Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 15
Burden of Metabolic Syndrome Among a Low-Income Population in China: A Population-Based Cross-Sectional Study
Authors Bao J, Wang L, Hu P , Liu J , Tu J, Wang J , Li J, Ning X
Received 5 June 2022
Accepted for publication 16 August 2022
Published 3 September 2022 Volume 2022:15 Pages 2713—2723
DOI https://doi.org/10.2147/DMSO.S377490
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Konstantinos Tziomalos
Jie Bao,1,* Lifeng Wang,2,* Peng Hu,3,* Jie Liu,2,4– 6 Jun Tu,2,4– 6 Jinghua Wang,2,4– 6 Jidong Li,2,7 Xianjia Ning2,4– 6
1Department of Rehabilitation Medicine, Tianjin Medical University General Hospital, Tianjin, 300052, People’s Republic of China; 2Center of Clinical Epidemiology & Evidence-Based Medicine, Tianjin Jizhou People’s Hospital, Tianjin, 301900, People’s Republic of China; 3Department of Acupuncture Encephalopathy, Binhai New Area Hospital of TCM, Tianjin, 300451, People’s Republic of China; 4Department of Neurology, Tianjin Medical University General Hospital, Tianjin, 300052, People’s Republic of China; 5Laboratory of Epidemiology, Tianjin Neurological Institute, Tianjin, 300052, People’s Republic of China; 6Tianjin Neurological Institute, Key Laboratory of Post-Neuroinjury Neuro-repair and Regeneration in Central Nervous System, Ministry of Education and Tianjin City, Tianjin, 300052, People’s Republic of China; 7Department of Neurosurgery, Tianjin Jizhou People’s Hospital, Tianjin, 301900, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xianjia Ning, Laboratory of Epidemiology, Tianjin Neurological Institute & Tianjin Neurological Institute, Key Laboratory of Post-Neuroinjury Neuro-repair and Regeneration in Central Nervous System, Ministry of Education and Tianjin City, Tianjin, 300052, People’s Republic of China, Tel +86-22-60817505, Fax +86-22-60817448, Email [email protected] Jidong Li, Department of Neurosurgery, Tianjin Jizhou People’s Hospital, Center of Clinical Epidemiology & Evidence-Based Medicine, Tianjin Jizhou People’s Hospital, 18 Nanhuan Road, Jizhou District, Tianjin, 301900, People’s Republic of China, Tel/Fax +86-22- 60733586, Email [email protected]
Introduction: Metabolic syndrome (MetS) is a chronic and complex disease associated with all-cause mortality, cardiovascular disease, and type 2 diabetes. The present study aimed to evaluate the prevalence of MetS and its risk factors among middle-aged and older adults in low-income, low-education rural areas with a high incidence of stroke.
Methods: This cross-sectional study of the general population was performed from April 2019 to June 2019 in rural areas of Tianjin, China. All eligible residents aged ≥ 45 years and without active malignant tumors, hepatic failure, and severe renal disease underwent routine medical examinations, which included a questionnaire, physical examination, and routine blood and biochemical tests. The modified International Diabetes Federation criteria for the Asian population was used to identify patients with MetS.
Results: A total of 3175 individuals (44.8% men, 55.2% women) were included in the final analysis. The prevalence of MetS was 52.8%, with higher prevalence in women than in men (62.4%and 40.9%, respectively). Of the five MetS components, high blood pressure and abdominal obesity were the two most prevalent in both women and men, accounting for 89.3% and 62.0%, respectively, followed by elevated fasting plasma glucose, low high-density lipoprotein cholesterol, and elevated triglycerides. Multivariate logistic regression analysis revealed the following traits to be risk factors for MetS: female sex, self-reported smoking, self-reported snoring, high body mass index, high waist-to-hip ratio, and high serum urate level.
Conclusion: The prevalence of MetS was quite high in rural areas with a low-income, low-education population. Implementing preventive and therapeutic interventions based on these risk factors is essential to prevent metabolic abnormalities.
