Back to Journals » ClinicoEconomics and Outcomes Research » Volume 9
Burden of uterine fibroids in Italy: epidemiology, treatment outcomes, and consumption of health care resources in more than 5,000 women
Authors Chiumente M , De Rosa M, Messori A, Proli EM
Received 10 April 2017
Accepted for publication 28 July 2017
Published 29 August 2017 Volume 2017:9 Pages 525—535
DOI https://doi.org/10.2147/CEOR.S139335
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Giorgio Colombo
Marco Chiumente,1 Mauro De Rosa,2 Andrea Messori,2 Enrica Maria Proli3
1Scientific Direction, SIFaCT – Italian Society for Clinical Pharmacy and Therapeutics, Milan, 2Board of directors, SIFaCT - Italian Society for Clinical Pharmacy and Therapeutics, Milan, 3Hospital Pharmacy, Policlinico Umberto I, Rome, Italy
Background and purpose: Epidemiological studies on uterine fibroids (UFs) are mostly based on surveys or analyses of small samples of patients. In 50% of women, the quality of life is worsened by disease-related symptoms; furthermore, treatments imply a remarkable health care cost. The aim of this observational study was to analyze a large sample of Italian patients with UFs and to assess the epidemiology, the appropriateness of treatments, and the consumption of disease-related resources.
Methods: Data were collected through a data-linkage technique from five administrative databases. Women aged between 18 and 55 years and resident in three local health authorities (north–central–south Italy) were selected over the period from 1st January 2009 to 31st December 2015. The inclusion criteria were a surgical procedure with diagnosis of UFs or a pharmacological treatment with gonadotropin-releasing hormone (GnRH) analogs or ulipristal acetate. Besides the overall descriptive analysis, two comparisons were evaluated: surgery versus no surgery and treatment with GnRH analogs versus ulipristal acetate.
Results: A total of 5,665 women with UFs were selected from an overall population of 2,400,000 people. In the north, 73.6% of patients underwent surgery, as opposed to only 16.7% in the south; 70% of surgeries were hysterectomies. The average cost per patient was €3,249 (duration of follow-up = up to 7 years). The southern district had the highest number of drug prescriptions; in particular, 49% of patients took >10 packages of GnRH analogs.
Conclusion: This study is the first on this topic conducted in Italy using a large sample size. The analysis of resource consumption revealed a high heterogeneity in the choice of drug treatments by gynecologists (especially in the south); in the north, marked variations were seen in the rates of surgery. The long-term use of GnRH was inappropriate.
Keywords: observational study, leiomyoma, ulipristal acetate, gonadotropin-releasing hormone, costs and cost analysis
Corrigendum for this article has been published
Introduction
Uterine fibroids (UFs), also known as leiomyomas or myomas, are solid tumors of the uterine smooth muscle cells.1 The etiology of this benign tumor is complex, and many hormonal and genetic factors are implicated. UFs have monoclonal proliferation, and their cells show chromosomal rearrangements that cause proliferation; however, as opposed to sarcomas, the differentiation persists.2
The diagnosis can generally be made starting from menarche, and the growth of the tumor stops with menopause. These tumors are often asymptomatic and their diagnosis can be the result of an incidental finding during a gynecological exam or ultrasound check.3 From 20% to 50% of the women with UF experience heavy menstrual bleeding and pelvic pressure; other common symptoms include infertility, increased urinary frequency or incontinence, constipation, abdominal bloating, dyspareunia, and fatigue (due to anemia from heavy bleeding).1,4
These symptoms determine a worsening in quality of life and consequently a loss of productivity.5 Moreover, UF is currently the most frequent indication for hysterectomy (2/3 of all hysterectomies are performed for this indication) and, therefore, is a leading cause of health care cost in gynecology.6
The prevalence of symptomatic UF increases with age: women between 35 and 55 years old account for 95% of the population affected by the disease.7 Several authors have studied epidemiology, burden of the disease, and quality of life both in European countries and in the rest of the world.5,7–10 Most of these epidemiological studies have used, as data source, surveys voluntarily filled in by patients or they examined samples of patients who were not representative of the entire national population; consequently, the estimated national prevalence of the disease is imprecise.
