Back to Journals » Infection and Drug Resistance » Volume 10
Burden of methicillin-resistant Staphylococcus aureus pneumonia among hospitalized patients in Lebanon and Saudi Arabia
Authors Althaqafi AO, Matar MJ, Moghnieh R, Alothman AF, Alenazi TH, Farahat F, Corman S, Solem CT , Raghubir N, Macahilig C, Haider S, Stephens JM
Received 30 September 2015
Accepted for publication 18 February 2016
Published 2 February 2017 Volume 2017:10 Pages 49—55
DOI https://doi.org/10.2147/IDR.S97416
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Professor Suresh Antony
Abdulhakeem O Althaqafi,1 Madonna J Matar,2 Rima Moghnieh,3 Adel F Alothman,4 Thamer H Alenazi,5 Fayssal Farahat,1 Shelby Corman,6 Caitlyn T Solem,6 Nirvana Raghubir,7 Cynthia Macahilig,8 Seema Haider,9 Jennifer M Stephens6
1Department of Infection Prevention and Control, King Abdullah International Medical Research Center, King Saud bin AbdulAziz University for Health Sciences, Jeddah, Kingdom of Saudi Arabia; 2Department of Infectious Disease, Notre Dame de Secours University Hospital, Byblos, 3Makassed General Hospital, Beirut, Lebanese Republic; 4Department of Medicine, King Abdulaziz Medical City, Central Region, Ministry of National Guard Health Affairs, 5Infection Prevention & Control Department, King Abdulaziz Medical City-Riyadh (KAMC), Kingdom of Saudi Arabia; 6Real World Evidence: Data Analytics Center of Excellence, Pharmerit International, Bethesda, MD, 7Medical Affairs, Pfizer, New York, NY, 8Medical Data Analytics, Parsippany, NJ, 9Outcomes & Evidence, Global Health and Value, Pfizer, Groton, CT, USA
Objectives: The objective of this study is to describe the real-world treatment patterns and burden of suspected or confirmed methicillin-resistant Staphylococcus aureus (MRSA) pneumonia in Saudi Arabia and Lebanon.
Methods: A retrospective chart review study evaluated 2011–2012 data from hospitals in Saudi Arabia and Lebanon. Patients were included if they had been discharged with a diagnosis of MRSA pneumonia, which was culture proven or suspected based on clinical criteria. Hospital data were abstracted for a random sample of patients to capture demographics (eg, age and comorbidities), treatment patterns (eg, timing and use of antimicrobials), hospital resource utilization (eg, length of stay), and clinical outcomes (eg, clinical status at discharge and mortality). Descriptive results were reported using frequencies or proportions for categorical variables and mean and standard deviation for continuous variables.
Results: Chart-level data were collected for 93 patients with MRSA pneumonia, 50 in Saudi Arabia and 43 in Lebanon. The average age of the patients was 56 years, and 60% were male. The most common comorbidities were diabetes (39%), congestive heart failure (30%), coronary artery disease (29%), and chronic obstructive pulmonary disease (28%). Patients most frequently had positive cultures from pulmonary (87%) and blood (27%) samples. All isolates were sensitive to vancomycin, teicoplanin, and linezolid, and only one-third of the isolates tested were sensitive to ciprofloxacin. Beta-lactams (inactive therapy for MRSA) were prescribed 21% of the time across all lines of therapy, with 42% of patients receiving first-line beta-lactams. Fifteen percent of patients did not receive any antibiotics that were considered to be MRSA active. The mean hospital length of stay was 32 days, and in-hospital mortality was 30%.
Conclusion: The treatment for MRSA pneumonia in Saudi Arabia and Lebanon may be suboptimal with inactive therapy prescribed a substantial proportion of the time. The information gathered from this Middle East sample provides important perspectives on the current treatment patterns.
