathology, Jiangxi Provincial Cancer Hospital, Nanchang, People’s Republic of China; 4Head and Neck Cancer Surgery, Jiangxi Provincial Cancer Hospital, Nanchang, People’s Republic of China
Correspondence: Lifei Deng
Head and Neck Cancer Surgery, Jiangxi Provincial Cancer Hospital, Nanchang 330006, People’s Republic of China
Tel +86 13657099876
Email [email protected]
Aim: To investigate the role of CAB39 in nasopharyngeal carcinoma (NPC) development and examine its expression level in NPC tumor samples.
Methods: Immunohistochemistry staining of NPC tissue microarray was conducted to detect the expression of CAB39 protein in NPC tissues, and the clinical significance of CAB39 was evaluated. Lentivirus-mediated over-expression of CAB39 was designed to increase CAB39 expression in CNE-1 cells. Cell colony formation, cell cycle and CCK-8 proliferation experiments were performed to compare the proliferation ability of CNE-1 cells with or without CAB39 over-expression. Western blotting was conducted to examine downstream targets of CAB39.
Results: CAB39 expression was higher in tumor samples compared to normal tissue and the higher CAB39 expression was positively correlated to higher TNM stage and distant metastasis rate and non-keratinized state. Further, CAB39 over-expression dramatically increased the proliferation and colony formation of CNE-1 cells. Finally, higher p-JNK protein level was found in CAB39 over-expressing cells.
Conclusion: CAB39 promotes the proliferation of CNE-1 cells via up-regulating p-JNK.
Keywords: CAB39, proliferation, nasopharyngeal carcinoma, p-JNK
Introduction
Nasopharyngeal carcinoma (NPC) is a type of head and neck malignant tumors with a high prevalence in Southern China and Asia, which can be categorized into keratinizing and nonkeratinizing squamous cell carcinoma according to the World Health Organization.1–3 Because of delayed diagnosis, high distant metastasis rate and resistance to radiotherapy and chemoradiotherapy, most NPC patients suffer from a terrible prognosis after systematic treatment.4,5 Gene therapy is recently applied as a therapy option for NPC patients with metastatic and recurrent disease.6 However, limited targets have been identified in NPC, which highlights the urgency of exploring new targets.
CAB39 (calcium-binding protein 39) was originally thought to be a highly conserved protein that expressed during the early stage of mouse embryogenesis.7 With more research on it, CAB39 has been identified as an activated factor of STE20 kinase, and affects the evolution of various type of diseases, including cancers.7–15 For instance, Jiang et al7 identified CAB39 as an oncogene in hepatocellular carcinoma by promoting the growth and metastasis of hepatocellular carcinoma through ERK pathways. Godlewski et al14 reported that miR-451 can modulate the adaptation to metabolic stress in glioma through regulating CAB39. These experiments demonstrated that CAB39 may be a key factor in tumor progression. However, its role in NPC remains unknown.
We first examined CAB39 protein levels in 53 NPC tumor tissues and 16 normal nasopharyngeal epithelial tissues with immunohistochemistry (IHC) staining. Then, we over-expressed CAB39 by lentivirus infection in CNE-1 cells, a NPC cell line, and compared the proliferation ability of NCE1 cells with or without CAB39 over-expression. Finally, Western blotting was employed to examine potential downstream targets of CAB39 in CNE-1 cells.
Materials and Methods
Specimens and Cell Lines
53 nasopharyngeal carcinoma tissues (31 keratinized squamous cell carcinoma cases and 22 non-keratinized squamous cell carcinoma cases) and 16 normal nasopharyngeal epithelial samples were obtained from volunteers with NPC who were diagnosed at People’s Hospital Affiliated to Nanchang University from January 2015 to December 2018. Tissue pathological grading was evaluated with reference to the 2002 Union for International Cancer Control (UICC) TMN staging and the World Health Organization (WHO) pathological grading standards. This project was approved by the Review Board of People’s Hospital Affiliated to Nanchang University. All participants enrolled signed informed consents.
NPC cell line (CNE-1) and 293T cell line were obtained from the Cell Bank of Chinese Academy of Sciences (Shanghai, China) and were cultured in DMEM (Corning, Manassas, VA, USA) with 10% FBS (Gibco, New York, USA) and penicillin/streptomycin in humidified atmosphere of 5% CO2 at 37°C.