Keywords: metabolic syndrome, epidemiology, risk factors, population-based, cross-sectional study
Introduction
Non-communicable diseases (NCDs) are the leading cause of death globally.1 One of the most common NCDs is metabolic syndrome (MetS), a disorder characterized by obesity, insulin resistance, hypertension, and hyperlipidemia.1 MetS can lead to the development of diseases including type 2 diabetes (T2DM), cardiovascular disease, nonalcoholic steatohepatitis, and cancer.2,3
Characterized by a cluster of interconnected cardiometabolic risk factors, including obesity, dyslipidemia, hypertension, and impaired glucose tolerance,4 MetS contributes to the development of coronary diseases, stroke, T2DM, and premature mortality.5 Previous research has shown that adults residing in rural China had a higher prevalence of MetS than urban adult residents in the United States.6 In addition, previous study reported that the prevalence of MetS in rural areas worldwide ranges from 2.45% to 39.70%.6
Despite the differing prevalence of MetS that depend on the various criteria used in different definitions, as well as the composition of the population studied, including sex, age, and ethnicity,7 the prevalence of MetS is alarmingly high, and a vast proportion of the population with MetS is at high risk of developing cardiovascular diseases and type 2 diabetes.8 Longer lifespans in China have accompanied a rapid increase in per-capita income, a rising proportion of the elderly, and progressive Westernization of lifestyle,9 and the lower socioeconomic status associated with the presence of MetS in a rural population in China.10 Accordingly, the resulting persistent increase in the prevalence of MetS in rural areas calls for further evaluation of modifiable risk factors for MetS.
We previously demonstrated a high incidence of stroke in rural areas in China, with an age-standardized incidence of first-ever stroke per 100,000 person-years of up to 318.2 in 2012 and an incidence of stroke that increased annually by 6.5% overall from 1992–2012.11 Considering the burgeoning epidemic of stroke worldwide, especially in rural areas of China, as well as the fact that MetS is a risk factor for stroke,12,13 early recognition of MetS risk factors and prompt intervention to curb disease progression is imperative. Therefore, the present study aimed to evaluate the prevalence of MetS among middle-aged and older adults in a low-income, low-education population in rural areas with a high incidence of stroke and identify potential risk factors for the development of MetS.
Methods and Materials
Study Population
This population-based cross-sectional study was derived from The Tianjin Brain Study, which is an ongoing longitudinal cohort study in Tianjin, China. Briefly, the Tianjin Brain Study is a population-based stroke surveillance project including 14,251 participants from 18 administrative villages in the Yangjinzhuang township of Ji country in Tianjin, China.14 The 95% local residents were farmers with relatively low levels of income and education. The main source of income was grain production, and the residents had an annual per capita income of <$100 in 1991 and <$1000 in 2010.15 100% of the population eats rice and wheat flour as staple food.
The study recruited all adults at least 45 years of age who resided in the villages between April and June 2019. In this study, all qualified residents who lived in this township more than five years were the potential participants. Exclusion criteria were age < 45 years, active malignant tumors, hepatic failure, and severe renal disease (defined as an estimated glomerular filtration rate of < 30 mL/min/1.73 m2). All eligible participants underwent routine medical examinations, which included a questionnaire, physical examination, and routine blood and biochemical tests.
Of the 4222 residents who met the inclusion criteria, 3182 eligible individuals enrolled in this study, with the response rate of 75.4%. After excluding seven residents with missing data (including physical examination results and biochemical parameters), 3175 participants were finally included in this study (Figure 1).
This study was approved by the Ethics Committee of the Tianjin Medical University General Hospital and complies with the Declaration of Helsinki. All participants provided written informed consent.
Data Collection and Identification of Risk Factors
Anthropometric Measurements
Personal information, including demographic characteristics (sex, age, education level, household income), personal/family medical history (coronary heart disease and stroke), and lifestyle factors (cigarette smoking, alcohol consumption, physical activity, and snoring) information were obtained through a specific questionnaire administered by trained researchers in person. Snoring status was ascertained based on the participants’ self-report and as assisted by family members. History of coronary heart disease and stroke were based on medical records.
Physical Examinations
Height, body weight, and hip and waist circumference (WC) were measured using a height scale and tape measure by trained staff. Blood pressure (BP) was measured using an electronic sphygmomanometer (HEM-741 C; Omron, Tokyo, Japan) a total of three times with the participant in a seated position at least 30 minutes after consumption of coffee, tea, and alcohol; cigarette smoking; and strenuous exercise. The mean values were used for analysis. Waist-to-hip ratio (WHR) was calculated as WC (cm) divided by the hip circumference (cm).