Keywords: MRSA, resource use, mortality, length of stay, antibiotics, Middle East
Introduction
In recent years, morbidity and mortality of nosocomial infections have increased, at least partly due to the increased frequency of antibiotic resistance in both Gram-positive and Gram-negative organisms. In the past, Gram-negative aerobes were the most frequently reported pathogens, but Staphylococcus aureus has been increasing in frequency, particularly methicillin-resistant S. aureus (MRSA) in intensive care units.1
Over 70% of S. aureus isolates are resistant to methicillin in the Middle East and with >85% of S. aureus pneumonia cases being MRSA pneumonia in the Arabian peninsula.2 In Saudi Arabia, 76% of health-care workers have tested positive for MRSA colonization despite being asymptomatic.3 On a separate note, a systematic review on the epidemiology of S. aureus in the Middle East indicates high incidence of community-acquired MRSA carried by healthy individuals, with positive MRSA samples obtained from random wound, throat, and nasal specimens.4
Glycopeptide antibiotics, vancomycin and teicoplanin, have been considered drugs of choice in treatment for infections due to suspected or documented MRSA.5–8 Burgeoning glycopeptide prescribing is believed to have led to the emergence of vancomycin-resistant enterococci and, more recently, S. aureus with reduced glycopeptide susceptibility. Treatment resistance of clinical MRSA has also been shown in this region against both β-lactam and non-β-lactam antibiotics (erythromycin, clindamycin, ciprofloxacin, and tetracycline), chloramphenicol, gentamicin, and sulfamethoxazole–trimethoprim.4
Even though there is a recent debate surrounding the use of vancomycin,9–11 it is still a common antibiotic for the treatment of nosocomial pneumonia and is recommended in the most recent guideline endorsed by the American Thoracic Society and the Infectious Disease Society of America.1 Despite guidelines, real-world practice patterns are known to vary substantially from those recommended. Furthermore, little is known in low- and middle-income countries of the Middle East regarding practice patterns and resource associated with MRSA pneumonia.
The objectives of this study were to describe the microbiologic profile of MRSA pneumonia in Lebanon and Saudi Arabia and assess the treatment received by these patients, in terms of drug selection, dosing, timing, and sensitivity of the infection pathogen, and to document hospital resource utilization and clinical outcomes among patients with this infection.
Methods
Study design
This study is a retrospective, observational medical chart review that took place at a total of five study sites in Saudi Arabia (two sites) and Lebanon (three sites). Per the study protocol, the study investigator (local physician responsible for data collection) assessed the need for ethics/IRB application at each site, IRB waiver or approval was completed at all sites based on local requirements. Specifically, approval was obtained from National Guard Health Affairs, King Abdalaziz Medical City- Jeddah and Riyadh, Kingdom of Saudi Arabia; Ain Wazein Hospital IRB; and Makassed General Hospital, Riad El-Solh Beirut, Lebanon.
Inclusion and exclusion criteria
In order to be included, patients had to have suspected or culture-proven MRSA pneumonia with appropriate respiratory symptoms. In the absence of culture results, suspected cases needed to have at least two of the following symptoms for inclusion: fever (body temperature ≥38.5˚C), hypothermia (body temperature ≤35.5˚C), respiratory rate (>30 breaths/min), systolic hypotension (<90 mmHg), heart rate (>120 beats/min), elevated peripheral white blood cell count (>10,000/mm3), leukopenia with total white blood cell count (<4,500 cells/mm3), and elevated inflammatory markers (eg, procalcitonin or C-reactive protein). Patients were excluded if they had inadequate data on the treatments and outcomes of interest or hospital resource utilization for the study, as judged by the investigators.
Data collection
Data were collected from patient medical records by hospital-based infectious disease specialists, medical microbiologists, internal medicine specialists, surgeons, and intensivists. All data collection instruments were translated into local language and, prior to roll out, pilot tested to ensure relevance and clarity of data points to be collected.