Immunohistochemistry
IHC staining was conducted on 53 nasopharyngeal carcinoma tissues and 16 normal nasopharyngeal epithelial samples. Anti-CAB39 was used and detailed samples preparation and antibodies incubating procedures were performed as previously described by Yeo et al.16 Then, images were taken with a tissue chip scanner and analyzed using paired software. Histochemistry score (H-score) based on the percentage of positive cells and degree of staining was for the scoring of CAB39 expression as previously described by Yeo et al.16 Patients were divided into two groups (low and high expression) based on the overall scores.
Establish a Stable CAB39 Over-Eexpression CNE-1 Cell Line
Lentiviral vectors were employed for CNE-1 cell transduction, and puromycin was hired to screen out stable CAB39 over-expression CNE-1 cells. All procedures were performed as previously described by Fang et al.17 The RPS15A-specific sequence and the negative control sequence were 5′GCTTCTCGGTGAACTACTACT3′ and 5ʹTTCTCCGAACGTGTCACGT3ʹ, respectively. CNE-1 cells were classified into three groups according to whether the sequences were infected and the type of sequences infected, which are sh-OE (RPS15A-specific sequence), sh-NC (the negative control sequence) and blank (without sequence) groups. Quantitative real-time PCR (qRT-PCR) and Western blotting were used to measure the infection efficiency.
Quantitative Real-Time PCR (qRT-PCR)
Total RNA was extracted from sh-OE and sh-NC CNE-1 cells and then reversed to cDNA using Trizol reagent (invitrogen, California, USA) and 2×EasyTaq PCR Super Mix kit (TransGen Biotech, Beijing, China), respectively. qPCR was conducted using the SYBR Master Mixture (TAKARA, Dalian, China). All experiments were based on the manufacturer’s protocol provided. β-actin was used as an endogenous control and quantification of CAB39 mRNA was calculated using 2−ΔΔCt method. The forward and reverse primer sequences for CAB39 were 5ʹGCATTTGCCACATTCAAGGATT3ʹ and 5ʹGCTGCGTCTTGTTAGGATTGG3ʹ, respectively. The forward and reverse primers sequences for β-actin were 5ʹCACCCAGCACAATGAAGATCAAGAT3ʹ and 5ʹCCAGTTTTTAAATCCTGAGTCAAGC3ʹ, respectively.
Western Blotting Assay
Total protein was extracted from sh-OE, sh-NC and blank CNE-1 cells and measured using cell membrane and cytoplasmic protein extraction kit and BCA protein concentration kit (Beyotime Biotechnology, Shanghai, China), respectively. This process was performed as previously described by Steinberger et al.18 Then, extracted proteins were transferred onto PVDF membranes, and primary and secondary antibodies were added in turn. The electrophoresis process was performed as previously described by Steinberger et al.18 The primary antibodies used here were anti-JNK (abcam, USA), anti-p-JNK (abcam, USA). Anti-β-actin (Servicebio, Wuhan, China, Cat. GB12001) was hired as an endogenous control.
Cell Counting Kit-8 (CCK-8) Assay
Blank, sh-OE- and sh-NC- CNE-1 cells were cultured in 96-well plates with 2×103 cells in each well, and 150µL medium were added into each well for cell culturing. 15µL CCK-8 solution, which was purchased from KeyGEN BioTECH company (Jiangsu China), was added into each well and cells were incubated at 37°C for 2 hours. The absorbance at 490nm was measured at 24h, 48h and 72h.
Cell Colony Formation Assay
Cell colony formation assay was conducted according to the manuscript handbook. Blank, sh-OE- and sh-NC- CNE-1 cells were seeded into 6-well plates with 600 cells in each well, and were well cultured for 14 days. Then, 1 mL 4% paraformaldehyde was added into each well to fix cells for 50 min. Finally, 1000µL crystal violet dye solution (0.1%) was seeded into each well and incubated for 20 min, and colonies were counted after washing the cells with ddH2O for several times.
Cell Cycle Assay
Cell cycle was measured using flow cytometry (FACS) (BD Biosciences, San Jose, CA, USA) technology. In brief, collected blank, sh-OE- and sh-NC- CNE-1 cells were fixed by 70% ethanol at 4°C overnight and stained by propidium iodide at 4°C for 30min. Then, cell cycle analysis was performed by FACS.
Statistic Analysis
Three experimental replicates were performed for each experiment. Data were expressed as mean ± standard deviation (SD). Comparisons were performed by two-sided independent Student’s test. The analysis was performed using SPSS software version 20.0 (IBM SPSS Inc, Chicago, IL, USA). Statistical significance was accepted when a P value is less than 0.05.
