Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 15
Bullous Pemphigoid Associated with Erythrodermic Psoriasis: A Case Report
Authors Chen W, Peng C, Zheng J, Lu X, Ding Y, Su L
Received 25 May 2022
Accepted for publication 31 August 2022
Published 7 September 2022 Volume 2022:15 Pages 1805—1808
DOI https://doi.org/10.2147/CCID.S374556
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Jeffrey Weinberg
Wenjuan Chen,1,2 Chen Peng,1,2 Jianfeng Zheng,1,2 Xiya Lu,1,2 Yangfeng Ding,1,2 Lina Su1,2
1Department of Dermatology, Shanghai Skin Disease Hospital, Tongji University School of Medicine, Shanghai, People’s Republic of China; 2Institute of Psoriasis, Tongji University School of Medicine, Shanghai, People’s Republic of China
Correspondence: Lina Su; Yangfeng Ding, Department of Dermatology, Shanghai Skin Disease Hospital, Tongji University School of Medicine, 1278 Baode Road, Jing’an District, Shanghai, 200443, People’s Republic of China, Email [email protected]; [email protected]
Abstract: Psoriasis is an immune-mediated systemic disease with multiple organs involvement, such as cardiovascular, live, gut, endocrine, and so on. Bullous pemphigoid (BP) is a rare comorbidity associated with psoriasis, while BP occurring in erythrodermic psoriasis (EP) is very rare. In this case, we reported a woman with severe EP who developed BP, in whom beta hemolytic streptococcus infection was a possible triggering factor. The complicated condition of such patients needs to draw attention of the physician and study further for better treatment.
Keywords: bullous pemphigoid, erythrodermic psoriasis, comorbidity
Introduction
Previous studies have demonstrated a bidirectional association between bullous pemphigoid (BP) and psoriasis, BP reported to associate with psoriasis vulgaris is rare.1 However, the association of BP with erythrodermic psoriasis (EP) is more scarce. The present case describes a 73-year-old woman who had EP with arthritis and Fatty Liver Disease and developed BP after infection with beta hemolytic streptococcus. Some scholars provide the idea that bullous is presumably a sign of psoriatic activity.2 EP is a severe type of psoriasis, which means the disease was in a progressive stage, the possible trigger was streptococcus pyogenes tonsillar infection, BP is probably another indication that psoriasis is in an active state.
Case Report
A 73-year-old Chinese woman with a 30-year history of chronic plaque psoriasis was admitted because of pruritic, erythematous, and bullous lesions on the basis of generalized psoriatic skin lesion. Five months ago, she developed scattered mung bean-sized blisters on the buttocks after a cold and fever, then the blisters gradually developed to the anterior neck, chest, axillae, groin, both upper limbs and oral mucosa. Meanwhile, the disease of psoriasis aggravated to almost whole body over the next few months. She also had psoriatic arthritis for over 20 years with joint pain/deformity. She does not have a history of hypertension, diabetes, or other chronic diseases. Previous medication included methotrexate 20 mg for 2 years in 1992 and discontinue due to abnormal liver function, Recombinant Human Tumor Necrosis Factor-α Receptor II :IgG Fc Fusion Protein 25mg twice a week for 3 years in 1995. Then she took irregular treatment with acitretin or traditional Chinese medicine for several years.
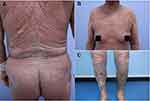
Physical examination revealed diffuse flushing, infiltrative swelling, and scaling lesions involving more than 90% of the total body surface area. Tense vesicles and blisters were distributed over her mouth, trunk, limb, and axillary regions; some of the bullae had ruptured and left behind eroded bases. Nikolsky’s sign was negative, and the Psoriasis Area and Severity Index (PASI) score was 29.8 (Figure 1). Abdominal ultrasound examination indicated fatty liver disease. Laboratory analysis indicated increased levels of BP180 (177.1 U/mL), anti-streptolysin-O (1285.1 IU/mL), and uric acid (397 µmol/L), but the other results were normal. Biopsy analysis demonstrated Munro’s microabscess, subepidermal blister under the psoriatic hyperplastic epidermis, and serous and inflammatory cells (including more eosinophils cells) in the blister (Figure 2). IgG, IgM, IgA, and C3 were negative, according to direct immunofluorescence analysis. Indirect immunofluorescence analysis revealed a 1:10 ratio. The patient was diagnosed with EP, psoriatic arthritis, and BP.
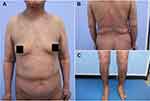
She was prescribed methotrexate with a dose of 10 mg per week, along with topical corticosteroids. While she did not respond to methotrexate and refused to increase the dose of methotrexate owing to abnormal liver function or undergo biological agent therapy because of financial constraints. Therefore, intravenous methylprednisolone (0.75mg/kg/d) was added. After 2 weeks, significant improvement of both BP and EP lesion was noted (Figure 3), blisters and vesicles have largely subsided, the PASI score of post-treatment was 1.6. Then, the dosage of methylprednisolone was reduced gradually in one year. Now she is taking 8 mg per day and is in stable condition with normal serum BP180 antibody.