Biochemical Parameters
For routine blood and biochemical tests, 10 mL of blood were drawn from all patients. Fasted venous blood samples were obtained in the morning after the participants had fasted for at least 12 hours. Serum was obtained by centrifuging the sample at 3000 RPM for 10 minutes and then taking the supernatant. Blood tests for all patients were performed at the Laboratory Department of Jizhou District People’s Hospital, which is a qualified laboratory center that meets the national standard. Blood biochemical indices including total cholesterol (TC: 2.58–5.17 mmol/L), triglyceride (TG: 0.56–1.70 mmol/L), low-density lipoprotein cholesterol (LDL-C< 3.12 mmol/L), high-density lipoprotein cholesterol (HDL-C> 1.04 mmol/L), fasting plasma blood glucose (FBG: 4.4–6.1 mmol/L), and serum uric acid (SUA) were analyzed at Guangzhou KingMed clinical laboratory in China. High SUA was defined as an SUA level ≥ 7.0 mg/dL (417 µmol/L) in men or ≥ 6.0 mg/dL (357 µmol/L) in women.16
Grouping and Definition of Variables
Education level was represented by three values: illiterate (without education), below high school (1–9 years), and high school (10–12 years). Household income was classified as < 2000, 2000–6000, and > 6000 yuan/year. Cigarette smoking was defined as smoking > 1 cigarette/day for ≥ 1 year and divided into never smoked, ever smoked, and currently smoked.17 Alcohol consumption was defined as drinking > 30 mL of alcohol per week for ≥ 1 year and was categorized as never drinking, ever drinking, and current drinking.18 Physical activity was defined as involvement in physical activity ≥ 2 days/week for ≥ 30 min/day. Body mass index (BMI) was calculated as weight (kg) divided by the square of height (m2) and included three categories: normal or underweight (BMI < 24 kg/m2), overweight (24.00 kg/m2 ≤ BMI < 28.00 kg/m2), and obese (BMI ≥ 28.00 kg/m2).19
Definitions of Metabolic Syndrome
According to the modified International Diabetes Federation criteria for the Asian population (2009), at least the following three conditions must be met to be defined as MetS:20 (1) abdominal obesity (WC ≥ 90 cm in men and ≥ 80 cm in women); (2) elevated TG (TG ≥ 1.70 mmol/L or medications to treat hypertriglyceridemia); (3) low HDL-C (HDL-C < 1.03 mmol/L in men and < 1.3 mmol/L in women, or using medications to regulate HDL-C); (4) high BP (systolic BP [SBP] ≥ 130 mmHg or diastolic BP [DBP] ≥ 85 mmHg, or medications to treat hypertension); or (5) elevated fasting plasma glucose ([FPG] ≥ 5.6 mmol/L or on antidiabetic treatment).
Statistical Analysis
Continuous variables were described using the mean and standard deviation and were compared using Student’s t-test; the distribution of these variables was assessed by the Kolmogorov–Smirnov test before performing Student’s t-test. Categorical variables were described as frequency and percentage and were compared using Pearson’s chi-squared test. Multivariate logistic regression was used to estimate the association between MetS and related factors by calculating the odds ratios (OR) and 95% confidence intervals (CIs) after adjustment for covariates with P <0.10 in the univariate analysis. We performed a sensitivity analysis for avoiding the effect of lifestyle intervention and medication on components of MetS among patients with the histories of coronary heart disease and/or stroke. Statistical analyses were conducted using IBM SPSS Statistics for Windows (version 25.0; IBM Corp, Armonk, NY, USA). All statistical tests were two-sided with a P value < 0.05 considered as significant.
Results
Demographic and Clinical Characteristics
Of the 3175 included individuals, 44.8% were men, and 55.2% were women. The mean age was 64 years. The 65-year and older age group accounted for 44.4%, the 55–64-year group accounted for 44.4%, and the 45–54-year group accounted for 14.5%. Altogether, 90% of the participants had ≤ 10-year education level; 73.5% had 1–9 year education, and 17.6% were illiterate. Moreover, 97% of the participants were of low and middle income, with household incomes less than 6000 yuan. In addition, 95.7% of women and 26.8% of men were never smokers and 98.0% and 46.7%, respectively, never drank alcohol. More than half of both men and women participated in physical activity and had self-reported snoring behavior. Furthermore, individuals with obesity, high SUA, and cardiovascular diseases accounted for 25.7%, 14.4%, and 16.1%, respectively. (Table 1).