Extracted chart data included demographics and comorbid conditions; antibiotic treatment patterns, including initial and subsequent inpatient antibiotic regimens prescribed and antibiotic therapy prescribed at discharge; health-care resource utilization, including diagnostic procedures, laboratory tests, and length of hospital stay; and clinical outcomes, including clinical response at discharge and rehospitalization. Pneumonia was classified into one of the four categories by the abstracting physician as follows:
Community-acquired pneumonia: Symptom onset prior to hospital admission but with no known patient history of 1) another hospital visit, 2) visit to another health-care institution, or 3) residence in a nursing home within 90 days prior to the present hospital admission
Health-care-associated pneumonia (HCAP): Symptom onset during 1) another hospital visit, 2) visit to another health-care institution, or 3) residence in a nursing home within 90 days prior to the present hospital admission
Nosocomial pneumonia: Symptom onset at least 48 hours after hospital admission
Unknown
Antibiotics received during hospitalization were categorized as MRSA active and not MRSA active. MRSA-active antibiotics as defined by guidelines included vancomycin (intravenous only), teicoplanin (intravenous only), clindamycin, doxycycline, trimethoprim/sulfamethoxazole, linezolid, fusidic acid, rifampin, and tigecycline.12 Antibiotics categorized as not MRSA active included beta-lactams (by definition), and in the absence of institution-specific antibiograms, fluoroquinolones and carbapenems were only categorized as MRSA active if culture results indicated sensitivity to the respective antibiotic.
Statistical analysis
Unless otherwise listed, binary and categorical end points were summarized in terms of percentages in each category, and continuous variables were presented using number of observations (when less than the entire sample), arithmetic mean, and standard deviation (SD).
Results
Demographic and clinical characteristics
Chart-level data were collected for 93 patients with MRSA pneumonia, 50 in Saudi Arabia and 43 in Lebanon. Of the 93 patients in the sample, 20% of the MRSA infections were community acquired, 32% health-care associated, 35% nosocomial, and 12% of unknown origin. Underlying chronic pulmonary disease was present in 28% of patients (Table 1). However, the most common comorbidities among these patients were diabetes (39%) and congestive heart failure (30%).
Antibiotic treatment patterns
Ninety of the 93 patients in the sample (97%) had at least one culture taken, and 82 of these (91%) had at least one culture positive for MRSA. As would be expected, only one-third of the isolates tested were sensitive to ciprofloxacin and only 15% were sensitive to beta-lactams. Patients most frequently had positive cultures from pulmonary (87%) and blood (27%) samples; nasal sites were also culture positive in 16% of patients. Table 2 shows the susceptibility profile for MRSA isolates from patients with pneumonia. All isolates tested were sensitive to vancomycin (n=80) and teicoplanin (n=32). Only ten isolates were tested for linezolid sensitivity, and all were found to be sensitive. Only one-third of the isolates tested were sensitive to ciprofloxacin.
  | Table 2 MRSA pneumonia susceptibility profile Abbreviation: MRSA, methicillin-resistant Staphylococcus aureus. |
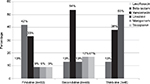
Drug selection for MRSA pneumonia, expressed as the percentage of patients receiving each antibiotic (with potential for multiple antibiotics per line of therapy), is summarized in Figure 1. Overall, 79 patients (85%) received at least one antibiotic considered to be MRSA active. The 93 MRSA pneumonia patients received a total of 140 antibiotic orders designated by study investigators to be first-line therapy (as some regimens consisted of multiple antibiotics). Of these, 28% were beta-lactams (thus representing inactive therapy), and the most frequently prescribed MRSA-active antibiotic was vancomycin (22% of orders). Among the 48 patients (52%) who were prescribed a total of 66 second-line antibiotic orders, vancomycin remained the most commonly prescribed antibiotic (39% of orders), followed by teicoplanin (12%) and linezolid (9%). Of the eleven third-line drugs received by eight patients, linezolid was the most commonly used (36% of orders), followed by vancomycin (27%) and ciprofloxacin (18%).
