Back to Journals » Clinical Ophthalmology » Volume 12
C-reactive protein may be useful to differentiate idiopathic orbital inflammation and orbital cellulitis in cases with acute eyelid erythema and edema
Authors Nishikawa Y, Oku H , Tonari M, Matsuo J, Sugasawa J, Ikeda T
Received 2 February 2018
Accepted for publication 18 April 2018
Published 26 June 2018 Volume 2018:12 Pages 1149—1153
DOI https://doi.org/10.2147/OPTH.S164306
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Yuko Nishikawa, Hidehiro Oku, Masahiro Tonari, Junko Matsuo, Jun Sugasawa, Tsunehiko Ikeda
Department of Ophthalmology, Osaka Medical College, Takatsuki, Osaka, Japan
Purpose: Idiopathic orbital inflammation (IOI) and orbital cellulitis can present similar clinical features, and the diagnoses of these two disorders are sometimes confused. The purpose of the present study was to determine whether or not inflammatory markers in the blood can be useful to differentiate between IOI and orbital cellulitis in cases with acute eyelid erythema and edema.
Subjects and methods: In this retrospective single-institute study, we reviewed the medical records spanning the past 10 years at the Department of Ophthalmology, Osaka Medical College Hospital, Takatsuki, Osaka, Japan, and found 45 cases, with patients >15 years of age, with presumed IOI. Their blood samples were obtained within 5 days after the onset of IOI. Of those cases, 15 patients (10 males, 5 females, mean age of 56.9 years; range 38–76 years) presented acute eyelid erythema and edema, and were initially misdiagnosed as orbital cellulitis. Thus, inflammatory markers in the blood (ie, white blood cells [WBCs] and C-reactive protein [CRP]) of those 15 patients were analyzed with 17 patients (10 males, 7 females) having orbital cellulitis. The receiver operating characteristic curve analysis was performed to determine the optimal cut-off values.
Results: The mean ± standard error (SE) levels of the WBC were 6.80±0.70×10³/µL in the IOI patients, and 8.54±0.91×10³/µL in the orbital cellulitis patients, and no significant differences were observed (P=0.15, Student’s t-test). However, the mean ± SE levels of CRP were 1.04±0.43 mg/dL in the IOI patients, yet were significantly increased to 4.65±1.21 mg/dL in the orbital cellulitis patients (P=0.01, Student’s t-test). The area under the curve value was 0.80 and the optimal cut-off value was 0.43 for orbital cellulitis, with sensitivity and specificity being 82% and 73%, respectively.
Conclusion: The findings of this study indicate that CRP may be useful in distinguishing patients with idiopathic orbital inflammation from those with orbital cellulitis.
Keywords: idiopathic orbital inflammation, orbital cellulitis, CRP, WBC, cut-off value
Introduction
Idiopathic orbital inflammation (IOI) is a non-infectious and nonspecific inflammatory condition in the orbit with no identifiable local or systemic causes.1 The clinical symptoms of IOI can affect any structure in the orbital region, including the lacrimal gland, eyelid, sclera, extraocular muscle, orbital fat, and optic nerve.2,3 Yamagami et al4 classified IOI into extraocular myositis, dacryoadenitis, optic perineuritis, intraorbital diffuse inflammation, tenonitis/scleritis, and orbital apex inflammation based on the clinical symptoms and imaging findings. Thus, although the clinical feature can vary highly, eye pain is the most common symptom.5 Anterior segment inflammation of the orbit causes eyelid swelling, ptosis, and injection of the eye. Intraorbital involvement may cause proptosis and diplopia,2,3,6 and intraocular inflammation can sometimes occur via scleral inflammation.7
On the other hand, orbital cellulitis is an infection of the orbital tissues, including the orbital fat and ocular muscles. Common features of orbital cellulitis are ocular pain, eyelid erythema, eyelid swelling, ophthalmoplegia, and proptosis.8
It should be noted that these two disorders are different in pathophysiology, and thus require distinct and specific medical management. Systemic steroid is the first-line treatment for cases of IOI, however, immunosuppressants or radiation therapy are needed for recurrent cases.3,9 In contrast, cases of orbital cellulitis require treatment with antibiotics, and systemic steroids are generally contraindicated. Thus, a differential diagnosis is very important for the proper management of these two conditions. However, considering the previously mentioned clinical features, IOI and orbital cellulitis share similar clinical features. Although proper imaging is important to distinguish between these two conditions,10 magnetic resonance imaging (MRI) is not always quickly available. If the diagnosis is confused due to a similarity between these two conditions, orbital cellulitis needs more urgent treatment with systemic antibiotics. Thus, a presence of acute eyelid erythema and edema often prompts physicians to prescribe systemic antibiotics.