Results
CAB39 Protein Expression Enhanced in NPC Tissues
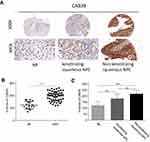
To measure CAB39 protein levels in NPC tumor tissues, IHC staining of CAB39 was performed for 53 nasopharyngeal carcinoma tissues and 16 normal nasopharyngeal epithelial samples. As shown in Figure 1A and B, increased CAB39 protein expression was detected in NPC tissues compared to normal ones (P<0.001). The positive CAB39 staining in NPC and normal specimens were 81.1% (45/53) and 25.0% (4/16), respectively.
Further, a subgroup analysis was performed to compare CAB39 expression in keratinizing and non-keratinizing NPC samples. As shown in Figure 1A and C, H-score and positive rates of CAB39 protein levels increased in non-keratinizing NPC tissues than those in keratinizing NPC samples (P<0.01). The positive expressive rates of CAB39 in non-keratinizing NPC and keratinizing NPC specimens were 95.5% (21/22) and 77.4% (24/31), respectively. Overall, enhanced CAB39 protein expression was detected in NPC tissues, especially in non-keratinizing NPC tissues.
The Correlation of CAB39 Expression with Clinical-Pathological Parameters of NPC Patients
To determine the clinical significance of CAB39 in NPC, the enrolled 53 nasopharyngeal carcinoma volunteers were divided into two groups based on the results of tissue array IHC staining-29 patients with high CAB39 expression and 24 patients with low CAB39 expression. The clinical details are presented in Table 1. There is no correlation between CAB39 levels and age and gender (both P>0.05). However, there is a positive correlation indicating that higher CAB39 expression is associated with higher TNM stage (P=0.022), distant metastasis rate (P=0.019) and non-keratinized state (P=0.026).
 |
Table 1 Correlation Between CAB39 and Clinicopathological Characteristics of NPC Patients |
Over-Expression of CAB39 Promotes the Proliferation of CNE-1 Cells
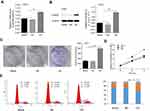
To study the biological function of CAB39 in NPC, stable CAB39 over-expressing CNE-1 cells were established. The transfection efficiency was determined by qPCR and Western blotting. CAB39 mRNA (P<0.01) and protein (P<0.001) expression increased by approximately 1.5- and 10-fold after transduction, respectively (Figure 2A and B).
Cell proliferation and colony formation of CNE-1 cells with or without CAB39 over-expression were measured using CCK-8 proliferation assay, FACS and cell colony formation assay. CAB39 over-expression promoted the cell proliferation of CNE-1 cells as shown by CCK-8 proliferation assay (Figure 2D, P<0.01) and enhanced the colony formation capacity as shown by the cell colony formation assay (Figure 2C, p<0.01). Moreover, the FACS analysis of CNE-1 cells showed that more cells are in G1 and S phase (indicative of proliferation) and less cells are in G2 phase after over-expressing CAB39 (Figure 2E) (P<0.001). Specially, the proliferation ability of CAB-39 and sh-CAB-39-NC cells was similar as shown by all the three experiments (Figure 2C–E) (all P>0.05). Therefore, over-expression of CAB39 promotes the proliferation of CNE-1 cells.
Downstream Targets of CAB39 in CNE-1 Cells
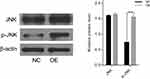
To analyze the potential mechanism of CAB39 in promoting the proliferation of CNE-1 cells, the protein levels of JNK and p-JNK were analyzed after over-expressing CAB39. As shown in Figure 3, although the total level of JNK protein in sh-OE-CAB39 and sh-NC-CAB39 cells was similar, the levels of p-JNK protein in sh-OE-CAB39 increased obviously (P<0.01). Overall, CAB39 could promote CNE-1 cells proliferation through positively regulating p-JNK protein expression.
Discussion
NPC is a malignant tumor with high distant metastasis rate and resistance to radiotherapy and chemoradiotherapy. Although gene therapy has brought hopes to NPC patients, it is still in its infancy due to the complex of gene interaction network. CAB39 (calcium-binding protein 39) is an activated factor of STE20 kinase that has been shown involved in the progression of various cancers, including hepatocellular carcinoma,7 gastric cancer,8 colorectal cancer,9,10 pancreatic cancer11 and glioma.12–15 However, its role in NPC is still elusive.