Other health-care resource utilization
Overall, the mean total length of stay (LOS) was 32.4 days, with the majority (20.4 days) spent in general wards (Figure 2). Thirty-six patients (39%) required mechanical ventilation, for a mean ± SD of duration of 18.7±24.4 days per patient. Four patients (4%) required hemodialysis, for a mean of 9.8 ± 11.9 days per patient. Of these patients, two patients were reported to have moderate-to-severe renal disease on admission, but whether these patients were receiving dialysis prior to admission is unknown.
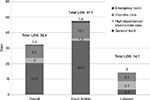  | Figure 2 Length of stay (LOS) among patients with MRSA pneumonia. Abbreviation: MRSA, methicillin-resistant Staphylococcus aureus. |
Other interventions required among patients with MRSA pneumonia included peripheral catheters (52%), central venous catheters (52%), and peripherally inserted central catheters (6%).
Clinical outcomes
Over one-fourth of the patients were considered treatment failures at the time of discharge (Table 3). The overall in-hospital mortality rate was 30%, with nearly one-third of deaths being MRSA related. Of the 28 patients who died, treatment response at discharge was failure in 24 (86%) cases, improved in one (3.5%), cure in two (7%), and indeterminate in one (3.5%). Relapse of MRSA pneumonia (3%) and rehospitalization for MRSA pneumonia (2%) were rare; however, all-cause rehospitalization occurred in 20% of patients.
Discussion
This real-world burden and treatment patterns study of MRSA pneumonia in the Middle East indicates a resource-intensive disease with a high LOS and mortality, consistent with other studies in high-income countries. Inappropriate/inactive therapy for MRSA nosocomial pneumonia was prescribed more than a quarter of the time over the entire course of therapy, and in first-line treatment was prescribed 42% of the time. The relatively high mortality rate in this study may have been influenced by the high proportion receiving suboptimal therapy as first-line treatment. In a multinational observational study in Asia and a randomized clinical trial in Canada, the frequency of prescribing inadequate initial antibiotic therapy in pneumonia may be >50%, and this significantly increases mortality rates.13,14 MRSA-targeted antibiotics were more frequently prescribed in second-line (vancomycin 54%) and third-line (linezolid 51%) treatments. Potential reasons for the administration of inappropriate antibiotics may be due to lack of physician knowledge, delay in receiving culture results, or availability of medications that cover MRSA. While the exact reason for prescribing inappropriate antibiotics cannot be determined from this study, a body of literature has focused on inadequate initial therapy as a driver of added morbidity and mortality.14,15
Administration of inappropriate empiric antibiotic therapy can increase mortality in patients with hospital-acquired pneumonia (defined in this study as occurring within 48 hours of admission) and ventilator-associated pneumonia (VAP, defined as occurring within 48 hours of endotracheal intubation). Results from a prospective surveillance study conducted through the Asian Network for Surveillance of Resistant Pathogens (ANSORP) found that of the 15.8% of patients with S. aureus hospital-acquired pneumonia or VAP, 50.4% received discordant therapy.13 Patients receiving discordant therapy had higher all-cause (44% vs 35%) and pneumonia-related (32% vs 21%) mortality than patients who received concordant therapy. Multivariate analysis results showed that discordant therapy was a risk factor for pneumonia-related mortality (OR, 1.542; 95% CI, 1.127–2.110; P=0.007).
Administration of inappropriate empiric antibiotic therapy can also increase mortality in patients with HCAP, defined as pneumonia in a patient admitted from a nursing home or other long-term care setting, who was hospitalized within the last 12 months, receiving dialysis or infusion therapy requiring regular visits to a hospital-based clinic, or who is immunocompromised. In a US-based study, patients with HCAP compared to community acquired pneumonia had higher mortality (24.6% vs 9.1%, P<0.001) and were more likely to receive inappropriate antimicrobial treatment (28.3% vs 13.0%, P<0.001).16 Mortality was higher for patients who received inappropriate compared to appropriate antibiotic therapy (32.2% vs 15.7%, P<0.001). Inappropriate initial antimicrobial therapy was identified as an independent risk factor for hospital mortality (OR, 2.19; 95% CI, 1.27–3.78; P<0.005).