In Italy, owing to the absence of a national disease registry, a suitable approach to generate epidemiological data can rely on the consumption of drugs and/or surgical treatments in relation to UFs. Surgical treatments, currently indicated for these benign tumors, include hysterectomy and myomectomy with a variable degree of invasiveness. At present, the drugs approved for the presurgical indication comprise gonadotropin-releasing hormone (GnRH) analogs and ulipristal acetate (UPA).11 The choice of the treatment is closely related to the attitudes of gynecologists.12
Owing to the debilitating effects of the disease and the frequent surgical treatments in addition to medical therapy, the disease-related health care costs are high and also difficult to study because of heterogeneity in treatments.6
In this context, we describe a retrospective observational experience conducted in three local health authorities of northern, central, and southern Italy in which we assessed the main determinants of health care resources in these patients.
Methods
Objectives and study design
Our retrospective observational study had the following aims: 1) to describe the characteristics of patients with UFs, in terms of demographic information and clinical characteristics; 2) to estimate the consumption of drugs and other health care resources charged to the National Health System (NHS), for example, surgery, diagnostic services, specialist visits, and hospitalizations. Our analysis comprised a main study (in which the use of health care resources was estimated from the overall population and compared between patients who underwent surgery and those treated with medical therapy) and an ancillary study (in which the resource consumption was compared between patients receiving different treatments).
Population
The sample of patients with UFs was extracted from the databases of three local health districts (northern, central, and southern Italy) that serve an overall population of 2.4 million people. The ethical committees of the three local health districts participating in the observational study provided their approvals. Each district provided administrative data related to drug consumption, diagnostic services, specialist visits, and hospitalizations. In particular, through a data-linkage technique, the information from five different administrative databases (population registry, local pharmaceutical consumption, hospital drug distribution, hospital discharge, and outpatient services) was included in a single database. Data were integrated through a procedure based on the patient’s identification code (i.e., social security number), thus enabling the tracking of individual patients along with chronological details and quantitative data. The time period chosen for the main study was from 1st January 2009 to 31st December 2015 (7 years). The time period chosen for ancillary study was from 1st September 2014 to 31st December 2015 because before September 2014 UPA 5 mg was not available in Italy.
Inclusion and exclusion criteria
The database of both the main study and the ancillary study included all patients with UFs identified through at least one of the following alternative criteria:
- one or more hospitalizations or surgical procedures in which the diagnosis (primary or secondary) at discharge was reported to be one of the following ICD-9-CM codes (International Classification of Diseases, ninth revision, Clinical Modification): 218 and 626, corresponding to the diagnosis of hospitalization for UFs and menometrorrhagia; 683.*, 684.*, 685.1, 681.2, 542.1, and 541 corresponding to hysterectomy, hysteroscopy, laparoscopy, and laparotomy, respectively;
- at least one specific drug for presurgical fibroids treatment (UPA [ATC code [Anatomical-Therapeutic-Chemical classification system]: G03XB02]) and/or (GnRH analogs [ATC code: L02AE]);
- at least one health care service univocally related to the UF disease.
All patients who changed local health authority during the study period were excluded.
Main study design
In the main study, the patients with UF aged between 18 and 55 years were divided into two cohorts: women who underwent surgery and women who did not. Owing to its design, this analysis included all patients in the database.
The descriptive analysis assessed, in each local health authority, the distribution of patients by age, the Charlson index, diagnostic tests, and drug consumption (both symptomatic and with indication of UF treatment). The two cohorts, surgery versus no surgery, were compared to investigate differences in distribution of patients, age, local health authority, type of surgery, and drug treatment.
Ancillary study design
The aim of the ancillary study was to evaluate medications taken by patients with UFs. Two cohorts were compared: 1) patients who took UPA and 2) patients who took GnRH analogs. The time period of this secondary analysis included only patients enrolled from 1st September 2014, when ulipristal was introduced into the Italian market, to 31st December 2015.