CAB39 has been identified as an oncogene and a favorable biomarker for the treatment of some cancers. Previous studies have shown an enhanced CAB39 expression in hepatocellular carcinoma and gastric cancer, and reported that CAB39 promotes the growth and metastasis of hepatocellular carcinoma, gastric cancer and colorectal cancer.7–10 Kelley et al10 measured the expression of CAB39 in rectal patients with or without response to radiotherapy and found a significantly higher CAB39 level in patients without response than those with complete or part response. However, CAB39 has been regarded as a tumor inhibitor in pancreatic cancer, which inhibits the proliferation and metastasis of pancreatic cancer cell lines (PANC-1, AsPC-1).11 The controversial view may due to the difference between these different types of tumors. Interestingly, the role of CAB39 in glioma has been studied in more detail. Two studies reported by Nan et al12 and Tian et al13 used LN229 and U87 cell lines and found that CAB39 promotes the progression of glioma. However, Godlewski et al14 concluded that CAB39 inhibits proliferation while promoting metastasis in LN229 cell line whereas Zhao et al15 drew a conclusion that CAB39 promotes proliferation while inhibiting metastasis in U87 cell line. More studies need to solve these differences. In this study, we found an increased CAB39 expression in NPC tissues and found that over-expressed CAB39 can promote the colony formation and proliferation of CNE-1 cells.
Limited researches have studied the mechanism of CAB39 in tumors. In detail, CAB39 can make up heterotrimers with serine/threonine kinase 11 (LKB1) and STE-related adaptor (STRAD). CAB39/STRAD/LKB1 cascade can modulate cell volume, immune response, cell migration, growth and apoptosis.19–22 Activated AMPK kinase adjusts cell morphology and polarity, thereby, promotes the conservation of energy and absorption of glucose.14,23 Hence, tumor cells can adapt to extreme adverse micro-environment occurred with the growth of tumors to keep surviving. Activated CAB39/STRAD/LKB1 can activate AMPK kinase. Thus, CAB39/STRAD/LKB1/AMPK cascade helps tumor cells to adjust themselves to adapt the micro-environment. The protective function of this cascade has been found in colorectal cancer9 and glioma.12,14,15 Besides that, some other oncogenes, including phosphoinositide 3-kinase (PI3K), AKT serine/threonine kinase 1 (AKT), mechanistic target of rapamycin kinase (mTOR), vascular endothelial growth factor (VEGF), matrix metalloproteinase 2 (MMP2) and matrix metalloproteinase 9 (MMP9), were also identified as downstream targets of CAB39 in glioma.12 Overall, CAB39 mainly functions as an activator of STRAD/LKB1 cascade to regulate tumor development. In this study, we detected the levels of JNK and p-JNK in CNE-1 cells with over-expression of CAB39, and found that p-JNK level increase after CAB39 over-expression.
There are several limitations in this study. Firstly, we only detected CAB39 protein levels in NPC, but failed to detect CAB39 mRNA expression in NPC. Secondly, animal experiments need to be done further verify the results in cells. Finally, only one NPC was enrolled for the experiment, and this study just preliminarily explores the mechanisms of CAB39 in NPC. All these limitations are what we need to solve in the future. Despite these limitations, we still can draw the conclusion that CAB39 protein expression is enhanced in NPC tumor tissues and CAB39 over-expression can promote the proliferation of CNE-1 cells via p-JNK.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Chua MLK, Wee JTS, Hui EP, et al. Nasopharyngeal carcinoma. Lancet. 2016;387(10022):
2. Li XH, Chang H, Xu BQ, et al. An inflammatory biomarker-based nomogram to predict prognosis of patients with nasopharyngeal carcinoma: an analysis of a prospective study. Cancer Med. 2017;6(1):310–319. doi:10.1002/cam4.947.
3. Kamran SC, Riaz N, Lee N. Nasopharyngeal carcinoma. Surg Oncol Clin N Am. 2015;24(3):547–561. doi:10.1016/j.soc.2015.03.008
4. Xu C, Zhang LH, Chen YP, et al. Chemoradiotherapy versus radiotherapy alone in stage II nasopharyngeal carcinoma: a systemic review and meta-analysis of 2138 patients. J Cancer. 2017;8(2):287–297. doi:10.7150/jca.17317
5. Huang CJ, Leung SW, Lian SL, et al. Patterns of distant metastases in nasopharyngeal carcinoma. Kaohsiung J Med Sci. 1996;12(4):
6. Hughes J, Alusi G, Wang Y. Gene therapy and nasopharyngeal carcinoma. Rhinology. 2012;50(2):115–121. doi:10.4193/Rhino11.239
7. Jiang L, Yan Q, Fang S, et al. Calcium binding protein 39 promotes hepatocellular carcinoma growth and metastasis by activating ERK signaling pathway. Hepatology. 2017;66(5):1529–1545. doi:10.1002/hep.29312
8. Xu Z, Li Z, Wang W, et al. MIR-1265 regulates cellular proliferation and apoptosis by targeting calcium binding protein 39 in gastric cancer and, thereby, impairing oncogenic autophagy. Cancer Lett. 2019;449:226–236. doi:10.1016/j.canlet.2019.02.026
9. Li HY, Zhang Y, Cai JH, et al. MicroRNA-451 inhibits growth of human colorectal carcinoma cells via downregulation of Pi3k/Akt pathway. Asian Pac J Cancer Prev. 2013;14(6):3631–3634. [PMID: 23886157]. doi:10.7314/APJCP.2013.14.6.3631
10. Kelley KA, Ruhl RA, Rana SR, et al. Understanding and resetting radiation sensitivity in rectal cancer. Ann Surg. 2017;266(4):610–616. doi:10.1097/SLA.0000000000002409 PMID: 28742699.