Discussion
The concept of psoriatic disease was recently proposed based on the systemic inflammatory nature of psoriasis. However, the real burden of psoriasis is significantly underestimated, especially the impact of psoriatic comorbidities on the social and personal lives of patients.3 Autoimmune bullous disease is a comorbid condition associated with psoriasis, and the prevalence rate of BP in patients with psoriasis is higher than that in control subjects, preexisting psoriasis was associated with a 50% increase in the risk of BP.1 Bullous pemphigoid is more likely to overlap with chronic psoriasis plaques, EP, a rare type of psoriasis, complicated with bullous pemphigoid has been reported infrequently. Ohata et al4 conducted a retrospective study of 145 patients with autoimmune bullous disease and psoriasis, 63.4% of which were BP, followed by anti-laminin  1 pemphigoid of 37.2%. Of these, 122 cases were psoriasis vulgaris, 7 cases were psoriasis erythroderma, and 3 cases were psoriatic arthritis.4
1 pemphigoid of 37.2%. Of these, 122 cases were psoriasis vulgaris, 7 cases were psoriasis erythroderma, and 3 cases were psoriatic arthritis.4
The most common causes of BP are drugs, ultraviolet radiation, infection, trauma, and depression, which are also the trigger factor of psoriasis. In the present case, the possible predisposing factor was streptococcal throat infection, which induces the eruption of psoriasis, the aggravation of psoriasis leads to BP further. Some scholars have suggested that pemphigoid diseases could be a sign of active psoriasis; conversely, psoriasis could trigger BP via antigen-altering factors.2
Viral infection may have a role in autoimmune bullous diseases which have been identified in several studies, while bacterial infection’s role has been reported few, the only report about bacterial infection is localized BP after two episodes of erysipelas in a 63-year-old man.5 In our case, whether streptococcal infection or psoriasis exacerbation is a trigger for BP is unclear.
However, the pathogenetic link between psoriasis and autoimmune bullous disorders is unclear. The role of IL-17 in the pathogenesis of BP has recently emerged, as Chakievska et al found that IL-17A and its related genes are upregulated in BP lesions and confirmed the protective effect of IL-17 antibodies in mouse BP models.6 Moreover, secukinumab has shown good treatment efficacy in several cases of BP complicated with chronic severe psoriasis.7,8 Disrupted basement membrane zone (BMZ) in psoriasis, structural alterations of BMZ may modify the antigenicity of BMZ.9 Neutrophils’ role in both diseases, metalloproteases secreted by neutrophils may be implicated in the degradation of matrix proteins, leading to subsequent exposure of antigenic epitopes.9
Currently, there are no standard treatment guidelines for EP. Although treatment with secukinumab was found to be effective for BP associated with psoriasis, the results were reported in a very small sample. Moreover, BP induced by biologic in treating psoriasis has been reported previously, such as adalimumab,10 ustekinumab,11 and guselkumab.12 The treatment experience is paradoxical due to many adverse events of different biologic treatments that induced BP in psoriasis. Further research is needed to identify which type of biological agent or other agents might be effective for the specific treatment of this condition.
Ethics Statement
Ethical committee approval was not required for this case report.
Consent Statement
The patient gave written informed consent for publication of clinical information and photographs.
Funding
1. National Natural Science Foundation of China, Grant/Award Number: 82103707; 2. Clinical Research Plan of SHDC, Number: No.SHDC2020CR1014B.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Kridin K, Ludwig RJ, Schonmann Y, Damiani G, Cohen AD. The bidirectional association between bullous pemphigoid and psoriasis: a population-based cohort study. Front Med. 2020;7:511. doi:10.3389/fmed.2020.00511
2. Iskandarli M, Gerceker Turk B, Yaman B, Ozturk G. Pemphigoid diseases as a sign of active psoriasis: a case report and brief review. Dermatology. 2015;231(4):319–321. doi:10.1159/000435912
3. Damiani G, Bragazzi NL, Karimkhani Aksut C, et al. The global, regional, and national burden of psoriasis: results and insights from the global burden of disease 2019 study. Front Med. 2021;8:743180. doi:10.3389/fmed.2021.743180
4. Ohata C, Ishii N, Koga H, et al. Coexistence of autoimmune bullous diseases (AIBDs) and psoriasis: a series of 145 cases. J Am Acad Dermatol. 2015;73(1):50–55. doi:10.1016/j.jaad.2015.03.016
5. Moro F, Fania L, Sinagra JLM, Salemme A, Di Zenzo G. Bullous pemphigoid: trigger and predisposing factors. Biomolecules. 2020;10(10):1432. doi:10.3390/biom10101432
6. Chakievska L, Holtsche MM, Kunstner A, et al. IL-17A is functionally relevant and a potential therapeutic target in bullous pemphigoid. J Autoimmun. 2019;96:104–112. doi:10.1016/j.jaut.2018.09.003
7. Yun JS, Scardamaglia L, Tan CG, McCormack CJ. Successful secukinumab treatment of active bullous pemphigoid and chronic severe psoriasis: a case report. Australas J Dermatol. 2022;63. doi:10.1111/ajd.13803
8. Kamata M, Asano Y, Shida R, et al. Secukinumab decreased circulating anti-BP180-NC16a autoantibodies in a patient with coexisting psoriasis vulgaris and bullous pemphigoid. J Dermatol. 2019;46(6):e216–e217. doi:10.1111/1346-8138.14760
9. McFadden JP, Powles A, Kimber I, Fry L. Psoriasis and basement-membrane laminin. Br J Dermatol. 2013;169(3):718–719. doi:10.1111/bjd.12400
10. Stausbol-Gron B, Deleuran M, Sommer Hansen E, Kragballe K. Development of bullous pemphigoid during treatment of psoriasis with Adalimumab. Clin Exp Dermatol. 2009;34(7):e285–286. doi:10.1111/j.1365-2230.2008.03204.x
11. Nakayama C, Fujita Y, Watanabe M, Shimizu H. Development of bullous pemphigoid during treatment of psoriatic onycho-pachydermo periostitis with ustekinumab. J Dermatol. 2015;42(10):996–998. doi:10.1111/1346-8138.12943
12. Burlando M, Capurro N, Herzum A, Cozzani E, Parodi A. Guselkumab-associated bullous pemphigoid in a psoriasis patient: a case report and review of the literature. Dermatol Ther. 2022;35(1):e15207. doi:10.1111/dth.15207
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.