UPA received the therapeutic indication of long-term intermittent treatment of UFs only in 2016; therefore, in this study, only presurgical indication could be considered. Patients with less than 6 months of follow-up were excluded. Women undergoing surgery could be present in both groups; in addition, as not all patients with UFs took UPA or GnRH analogs, fewer patients were included in the ancillary study than in the overall population.
The data analysis procedure was similar to that of the main study and consisted first of an overall description of patients’ characteristics followed by further analyses focused on age and local health authority. Compared with the main study, the ancillary one explored in more detail the utilization of UPA and GnRH analogs also outside their therapeutic indication.
Cost analysis
The perspective of the analysis was that of our NHS. Hence, only direct medical costs were considered; nonmedical direct costs, indirect costs, and intangible costs were excluded. Unit costs for drugs, diagnostics, hospitalization, specialist consultations, and surgeries are listed in Table 1. The total cost for each individual patient was calculated by multiplying the amount of resources consumed by their unit cost.
  | Table 1 Unit costs included in the analysis Abbreviation: GnRH, gonadotropin-releasing hormone. |
Statistical analysis
Quantitative variables were expressed as mean±SD. Qualitative variables were expressed as absolute and relative frequencies. To test for differences between groups, chi-square test and Student’s t-test were used for categorical variables and continuous variables, respectively.
Using the dependent variables, a multivariate analysis was performed based on binary logistic regression. Final results were expressed as adjusted odds ratio (with 95% CI).
All statistical tests used a significance level of 0.05. All calculations were performed using STATA 12 software.
Results
In an overall population of 2.4 million people from three local health authorities (northern, central, and southern Italy), 5,665 women with UFs were identified over the period from 1st January 2009 to 31st December 2015.
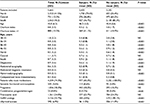
The descriptive analysis of all patients with UFs is shown in Table 2. People assisted in the health care districts were 1.1 million in the north, 0.5 million in the center, and 0.8 million in the south. The mean age was 45.8; the Charlson index was ~1, indicating a low comorbidity; only in 15.5% of cases was the Charlson index >1.
Most of the patients underwent a medical echography; other, more expensive tests like magnetic resonance, hysterosalpingography, and bone mineralometry were prescribed less frequently.
A total of 74.3% of patients with UFs took medications such as tranexamic acid for symptom control and for therapeutic purposes (e.g., UPA and GnRH analogs).
Main study
Table 2 summarizes our descriptive analysis of all patients with UFs along with the data of the two cohorts of the main study (i.e., patients who underwent surgery versus patients who did not undergo surgery). Most of the patients with UFs undergoing surgery were in the north (n=1,730, equivalent to 73.6%), whereas the situation in the south was the opposite (n=427, 16.7% of the patients). The mean age did not differ between the two groups.
Regarding comorbidity, the Charlson index was significantly higher in patients who did not undergo surgery. In fact, these patients received a closer monitoring of the disease over time compared to surgical patients (on average an index of 1.1 versus 0.6). Furthermore, 40.2% of patients who underwent surgery took the index drugs, and only 11.5% took drugs with presurgical therapeutic indication.
Figure 1 shows how the patients included in our database were divided into subgroups based on local health care authorities, age, type of surgery, drug therapy, and treatment with UPA or GnRH analogs. Panel 1A shows that most patients taking GnRH analogs or UPA, in the absence of surgery, lived in the south.
In Panel 1B, the age distribution of patients who did not undergo surgery shows that the frequency of drug treatments increased with age (from a minimum of 1.2% under 25 years, up to 58.1% in the range of 46–55 years).
Panel 1C shows that the most frequent surgery was hysterectomy (70.1%, 1,785 of 2,545 patients).
Finally, panel 1D shows that 95% of surgeries were in the 36–45 and 46–55 age groups.
Ancillary study
Table 3 shows the analysis of patients according to the medical treatment. The total number of patients included in the ancillary study was 1,352, 61.2% of whom resided in the south. Patients had a mean age of 45 years; Charlson index was about 1% and 10.9% after medical therapy underwent surgery.