Results from separate studies conducted at the same institution found that treatment escalation did not decrease mortality in patients with HCAP treated with inappropriate empiric antibiotic therapy17 and that initial therapy for HCAP should include an antibiotic that targets MRSA.16
The resource use findings were quite disparate between countries in this study where the mean LOS was 30 days longer in Saudi Arabia than in Lebanon. It is unclear whether this difference results from an underlying disparity in the severity of infection between groups, or a difference in health-care infrastructure. A surveillance study in Saudi Arabia looking at 2,800 patients admitted to the medical-surgical intensive care unit suggests that long LOS is not uncommon for patients that are severely ill. They found that patients with VAP had a longer mean LOS of 85 days versus 61 days in those without VAP.18 This population had primarily Gram-negative pneumonias, but 20% were attributed to Gram-positive infection.
The study is naturally limited by its retrospective design and reliance on data that are documented in the medical chart. Since inclusion criteria required that the patients were diagnosed with MRSA pneumonia, we do not have details on other patients that may have been colonized and not developed active infection. However, the information gathered from the charts represents a snapshot of the real-world treatment patterns and supplements the limited data we have in this indication and region of the world.
Conclusion
MRSA pneumonia in Saudi Arabia and Lebanon may be treated with antibiotics that are inactive against MRSA in a majority of patients for first-line therapy, resulting in an added resource use burden and mortality. The information gathered in this Middle East population provides important real-world perspectives on the current treatment patterns and opportunities to improve care. Future studies should more closely examine the appropriateness of initial therapy in MRSA pneumonia patients.
Acknowledgment
The authors thank Monica Katyal for her assistance with data collection and analysis.
Author contributions
Adel F Alothman, Abdulhakeem O Althaqafi, Madonna J Matar, Rima Moghnieh, Nirvana Raghubir, Seema Haider, Caitlyn T Solem, and Jennifer M Stephens contributed to study design. Cynthia Macahilig, Adel F Alothman, Abdulhakeem O Althaqafi, Madonna J Matar, Rima Moghnieh, Thamer H Alenazi, and Fayssal Farahat were involved in data acquisition. Caitlyn T Solem and Shelby Corman undertook data analysis. All authors contributed to data interpretation, manuscript drafting, and approved the final manuscript.
Disclosure
This study was sponsored by Pfizer. Shelby Corman, Jennifer M Stephens, and Caitlyn T Solem are employees of Pharmerit International, who were paid consultants to Pfizer in connection with the development of this manuscript and study design, management, and statistical analysis for the study. Cynthia Macahilig is an employee of Medical Data Analytics, a subcontractor to Pharmerit International, who was a paid consultant to Pfizer in the study design, management, and data collection for the study. Nirvana Raghubir and Seema Haider are employees and shareholders of Pfizer. Adel F Alothman received honoraria for several presentations from Pfizer, MSD, and Al Hikma; received travel support from Pfizer, MSD, Gilead, and Al Hikma to attend symposia; and received honoraria for patient data collection related to this study from Pfizer. Abdulhakeem O Althaqafi received a research grant (RR13/248/J) sponsored by Pfizer and received honoraria for patient data collection related to this study from Pfizer. Fayssal Farahat received honoraria for patient data collection related to this study from Pfizer. Madonna J Matar received travel support for attending meetings and received honoraria for patient data collection related to this study from Pfizer. Rima Moghnieh received honoraria for patient data collection related to this study from Pfizer and sponsorship for attending medical meetings from Pfizer and MSD. Thamer H Alenazi received travel support for attending medical meetings from MSD and Pfizer. The authors report no other conflicts of interest in this work.