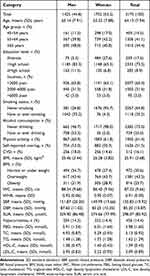 |
Table 1 Characteristics of Study Participants by Sex |
Prevalence of MetS and Its Components
The total prevalence of MetS in this study was 52.8%, and the prevalence was higher in women (62.4%) than in men (40.9%). Individuals with two and three MetS components comprised the highest proportion, while those with five components comprised the lowest proportion. Among single MetS components, the major component was high BP (89%), followed by abdominal obesity (62.0%), elevated FBG (47.7%), low HDL-C level (33.7%), and elevated TG level (35.1%). Moreover, the values of MetS components, including abdominal obesity, low HDL-C level, and elevated TG level in women, seemed to be higher than those in men (Table 2).
 |
Table 2 Characteristics of Metabolic Syndrome and Its Components |
Risk Factors for MetS
In general, participants with MetS were more likely to be female, older, non-smokers, and non-drinkers. Moreover, these individuals were more likely to have a history of self-reported snoring, overweight and obesity, and high SUA level. Higher levels of BMI, WHR, SUA level, LDL-C were also found in participants with MetS than in those without MetS (all P < 0.05; Table 3).
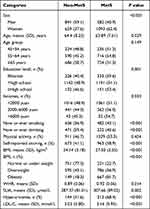 |
Table 3 The Univariate Analysis of Influence Factors for Metabolic Syndrome |
The significant factors of MetS in the multivariate analysis are presented in Table 4. After multivariable adjustment for confounding factors including sex, age, education level, income level, smoking status, alcohol consumption, BMI, WHR, self-reported snoring, SUA level, and LDL-C level at baseline, compared with the reference group, the risk factors for MetS included female sex, current or ever smoking, BMI, WHR, self-reported snoring, and SUA level. Female sex (OR = 4.791, 95% CI: 3.597, 6.271), smoking (OR = 1.363; 95% CI: 1.035, 1.794), high BMI (OR = 2.565; 95% CI: 2.285, 2.879), high WHR (OR = 1.929; 95% CI: 1.740, 2.138), self-reported snoring (OR =1.221; 95% CI: 1.027, 1.451), and elevated SUA level (OR = 1.291; 95% CI: 1.178, 1.415) were significantly associated with a higher likelihood of developing MetS (all P < 0.05).
 |
Table 4 Risk Factors of Metabolic Syndrome in Multivariate Analysis |
In the sensitivity analyses, the risk factors for MetS remained similar to those in the main analysis (Table 5).
 |
Table 5 The Sensitivity Analyses of the Association of Metabolic Syndrome with Relative Risk Factors |
Discussion
This is the first investigation into the prevalence and risk factors for metabolic abnormalities in rural areas of China with a high stroke risk. The total prevalence of MetS was 52.8%, of which high BP and abdominal obesity were the major components in both sexes, accounting for 89.3% and 62.0%, respectively. Female sex, smoking, self-reported snoring, high BMI, WHR, and SUA level were independent risk factors for MetS.
MetS often accompanies obesity and type 2 diabetes and is associated with a high risk of cardiovascular diseases and stroke independent of the diagnostic criteria used.17–19 The high prevalence of MetS in rural areas based on epidemiological studies has caused great concern in the aging society in China. Two health surveys conducted in southern China reported that the age-standardized prevalence of MetS increased fourfold (from 5.4% in 2002 to 21.3% in 2010) in individuals aged ≥ 20 years.21 An earlier study conducted in 2010 showed that MetS was prevalent at 10.8% among rural adults in northwest China according International Diabetes Federation criteria.21 In the current study conducted in 2019, a high total prevalence of MetS was observed, ie, up to 52%, in the low-income, low-education population aged ≥ 45 years, which is a higher prevalence than that reported in previous reports. However, these data are consistent with those reported in a 2004 study based on the same diagnostic criteria for MetS, which demonstrated a prevalence of 56.9% among a rural older population in China.22 In view of the rising prevalence of MetS, rapid economic development and accelerated changes in dietary patterns and lifestyles may play an important role.21 For instance, a higher consumption of snacks and fried food was observed among rural residents.23
The current results show a sex-specific prevalence of MetS, which was higher in women than in men, similar to previous findings.21,24 The China Health and Retirement Longitudinal Study (CHARLS) illustrated that women had a 2.94-fold greater risk of MetS (95% CI 2.55–3.39, P < 0.001) compared with that of men. In contrast, the findings of the Third National Health and Nutrition Examination Survey revealed that MetS was more prevalent in men than in women (30.53% vs 20.44%).25 Given that sex-related factors are susceptible to social and cultural behaviors, dietary habits, and psychosocial factors, women are more prone than men to developing MetS in response to work stress and lower socioeconomic status,26 especially in rural areas. The Fourth Korea National Health and Nutrition Examination Surveys conducted in 2014 also revealed that sex-specific socioeconomic disparities in adulthood have differential effects on the prevalence of MetS.9 A previous study has shown that in both mice and humans, adipose mitochondrial functions are elevated in females and are strongly associated with adiposity, insulin resistance, and plasma lipids.27 Since sex differences in the prevalence of MetS may translate to different cardiovascular-associated risks,25 more attention should be paid to the consequence of sex disparities on metabolic disorders.