Based on the different pathophysiologies (ie, nonspecific inflammation and infection), we hypothesized that the inflammatory biomarkers in the blood may also be different. The purpose of this present study was to determine whether or not inflammatory markers are significantly different enough to distinguish between IOI and orbital cellulitis. Thus, we reviewed the medical charts and selected IOI cases that were initially misdiagnosed as orbital cellulitis. Levels of white blood cell (WBC) and C-reactive protein (CRP) were selected as inflammatory markers, and receiver operating characteristic (ROC) curve analysis was performed to determine the optimal cut-off values.
Subjects and methods
In this study, we reviewed the medical records of the patients >15 years of age with a diagnosis of IOI including orbital pseudo-tumor, dacryoadenitis, and extraocular myositis, who were seen from January 1, 2007 and December 31, 2016 at the Department of Ophthalmology, Osaka Medical College Hospital, Takatsuki, Osaka, Japan. The study was approved by the Ethics Committee of Osaka Medical College. Since this research was retrospective, using already existing information, patient’s informed consent was not needed. Instead, information on the research was disclosed to patients and guarantees opportunities for research subjects to refuse to participate. We handle patients’ personal information carefully. Each case was anonymized, and an electronic file with a password so that only a specific researcher can log in, was stored in a specific office computer separated from Internet access. In the manuscript submission or conference presentation, personal information were de-identified. Excluded from the study were all cases with possible thyroid eye disease, malignant lymphoma, antineutrophil cytoplasmic antibody-associated disease, IgG4-related disease, and sarcoidosis. A final diagnosis of IOI was verified by the findings of clinical symptoms, computed tomography (CT) and MRI imaging, and clinical outcomes of treatment with systemic corticosteroids.
Of the reviewed medical records, we found 45 cases aged >15 years, with presumed IOI, and in whom blood samples were obtained within 5 days from the onset of subjective symptoms. Of those 45 cases, 15 patients (10 males and 5 females; mean age of 56.9 years; range 38–76 years) presented with acute eyelid erythema and edema with eye pain, and were initially misdiagnosed as orbital cellulitis. Of these 15 cases, intraorbital diffuse inflammation was seen in 1 case. In the other 14 cases, although some overlaps existed, inflammatory lesions were located at the lacrimal glands in 4 cases, at the extra ocular muscles in 5 cases, and at the episcleral regions in 7 cases.
In the present study, the final diagnosis of IOI was basically verified by confirming a lack of response to systemic antibiotics and a good response to systemic steroids.
Orbital cellulitis was also diagnosed based on the findings of clinical symptoms (acute eyelid erythema, edema, eye pain, and proptosis), CT and MRI imaging. Excluded in this study were patients with preseptal cellulitis, in which the infectious lesion was restricted to the anterior portion of the eyelid. There were 17 definite cases with orbital cellulitis (10 males and 7 females, mean age of 58.4 years; range 16–88 years) that were verified by clinical symptoms, CT or MRI imaging findings, and clinical responses to antibiotics. Specifically, although the presence of sinusitis could support the probability of orbital cellulitis, the diagnosis was basically verified by the clinical responses to systemic antibiotics. In all cases, blood samples were obtained within 5 days from the onset of their subjective symptoms.
We analyzed the levels of the WBC and CRP in the blood as inflammatory markers. Moreover, we generated a ROC curve, and a cut-off value, area under the curve (AUC) value, sensitivity, and specificity were calculated. The ROC curve analysis was done using JMP® software (SAS Institute Inc., Cary, NC, USA). Data in this study are expressed as mean ± standard error (SE). Student’s t-test was used to determine the significance of differences, and a P-value of <0.05 was considered statistically significant.
In addition, prior to obtaining blood samples, patient profiles were reviewed in regard to the usage of antibiotics or non-steroidal anti-inflammatory drugs (NSAIDs), as well as the presence or absence of obesity and diabetes.