References
American Thoracic Society, Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171(4):388–416. | ||
Zigmond J, Pecan L, Hájek P, Raghubir N, Omrani A. MRSA infection and colonization rates in Africa and Middle East: a systematic review & meta-analysis. 16th International Congress on Infectious Diseases. Cape Town: 2014. | ||
Iyer A, Kumosani T, Azhar E, Barbour E, Harakeh S. High incidence rate of methicillin-resistant Staphylococcus aureus (MRSA) among healthcare workers in Saudi Arabia. J Infect Dev Ctries. 2014;8(3):372–378. | ||
Tokajian S. New epidemiology of Staphylococcus aureus infections in the Middle East. Clin Microbiol Infect. 2014;20(7):624–628. | ||
Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52:1–38. | ||
Gould IM, Cauda R, Esposito S, Gudiol F, Mazzei T, Garau J. Management of serious meticillin-resistant Staphylococcus aureus infections: what are the limits? Int J Antimicrob Agents. 2011;37(3):202–209. | ||
Morgan M. Treatment of MRSA soft tissue infections: an overview. Injury. 2011;42(suppl 5):S11–S17. | ||
Rodvold KA, McConeghy KW. Methicillin-resistant Staphylococcus aureus therapy: past, present, and future. Clin Infect Dis. 2014;58(suppl 1):S20–S27. | ||
Georges H, Leroy O, Alfandari S, et al. Pulmonary disposition of vancomycin in critically ill patients. Eur J Clin Microbiol Infect Dis. 1997;16(5):385–388. | ||
Lodise TP, Patel N, Lomaestro BM, Rodvold KA, Drusano GL. Relationship between initial vancomycin concentration-time profile and nephrotoxicity among hospitalized patients. Clin Infect Dis. 2009;49(4):507–514. | ||
Patel N, Pai MP, Rodvold KA, Lomaestro B, Drusano GL, Lodise TP. Vancomycin: we can’t get there from here. Clin Infect Dis. 2011;52(8):969–974. | ||
Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the infectious diseases society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52(3):e18–e55. | ||
Chung DR, Song JH, Kim SH, et al; Asian Network for Surveillance of Resistant Pathogens Study Group. High prevalence of multidrug-resistant nonfermenters in hospital-acquired pneumonia in Asia. Am J Respir Crit Care Med. 2011;184(12):1409–1417. | ||
Muscedere JG, Shorr AF, Jiang X, Day A, Heyland DK; Canadian Critical Care Trials Group. The adequacy of timely empiric antibiotic therapy for ventilator-associated pneumonia: an important determinant of outcome. J Crit Care. 2012;27(3):322.e7–322.e14. | ||
Zilberberg MD, Shorr AF, Micek ST, et al. Hospitalizations with healthcare-associated complicated skin and skin structure infections: impact of inappropriate empiric therapy on outcomes. J Hosp Med. 2010;5(9):535–540. | ||
Micek ST, Reichley RM, Kollef MH. Health care-associated pneumonia (HCAP): empiric antibiotics targeting methicillin-resistant Staphylococcus aureus (MRSA) and Pseudomonas aeruginosa predict optimal outcome. Medicine (Baltimore). 2011;90(6):390–395. | ||
Zilberberg MD, Shorr AF, Micek ST, Mody SH, Kollef MH. Antimicrobial therapy escalation and hospital mortality among patients with health-care-associated pneumonia: a single-center experience. Chest. 2008;134(5):963–968. | ||
Al-Dorzi HM, El-Saed A, Rishu AH, Balkhy HH, Memish ZA, Arabi YM. The results of a 6-year epidemiologic surveillance for ventilator-associated pneumonia at a tertiary care intensive care unit in Saudi Arabia. Am J Infect Control. 2012;40(9):794–799. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.