The prevalence of high blood pressure and abdominal obesity is of great concern in rural areas. These findings are consistent with those of previous reports.28 Data from the 2017 Beijing Chronic Disease and Risk Factors Surveillance, which examined a representative sample of 12,597 urban residents, found that the proportion of high blood pressure and central obesity were 42.02 and 43.06%, respectively. Strategies aiming to prevent and control the epidemic of high blood pressure and obesity should be prioritized to reduce the occurrence and progression of MetS in rural areas.
Previous epidemiological evidence suggests that chronic smoking is associated with the development of MetS29,30 and is a risk factor for cardiovascular events.31 Moreover, a meta-analysis of prospective studies reported that active smoking is associated with the development of MetS;29 however, the pathophysiological interaction between cigarette smoking and MetS requires further elucidation. Similar to the previous results, current or ever-smoking based on self-reported questionnaire has been associated with a high risk of MetS after adjustment in multivariate analysis and sensitivity analysis in this study.
Obesity, which is associated with metabolic disturbances, is rapidly becoming more prevalent in developing countries, leading to increased morbidity and mortality due to cardiovascular disease and T2DM.32–34 BMI, as well as indices of abdominal obesity (WC and WHR), have been associated with a higher cardiometabolic risk.35–37 In the present study, BMI and WHR were found to be linked to MetS. A study on Nigerian population showed that both WC and WHR were good predictors of MetS.38 Further, data from a cross-sectional study in China showed that BMI and WC were more useful than WHR in predicting two or more non-adipose components of MetS.39 These findings suggest the need to further explore the role of obesity measurement tools in assessing MetS risk.
Snoring, a common but weak specific marker of obstructive sleep apnea, is associated with potential cardiovascular events.40 We observed that 51.5% of middle-aged or older individuals reported a habit of snoring. Several epidemiological studies have suggested a significant association between self-reported snoring and MetS and its components.41,42 A meta-analysis of 40 studies with 966,652 participants showed that snoring was a risk factor of MetS, and a linear trend was detected in the association between snoring and all MetS components except low-HDL.43 In a Korean multi-rural communities cohort study, snoring was found to be significantly and linearly associated with MetS.44 Similarly, our data showed that self-reported snoring was a relevant determinant for MetS, with an OR of 1.221. The long-term effects of snoring on metabolic disorders and their underlying mechanisms deserve further investigation.
The prevalence of high SUA in adults was 14.4%. At normal SUA levels, uric acid can scavenge oxygen radicals and protect the erythrocyte membrane from lipid oxidation,45 while high SUA levels are associated with cardiovascular disease, diabetes mellitus, and MetS.46–48 Our findings are similar to those of a study conducted among Bangladeshi adults, which showed that elevated SUA was significantly associated with the prevalence of MetS and its components.49 However, whether elevated SUA levels are a risk factor or only a biomarker in the progression and development of MetS remains controversial. Further longitudinal studies are required to determine if a high SUA level is an additional component of MetS.
The present study has several limitations. First, as is inherent in a cross-sectional study, our findings are subject to the effects of confounding factors, and no conclusions regarding causality can be drawn. More relevant prospective studies are therefore necessary. Second, the present findings are based on a rural population aged ≥45 years. It is important to include populations with different ethnic and socioeconomic backgrounds and of a greater age range for more accurate and generalizable results. Third, other confounders, such as differing noise pollution, drugs, and a more detailed diet analysis may also influence the results and need further investigation.