The cohort receiving GnRH analogs included 1,075 patients, 66.5% of whom resided in the south; 277 patients were treated with UPA, 41.5% in the north, 40.8% in the south, and 17.7% in central Italy. Age did not vary significantly between the two groups while the Charlson index was lower in the UPA group. While 92.2% of patients treated with GnRH analogs did not undergo surgery, 76.9% of patients were treated with UPA.
The two cohorts were further investigated considering the number of prescribed (and reimbursed) medication packages (Figure 2). Forty percent of patients of the UPA group took three drug packages, 24% <3 packages and only 6% >6 packages. In the GnRH analogs group, 25.6% of patients received 1 package, 9.0% 2 packages, and 62.5% >4 packages (48.9% >10 packages).
  | Figure 2 Number of prescribed and reimbursed medication packages per patient. Ulipristal (A) and GnRH analogs (B). Abbreviation: GnRH, gonadotropin-releasing hormone. |
Cost analysis – main study
In the main study (5,665 patients), the mean cost per patient was €3,249.31, with a median of €2,978.71. Figure 3A shows the distribution of the total cost per patient.
  | Figure 3 Distribution of total direct costs per patients in the main study: overall (A) and divided by surgical and nonsurgical patient groups (B). |
The shape of the costs distribution curve was trimodal.
To investigate the types of patients who generated a high cost for the NHS, the cost distribution has been divided for patients who underwent surgery and patients who did not (Figure 3B).
The mean costs of the two arms were similar: €3,290.28 for surgery patients versus €3,218.46 for no surgery.
The cost distribution was different in the two arms. In patients who underwent surgery, the distribution is Gaussian, with the median very similar to the mean, €3,067.00.
The costs distribution in patients who did not undergo surgery was bimodal and the median was lower than the mean.
Cost analysis – ancillary study
Figure 4 shows the cost distribution for the ancillary study. This analysis excluded the cost of surgery in order to evaluate only the medication costs related to the treatment of UFs. Panel 4A about total costs distribution included all patients of the ancillary study and showed that there was a high number of patients in the ranges between €0 to €500 and €1,000 to €1,500. Panel 4B shows the cost differences in patients receiving GnRH versus those taking UPA: 67% of patients of the former group cost <€500 while in the latter group (277 patients) the mean cost was €487. In patients treated with GnRH, 43.9% was in the range from €1,001 to €1,500 with a mean cost of €751.
Discussion
This retrospective observational study is the first that provided epidemiological data on a very large sample of patients representative of our peninsula.
The data-linkage technique used to create our patient sample from administrative databases allowed us to obtain quite detailed information about real-world patients. The perspective of the NHS was useful to quantify the impact of the disease in a public health system like the Italian one, even though we could not entirely clarify the resource consumption related to the disease. In fact, our study design did not consider nonmedical direct, indirect, and intangible costs that are likely to be high in this disease.13
Furthermore, unlike several other diseases (for example, cancer), out-of-pocket expenses of women with UF are high, as several gynecological examinations are performed privately. Another feature of this disease, which influences the epidemiological evidence, is the frequent absence of symptoms directly related to the disease in a significant number of patients (which could even reach 50%).14 Hence, our epidemiological data might be an underestimation of the real data.
One of the main objectives of our study, that is, investigating misuse of surgery and of medical treatment, provided quite unexpected results. The surgical approach is employed mainly in northern Italy, and this finding emphasizes, also for Italy, the results by Pazzaglia et al concerning the subjectivity of choosing either the surgical or the medical approach in this disease.12 Furthermore, the prevalence of the most invasive surgical technique, hysterectomy, is a factor that deserves to be assessed in terms of patients’ quality of life and posthospitalization costs. The rationale behind the high rate of hysterectomies in northern Italy is not known, but a reduction in surgical interventions due to the new UPA long-term therapeutic indication can be expected. In the ancillary study, we carried out an analysis focused on medical therapies to investigate the heterogeneity of therapeutic choices and the potential areas of inappropriate prescription. UPA has been commercially available in Italy since 2012 and has been reimbursed by the NHS since 2014.