11. Guo R, Gu J, Zhang Z, et al. MiR-451 promotes cell proliferation and metastasis in pancreatic cancer through targeting CAB39. Biomed Res Int. 2017;2017:2381482. PMID: 28197410. doi:10.1155/2017/2381482
12. Nan Y, Guo H, Guo L, et al. MiRNA-451 inhibits glioma cell proliferation and invasion through the mTOR/HIF-1a/VEGF signaling pathway by targeting CAB39. Hum Gene Ther Clin Dev. 2018;29(3):156–166. doi:10.1089/humc.2018.133 PMID: 30180756.
13. Tian Y, Nan Y, Han L, et al. MicroRNA miR-451 downregulates the PI3K/AKT pathway through CAB39 in human glioma. Int J Oncol. 2012;40(4):1105–1112. doi:10.3892/ijo.2011.1306 PMID: 22179124.
14. Godlewski J, Nowicki MO, Bronisz A, et al. MicroRNA-451 regulates LKB1/AMPK signaling and allows adaptation to metabolic stress in glioma cells. Mol Cell. 2010;37(5):620–632. doi:10.1016/j.molcel.2010.02.018 PMID: 20227367.
15. Zhao K, Wang L, Li T, et al. The role of miR-451 in the switching between proliferation and migration in malignant glioma cells: AMPK signaling, mTOR modulation and Rac1 activation required. Int J Oncol. 2017;50(6):1989–1999. doi:10.3892/ijo.2017.3973 PMID: 28440461.
16. Yeo W, Chan SL, Mo FK, et al. Phase I/II study of temsirolimus for patients with unresectable Hepatocellular Carcinoma (HCC)-a correlative study to explore potential biomarkers for response. BMC Cancer. 2015;15:395. PMID: 25962426. doi:10.1186/s12885-015-1334-6
17. Fang KP, Dai W, Ren YH, et al. Both Talin-1 and Talin-2 correlate with malignancy potential of the human hepatocellular carcinoma MHCC-97 L cell. BMC Cancer. 2016;16:45. PMID:26822056. doi:10.1186/s12885-016-2076-9
18. Steinberger B, Brem G, Mayrhofer C. Evaluation of SYPRO Ruby total protein stain for the normalization of two-dimensional Western blots. Anal Biochem. 2015;476:17–19. doi:10.1016/j.ab.2015.01.015 PMID: 25640586.
19. Leberer E, Dignard D, Harcus D, et al. The protein kinase homologue Ste20p is required to link the yeast pheromone response G-protein beta gamma subunits to downstream signalling components. EMBO J. 1992;11(13):4815–4824. [PMID: 1464311]. doi:10.1002/j.1460-2075.1992.tb05587.x
20. Filippi BM, de Los Heros P, Mehellou Y, et al. MO25 is a master regulator of SPAK/OSR1 and MST3/MST4/YSK1 protein kinases. EMBO J. 2011;30(9):1730–1741. doi:10.1038/emboj.2011.78.
21. Dan I, Watanabe NM, Kusumi A. The Ste20 group kinases as regulators of MAP kinase cascades. Trends Cell Biol. 2001;11(5):220–230. doi:10.1016/S0962-8924(01)01980-8
22. Strange K, Denton J, Nehrke K. Ste20-type kinases: evolutionarily conserved regulators of ion transport and cell volume. Physiology (Bethesda). 2006;21:61–68. doi:10.1152/physiol.00139.2005
23. Lee JH, Koh H, Kim M, et al. Energy-dependent regulation of cell structure by AMP-activated protein kinase. Nature. 2007;447(7147):1017–1020. doi:10.1038/nature05828.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.