Conclusions
In summary, MetS is highly prevalent in a low-income and low-education Chinese population with a high incidence of stroke. Appropriate management of high blood pressure and abdominal obesity, major components in both sexes, is necessary; blood pressure should be maintained within the normal range and visceral fat reduced. Early preventive and therapeutic interventions for these significant risk factors for MetS are crucial to curb metabolic abnormalities. Targeted prevention and treatment plans should be formulated based on individual differences.
Data Sharing Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
This study was approved by the Ethics Committee of the Tianjin Medical University General Hospital; this study complies with the Declaration of Helsinki. All participants provided written informed consent.
Acknowledgments
We thank all participants of the Tianjin Brain Study, and local medical care professionals for their valuable contributions.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
The authors declare no competing financial interests.
References
1. Girum T, Mesfin D, Bedewi J, Shewangizaw M. The burden of noncommunicable diseases in Ethiopia, 2000–2016: analysis of evidence from global burden of disease study 2016 and global health estimates 2016. Int J Chronic Dis. 2020;2020:3679528. doi:10.1155/2020/3679528
2. Esposito K, Chiodini P, Colao A, Lenzi A, Giugliano D. Metabolic syndrome and risk of cancer: a systematic review and meta-analysis. Diabetes Care. 2012;35(11):2402–2411. doi:10.2337/dc12-0336
3. Mili N, Paschou SA, Goulis DG, Dimopoulos MA, Lambrinoudaki I, Psaltopoulou T. Obesity, metabolic syndrome, and cancer: pathophysiological and therapeutic associations. Endocrine. 2021;74(3):478–497. doi:10.1007/s12020-021-02884-x
4. Vona R, Gambardella L, Cittadini C, Straface E, Pietraforte D. Biomarkers of oxidative stress in metabolic syndrome and associated diseases. Oxid Med Cell Longev. 2019;2019:8267234. doi:10.1155/2019/8267234
5. Spahis S, Borys JM, Levy E. Metabolic syndrome as a multifaceted risk factor for oxidative stress. Antioxid Redox Signal. 2017;26(9):445–461. doi:10.1089/ars.2016.6756
6. Trivedi T, Liu J, Probst JC, Martin AB. The metabolic syndrome: are rural residents at increased risk. J Rural Health. 2013;29(2):188–197. doi:10.1111/j.1748-0361.2012.00422.x
7. Samson SL, Garber AJ. Metabolic syndrome. Endocrinol Metab Clin North Am. 2014;43(1):1–23. doi:10.1016/j.ecl.2013.09.009
8. Kassi E, Pervanidou P, Kaltsas G, Chrousos G. Metabolic syndrome: definitions and controversies. BMC Med. 2011;9:48. doi:10.1186/1741-7015-9-48
9. Cheng J, Zhao D, Zeng Z, et al. The impact of demographic and risk factor changes on coronary heart disease deaths in Beijing, 1999–2010. BMC Public Health. 2009;9:30. doi:10.1186/1471-2458-9-30
10. Yang JJ, Yoon HS, Lee SA, et al. Metabolic syndrome and sex-specific socio-economic disparities in childhood and adulthood: the Korea National Health and Nutrition Examination Surveys. Diabet Med. 2014;31(11):1399–1409. doi:10.1111/dme.12525
11. Wang J, An Z, Li B, et al. Increasing stroke incidence and prevalence of risk factors in a low-income Chinese population. Neurology. 2015;84(4):374–381. doi:10.1212/WNL.0000000000001175
12. Mottillo S, Filion KB, Genest J, et al. The metabolic syndrome and cardiovascular risk a systematic review and meta-analysis. J Am Coll Cardiol. 2010;56(14):1113–1132. doi:10.1016/j.jacc.2010.05.034
13. Cabré JJ, Martín F, Costa B, et al. Metabolic syndrome as a cardiovascular disease risk factor: patients evaluated in primary care. BMC Public Health. 2008;8:251. doi:10.1186/1471-2458-8-251
14. Ren L, Shi M, Wu Y, et al. Correlation between hypertension and common carotid artery intima-media thickness in rural China: a population-based study. J Hum Hypertens. 2018;32(8–9):548–554. doi:10.1038/s41371-018-0074-x
15. Administration NHS. Statistical bulletin on healthcare security 2018; 2019. Available from: http://www.nhsa.gov.cn/art/2019/.