Regarding the GnRH analog cohort, there was, particularly in the south, the prescription of a high number of packages. Considering that most of the patients treated with medications belonged to the highest age group, studying the prescription pattern was of interest. The approved dosing schedules of UPA, consisting of one or two 3-month cycles of drug treatment before surgery, were observed. Conversely, the use of >10 packages in 49% of GnRH analogs patients is inappropriate and does not reflect the approved dosing schedule; one interpretation of this finding is that the drug was administered off label until menopause without considering adverse events that cannot be adequately managed with complementary therapies.15 This off-label long-term treatment of GnRH is an unexpected and surprising deviation from the international standard and recommendations. All in all, the multivariate results emphasized the critical role played by the geographic jurisdiction. In addition, after adjusting for covariates, concomitant medications and surgery proved to be more frequent in the patients’ group treated with UPA than in the controls.
Since late 2016, only UPA has been approved for long-term intermittent treatment of UFs, based on the findings of the PEARL IV trial.16 In the future, off-label treatments with GnRH, observed herein in a real setting, might be replaced by intermittent long-term therapy with UPA, considering the safety and efficacy data that led to its long-term therapeutic indication. The last part of our study concerning costs was interesting to better understand the economic impact of the disease on the Italian NHS.
The mean cost per patient of €3,249 did not vary significantly between patients who underwent surgery and those who did not, but the costs distribution clarified the different pattern of resource consumption. The patients who underwent surgery substantially consumed resources related to surgery, while in the patients who did not undergo surgery, these figures were divided substantially between either low cost (52% <€1,500) or high cost (22.1% >€5500). The heterogeneity of cost data is even more evident in the ancillary study, which considered only patients from 2014 and excludes surgical costs. Figure 4 shows that treatment with GnRH costs 54% more than ulipristal (€751.10 versus €487.07), and this might be due to the inappropriate long-term use of GnRH.
The main limitation of our study is related to the observation period that, regarding the pharmacological treatment with UPA, did not allow us to perform an adequate follow-up of the patients. In addition, the cost data are likely to be an underestimation of the real economic impact of the disease, likely because the analysis only considered direct medical costs of the NHS, so that women with UFs might have paid for some health care resources on their own. The strengths of this study include its large sample and the representativeness of the three patient cohorts living in northern, central, and southern areas of Italy.
Conclusion
Evidence from real-world studies should be combined with randomized clinical trials evidence to provide a more complete picture of the intervention effectiveness and realistic treatment outcomes. This is the first retrospective real-world observational study conducted in Italy on a large sample of patients with UFs. Our study showed a high prevalence of UFs in Italy with high related costs for the NHS. Surgery was the favorite treatment in northern Italy, whereas drugs for UF therapy were taken mostly in the south; GnRH analogs were often prescribed off label leading to too many packages per patient.
Our findings provide new epidemiological data on the disease and highlight both medical and surgical treatment data that would require more surveillance by regulatory agencies. Further epidemiological studies would be required, considering that the new long-term intermittent treatment of UPA for UFs will likely have an important impact in the optimization of resource consumption to satisfy a clinical unmet need.
Acknowledgments
Analyses provided in this manuscript were conducted by CliCon S.r.l., a private firm specialized in outcomes research analyses. Data for analyses were retrieved from administrative databases of a sample of local health units. To comply with Italian regulation on the conduction of observational analysis, CliCon S.r.l. notified the study to the local ethics committee of each participating local health units and, after approval, was entitled by contract as the body for the conduction of the analyses. Data were extracted by the staff of each local health unit and their databases were anonymized in full compliance with the Italian code of protection of personal data (Legislative Decree, 196/03). All results of the analyses provided by CliCon S.r.l. to researchers were provided at an aggregated level, which were not possible to assign, either directly or indirectly, to the individual patients. The authors thank the staff of Clicon S.r.l. (Luca Degli Esposti, Valentina Perrone, Chiara Veronesi, Davide Alessandrini, Valerio Blini, and Stefano Buda) for their contributions to the scientific advancement of the study.
Italian Society for Clinical Pharmacy and Therapeutics (SIFaCT) has received research grants from Gedeon Richter Italia srl.
Disclosure
The authors report no conflicts of interest in this work.
References
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.