Results
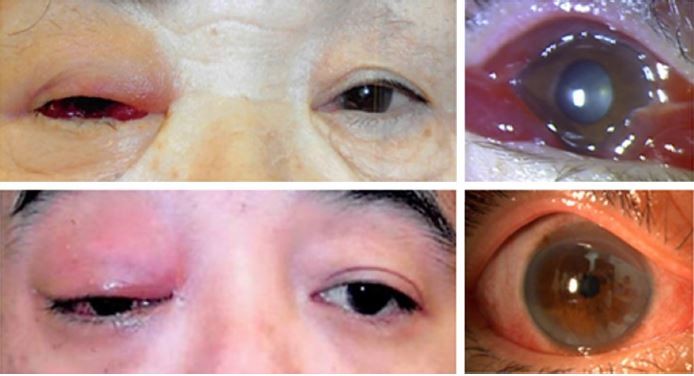
We found representative facial photographs of cases with IOI who presented with eyelid erythema, eyelid edema, and conjunctival hyperemia. Those cases were initially confused with orbital cellulitis in their medical records. Antibiotics were found to not be effective, and their symptoms were quickly resolved by systemic steroids. The 15 cases with IOI analyzed in this study demonstrated similar findings (Figure 1).
The results are summarized in Figure 2. The mean ± SE levels of WBC were 6.80±0.70×103/μL in the patients with IOI, and 8.54±0.91×103/μL in the patients having orbital cellulitis, and no significant differences were observed (P=0.15, Student’s t-test). The WBC levels increased over the normal limits (>8.19×103/μL) in 3 IOI patients (20%) and 7 orbital cellulitis patients (41%), respectively. Thus, WBC levels remained within the normal limits in 59% of the cases with orbital cellulitis. The area under the ROC curve of WBC was 0.63. The best cut-off value of WBC was estimated at 9.66×103/μL. Sensitivity was as low as 41%, and specificity was 87%, thus suggesting a poor association with orbital cellulitis.
On the other hand, the mean ± SE levels of CRP were 1.04±0.43 mg/dL in the IOI patients, yet increased significantly to 4.65±1.21 mg/dL in the patients with orbital cellulitis (P=0.01, Student’s t-test). The area under the ROC curve of CRP was 0.8. The best cut-off value of CRP was 0.43. Sensitivity was 82%, and specificity was 73%, thus indicating a better association with orbital cellulitis. The positive predictive value was 78%, and the negative predictive value was 79%.
The mean age of each group was similar (IOI group, 56.9 years; orbital cellulitis group, 58.4 years). However, it should be noted that in 3 cases (20.0%) with IOI and 5 cases (29.4%) with orbital cellulitis, antibiotics had already been administered prior to when their blood samples were obtained. NSAIDs were also given in 1 case with IOI and 2 cases with orbital cellulitis before obtaining their blood samples. One patient with IOI and 2 cases with orbital cellulitis were obese (BMI >25%). In addition, 2 cases with IOI and 1 case with orbital cellulitis were diabetic.
Discussion
The most important finding of this study was that normal WBC levels could not exclude the possibility of orbital cellulitis. The levels of WBC were within the normal range (3.30–8.19×103/μL) in 59% of the cases with orbital cellulitis. Since orbital cellulitis needs urgent treatment with antibiotics to prevent serious complications including visual loss, the use of antibiotics cannot be criticized in some cases without leukocytosis that were later identified as IOI.
On the other hand, the CRP levels were significantly higher in the cases with orbital cellulitis than in the cases with IOI. In addition, since AUC of CRP (0.8) was higher than that of WBC (0.63), higher CRP levels were more associated with orbital cellulitis. The cut-off value of CRP obtained in this study was 0.43, but the level of increase was quite modest compared with the normal limit (ie, 0.25 mg/dL). However, considering the vision-threatening nature of orbital cellulitis, beyond that level can be an indication to prescribe systemic antibiotics.
It should be noted that these findings seem to be contradictory when considering their diagnostic value. Elevated WBC levels usually indicate bacterial infection, while CRP is elevated in both the infection and inflammation. Reportedly, IOI can be caused by viral infection and immune-related abnormalities that may increase CRP levels.11–14 Thus, we initially thought the WBC level would be a better indicator of orbital cellulitis considering its nature of infectious disease.
Several factors that affect the WBC and CRP levels have been reported.15,16 Those include age, bone marrow function, and physical exercise, as well as obesity and diabetic conditions. Perhaps, the WBC level has a wide normal range, and localized infection in the orbit may not be able to elevate WBC levels beyond the normal limits, even though they may exceed individual healthy levels. In addition, antibiotics that were prescribed in 3 cases (20%) with IOI and 5 cases (29.4%) with orbital cellulitis by their previous doctors might have quickly decreased the WBC levels, while the CRP levels tended to remain at higher levels for a longer period of time. Compared to this, NSAIDs that may decrease CRP levels were given in a few cases in each group. However, few cases with IOI or orbital cellulitis are obese or diabetic. These may account for the discrepancy.