16. Redon P, Maloberti A, Facchetti R, et al. Gender-related differences in serum uric acid in treated hypertensive patients from central and east European countries: findings from the Blood Pressure control rate and CArdiovascular Risk profilE study. J Hypertens. 2019;37(2):380–388. doi:10.1097/HJH.0000000000001908
17. Lou Y, Li B, Su L, et al. Association between body mass index and presence of carotid plaque among low-income adults aged 45 years and older: a population-based cross-sectional study in rural China. Oncotarget. 2017;8(46):81261–81272.
18. Turner C. How much alcohol is in a “standard drink”? An analysis of 125 studies. Br J Addict. 1990;85(9):1171–1175. doi:10.1111/j.1360-0443.1990.tb03442.x
19. Zhou BF. Effect of body mass index on all-cause mortality and incidence of cardiovascular diseases–report for meta-analysis of prospective studies open optimal cut-off points of body mass index in Chinese adults. Biomed Environ Sci. 2002;15(3):245–252.
20. Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120(16):1640–1645. doi:10.1161/CIRCULATIONAHA.109.192644
21. Lao XQ, Ma WJ, Sobko T, et al. Dramatic escalation in metabolic syndrome and cardiovascular risk in a Chinese population experiencing rapid economic development. BMC Public Health. 2014;14:983. doi:10.1186/1471-2458-14-983
22. Yan Z, Liang Y, Jiang H, Cai C, Sun B, Qiu C. Metabolic syndrome and subclinical carotid atherosclerosis among Chinese elderly people living in a rural community. Metab Syndr Relat Disord. 2014;12(5):269–276. doi:10.1089/met.2013.0135
23. Wang Z, Zhai F, Du S, Popkin B. Dynamic shifts in Chinese eating behaviors. Asia Pac J Clin Nutr. 2008;17(1):123–130.
24. Ge H, Yang Z, Li X, et al. The prevalence and associated factors of metabolic syndrome in Chinese aging population. Sci Rep. 2020;10(1):20034. doi:10.1038/s41598-020-77184-x
25. Lin JW, Caffrey JL, Chang MH, Lin YS. Sex, menopause, metabolic syndrome, and all-cause and cause-specific mortality–cohort analysis from the Third National Health and Nutrition Examination Survey. J Clin Endocrinol Metab. 2010;95(9):4258–4267. doi:10.1210/jc.2010-0332
26. Pucci G, Alcidi R, Tap L, Battista F, Mattace-Raso F, Schillaci G. Sex- and gender-related prevalence, cardiovascular risk and therapeutic approach in metabolic syndrome: a review of the literature. Pharmacol Res. 2017;120:34–42. doi:10.1016/j.phrs.2017.03.008
27. Chella Krishnan K, Vergnes L, Acín-Pérez R, et al. Sex-specific genetic regulation of adipose mitochondria and metabolic syndrome by Ndufv2. Nat Metab. 2021;3(11):1552–1568. doi:10.1038/s42255-021-00481-w
28. Ma A, Fang K, Dong J, Dong Z. Prevalence and related factors of metabolic syndrome in Beijing, China (Year 2017). Obes Facts. 2020;13(6):538–547. doi:10.1159/000508842
29. Sun K, Liu J, Ning G, Barengo NC. Active smoking and risk of metabolic syndrome: a meta-analysis of prospective studies. PLoS One. 2012;7(10):e47791. doi:10.1371/journal.pone.0047791
30. Kim BJ, Kim BS, Sung KC, Kang JH, Lee MH, Park JR. Association of smoking status, weight change, and incident metabolic syndrome in men: a 3-year follow-up study. Diabetes Care. 2009;32(7):1314–1316. doi:10.2337/dc09-0060
31. Ishizaka N, Ishizaka Y, Toda E, Hashimoto H, Nagai R, Yamakado M. Association between cigarette smoking, metabolic syndrome, and carotid arteriosclerosis in Japanese individuals. Atherosclerosis. 2005;181(2):381–388. doi:10.1016/j.atherosclerosis.2005.01.026
32. Misra A, Khurana L. Obesity and the metabolic syndrome in developing countries. J Clin Endocrinol Metab. 2008;93(11 Suppl 1):S9–30. doi:10.1210/jc.2008-1595