It should be noted that this study had several limitations. First, this was a retrospective single-institute study with a small number of subjects. Second, although blood samples were collected within 5 days from the onset of subjective symptoms, antibiotics had already been administered in some cases. Hence, these limitations might have skewed our results. A future prospective study is necessary to confirm the present results, and to also find more useful biomarkers including procalcitonin,17 higher levels of which are known to indicate sepsis.
Conclusion
Our results suggest that normal WBC levels cannot exclude the possibility of orbital cellulitis. However, quite high levels of sensitivity (82%) and specificity (73%) may support the idea that CRP levels higher than 0.43 can be useful to differentially diagnose cases of orbital cellulitis from IOI.
Acknowledgment
The authors wish to thank John Bush for editing the manuscript.
Disclosure
The authors declare no conflicts of interest in this work.
References
Gleason JE. Idiopathic myositis involving the extraocular muscles. Ophthalmol Rec. 1903;12:471–478. | ||
Swamy BN, McCluskey P, Nemet A, et al. Idiopathic orbital inflammatory syndrome: clinical features and treatment outcomes. Br J Ophthalmol. 2007;91(12):1667–1670. | ||
Yuen SJ, Rubin PA. Idiopathic orbital inflammation: distribution, clinical features, and treatment outcome. Arch Ophthalmol. 2003;121(4):491–499. | ||
Yamagami A, Wakakura M, Inoue K. Evaluation of clinical presentation of idiopathic orbital inflammation. Neuro-Ophthalmol Jpn. 2016; 33(3):242–248. | ||
Li Y, Lip G, Chong V, Yuan J, Ding Z. Idiopathic orbital inflammation syndrome with retro-orbital involvement: a retrospective study of eight patients. PLoS One. 2013;8(2):e57126. | ||
Markowski J, Jagosz-Kandziora E, Likus W, et al. Primary orbital tumors: a review of 122 cases during a 23-year period: a histo-clinical study in material from the ENT Department of the Medical University of Silesia. 2014;20:988–994. | ||
Chaudhry IA, Al-Obaisi S, Al-Sheikh O, Galvez A. Unilateral optic neuritis, scleritis and exudative retinal detachment due to recurrent orbital pseudotumor. Saudi J Ophthalmol. 2012;26(4):449–451. | ||
Ferguson MP, McNab AA. Current treatment and outcome in orbital cellulitis. Aust N Z J Ophthalmol. 1999;27(6):375–379. | ||
Leone CR Jr, Lloyd WC 3rd. Treatment protocol for orbital inflammatory disease. Ophthalmology. 1985;92(10):1325–1331. | ||
Kubota T. Corticosteroids or biopsy for idiopathic orbital inflammation. Surv Ophthalmol. 2017;62(2):253–255. | ||
Rico M, Díaz-López JB, Peña J, Oliva-Nacarino P. Latent orbital pseudotumor secondary to systemic lupus erythematosus. Clin Case Rep. 2016;4(11):1065–1067. | ||
Ramalho J, Castillo M. Imaging of orbital myositis in Crohn’s disease. Clin Imaging. 2008;32(3):227–229. | ||
Cheng S, Vu P. Recurrent orbital myositis with radiological feature mimicking thyroid eye disease in a patient with Crohn’s disease. Orbit. 2009;28(6):368–370. | ||
Ren MW, Du Y, Ren S, Tang CY, He JF. Epstein-Barr virus-encoded small RNAs in idiopathic orbital inflammatory pseudotumor tissues: a comparative case series. Int J Ophthalmol. 2017;10(8):1268–1272. | ||
Nakanishi N, Suzuki K, Tatara K. Association between lifestyle and white blood cell count: a study of Japanese male office workers. Occup Med (Lond). 2003;53(2):135–137. | ||
Lim LS, Tai ES, Mitchell P, et al. C-reactive protein, body mass index, and diabetic retinopathy. Invest Ophthalmol Vis Sci. 2010;51(9):4458–4463. | ||
Barati M, Alinejad F, Bahar MA, et al. Comparison of WBC, ESR, CRP and PCT serum levels in septic and non-septic burn cases. Burns. 2008;34(6):770–774. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.