33. Martin KA, Mani MV, Mani A. New targets to treat obesity and the metabolic syndrome. Eur J Pharmacol. 2015;763(Pt A):64–74. doi:10.1016/j.ejphar.2015.03.093
34. Moore KJ, Shah R. Introduction to the obesity, metabolic syndrome, and CVD compendium. Circ Res. 2020;126(11):1475–1476. doi:10.1161/CIRCRESAHA.120.317240
35. Huxley R, Mendis S, Zheleznyakov E, Reddy S, Chan J. Body mass index, waist circumference and waist: hipratio as predictors of cardiovascular risk–a review of the literature. Eur J Clin Nutr. 2010;64(1):16–22. doi:10.1038/ejcn.2009.68
36. Czernichow S, Kengne AP, Stamatakis E, Hamer M, Batty GD. Body mass index, waist circumference and waist-Hip ratio: which is the better discriminator of cardiovascular disease mortality risk?: evidence from an individual-participant meta-analysis of 82 864 participants from nine cohort studies. Obes Rev. 2011;12(9):680–687. doi:10.1111/j.1467-789X.2011.00879.x
37. Peer N, Lombard C, Steyn K, Levitt N. Waist-to-height ratio is a useful indicator of cardio-metabolic risk in South Africa. Fam Pract. 2020;37(1):36–42. doi:10.1093/fampra/cmz044
38. Ghazali SM, Sanusi RA. Waist circumference, waist to Hip ratio, and body mass index in the diagnosis of metabolic syndrome in Nigerian subjects. Niger J Physiol Sci. 2010;25(2):187–195.
39. Wang F, Wu S, Song Y, et al. Waist circumference, body mass index and waist to Hip ratio for prediction of the metabolic syndrome in Chinese. Nutr Metab Cardiovasc Dis. 2009;19(8):542–547. doi:10.1016/j.numecd.2008.11.006
40. Nagayoshi M, Tanigawa T, Yamagishi K, et al. Self-reported snoring frequency and incidence of cardiovascular disease: the Circulatory Risk in Communities Study (CIRCS). J Epidemiol. 2012;22(4):295–301. doi:10.2188/jea.JE20110109
41. Sabanayagam C, Zhang R, Shankar A. Markers of sleep-disordered breathing and metabolic syndrome in a multiethnic sample of US adults: results from the National Health and Nutrition Examination Survey 2005–2008. Cardiol Res Pract. 2012;2012:630802. doi:10.1155/2012/630802
42. Sun L, Pan A, Yu Z, et al. Snoring, inflammatory markers, adipokines and metabolic syndrome in apparently healthy Chinese. PLoS One. 2011;6(11):e27515. doi:10.1371/journal.pone.0027515
43. Ma J, Zhang H, Wang H, et al. Association between self-reported snoring and metabolic syndrome: a systematic review and meta-analysis. Front Neurol. 2020;11:517120. doi:10.3389/fneur.2020.517120
44. Shin MH, Kweon SS, Choi BY, et al. Self-reported snoring and metabolic syndrome: the Korean multi-rural communities cohort study. Sleep Breath. 2014;18(2):423–430. doi:10.1007/s11325-013-0902-8
45. Li C, Hsieh MC, Chang SJ. Metabolic syndrome, diabetes, and hyperuricemia. Curr Opin Rheumatol. 2013;25(2):210–216. doi:10.1097/BOR.0b013e32835d951e
46. Lim JH, Kim YK, Kim YS, Na SH, Rhee MY, Lee MM. Relationship between serum uric Acid levels, metabolic syndrome, and arterial stiffness in Korean. Korean Circ J. 2010;40(7):314–320. doi:10.4070/kcj.2010.40.7.314
47. Lin SD, Tsai DH, Hsu SR. Association between serum uric acid level and components of the metabolic syndrome. J Chin Med Assoc. 2006;69(11):512–516. doi:10.1016/S1726-4901(09)70320-X
48. Tani S, Matsuo R, Imatake K, et al. The serum uric acid level in females may be a better indicator of metabolic syndrome and its components than in males in a Japanese population. J Cardiol. 2020;76(1):100–108. doi:10.1016/j.jjcc.2020.01.011
49. Ali N, Miah R, Hasan M, et al. Association between serum uric acid and metabolic syndrome: a cross-sectional study in Bangladeshi adults. Sci Rep. 2020;10(1):7841. doi:10.1038/s41598-020-64884-7
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

