Back to Journals » Drug Design, Development and Therapy » Volume 17
Bulevirtide in the Treatment of Hepatitis Delta: Drug Discovery, Clinical Development and Place in Therapy
Authors Soriano V , Moreno-Torres V, Treviño A, Corral O, de Mendoza C
Received 25 December 2022
Accepted for publication 14 January 2023
Published 21 January 2023 Volume 2023:17 Pages 155—166
DOI https://doi.org/10.2147/DDDT.S379964
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Tuo Deng
Vicente Soriano,1 Victor Moreno-Torres,1,2 Ana Treviño,1 Octavio Corral,1 Carmen de Mendoza2
1Health Sciences School & Medical Center, Universidad Internacional La Rioja (UNIR), Madrid, Spain; 2Puerta de Hierro University Hospital & Research Institute, Madrid, Spain
Correspondence: Vicente Soriano, UNIR Health Sciences School & Medical Center, Calle García Martín 21, Pozuelo de Alarcón 28224, Madrid, Spain, Tel +34 659687981, Email [email protected]
Abstract: It has been ten years since the identification of NTCP as the cell surface receptor for HBV and HDV entry into hepatocytes. The search for molecules interfering with the binding of NTCP and HBV/HDV led to design bulevirtide (BLV). This large polypeptide mimics a region of the pre-S1 HBsAg and blocks viral entry by inhibitory competition. BLV was initially tested in cell cultures, animal models and more recently in Phase I–III human trials (called ‘MYRS’). As monotherapy or in combination with peginterferon, BLV is well tolerated and exhibits potent antiviral activity. Plasma viremia significantly declines and/or becomes undetectable in more than 75% of patients treated for > 24 weeks. However, serum HBsAg concentrations remain unchanged. No selection of BLV resistance in HBV/HDV has been reported in vivo to date. BLV is administered subcutaneously once daily at doses between 2 and 10 mg. BLV received conditional approval in Europe in 2020 to treat chronic hepatitis delta. The advent of peginterferon lambda or new specific anti-HDV antivirals (lonafarnib, etc.) will open the door for combination therapies with BLV. Since there is no stable reservoir for HDV-RNA within infected hepatocytes, viral clearance might be achieved using antivirals for a minimum timeframe. This is what happens in hepatitis C combining several antivirals, curing nearly all patients treated for 3 months. Clearance of HDV-RNA genomes may occur despite HBV persistence as cccDNA or chromosome integrated HBV-DNA within hepatocytes. This is supported by cases of HDV elimination using BLV despite persistence of serum HBsAg. Another path for HDV cure will derive from achieving HBsAg clearance, the goal of new promising anti-HBV gene therapies (bepirovirsen, etc.). In summary, the advent of BLV has triggered a renovated interest for antiviral therapy in hepatitis delta. We envision combination therapies that will lead to HDV cure in the near future.
Keywords: hepatitis delta, bulevirtide, lonafarnib, tenofovir, peginterferon lambda, hepatitis B functional cure, MYR trials
Introduction
Hepatitis D virus (HDV) infection causes the most severe forms of both acute and chronic viral hepatitis. Approximately 12–15 million people are chronically infected with HDV worldwide.1 HDV is a small defective virus that only produces human infection along with the hepatitis B virus (HBV). HDV requires HBV-encoded envelope proteins for dissemination and de novo cell entry. However, it can also spread by cell division of infected hepatocytes in carriers.2,3 HDV replicates its 1.7 kb circular single-stranded RNA genome in the nucleus of hepatocytes using a human polymerase.4
Simultaneous exposure to HBV and HDV (co-infection) mostly occurs in young persons and frequently produces icteric acute hepatitis. Fulminant hepatitis may occur rarely,5 resolving most acute episodes without progressing to chronicity. In contrast, HDV super-infection may occur anytime in chronic hepatitis B patients; then HDV uniformly establishes persistence.6,7 Chronic hepatitis delta is characterized by accelerated course to cirrhosis and more frequent development of liver cancer.8 No specific antivirals to treat hepatitis delta existed until recently. Interferon alfa has been used for decades, but poor drug tolerance and low efficacy have discouraged its broader use.4,9
The small HDV does not codify for any replication enzyme, as do other RNA viruses such as hepatitis C or HIV, for which drugs targeting viral enzymes (ie, polymerases and proteases) have successfully been developed. Therefore, the search for antivirals against HDV has been an important challenge. A few host proteins have been identified as potential antiviral targets. Indeed, it has been ten years since the identification of NTCP as the receptor for both HBV and HDV entry into hepatocytes.10
Drug Discovery
The search for molecules that interfere with the binding of NTCP and HBV/HDV led to design bulevirtide (BLV), formerly known as myrcludex-B, by researchers at the University of Heidelberg, Germany.11 It is a very large lipopeptide comprising 47 amino acids in its sequence and a myristoylation at the N-terminus. BLV mimics a region of the pre-S1 hepatitis B surface antigen (HBsAg) and blocks viral entry by inhibitory competition. Figure 1 shows the viral replication cycle for HDV and other hepatitis viruses.
 |
Figure 1 Major differences in the replication of hepatitis viruses. Abbreviations: NTCP, sodium taurocholate co-transporter polypeptide; Pol, human RNA polymerase II. |
Drug Development
In vitro experiments using hepatoma cell lines and studies in animal models demonstrated the efficacy of BLV, producing rapid and significant declines in HDV-RNA.12,13 After the release of new NIH regulations restricting the use of chimpanzees on experiments, studies using chimeric small animals with hepatocytes expressing the NTCP receptor have provided helpful models.14,15
Clinical Development: The MYR Trials
Phase 1 trials in humans testing BLV started in 2015. One of these trials confirmed a good safety profile of BLV subcutaneously at multiple doses up to 10 mg daily in 36 healthy volunteers.16 The drug was well tolerated, and no serious adverse events representing off-target effects nor immunogenic reactions were observed up to the highest applied dose of 20 mg intravenously. BLV showed dose-dependent pharmacokinetics, best described by a 2-compartment target-mediated drug disposition model. Furthermore, the bioavailability of the subcutaneous administration was large (85%) and interindividual variability was moderate. Overall, BLV pharmacokinetics showed that subcutaneous doses of 10 mg and above reached a target saturation >80% for at least 15 h.16,17
Further Phase II–III human studies were conducted testing the safety and efficacy of BLV as monotherapy or in combination with tenofovir and/or pegylated interferon α-2a (pegIFN). These studies are known as the “MYR” trials, acknowledging that the drug was formerly named myrcludex-B. Table 1 summarizes the main features of these studies.
 |
Table 1 Bulevirtide (“MYR”) Clinical Trials |
The MYR-201 trial was a phase Ib/II study that tested BLV safety and efficacy at doses of 2 mg sc/day.18 It was the first proof-of-concept for BLV efficacy in humans infected with HDV. A total of 24 patients with chronic hepatitis delta were randomized into 3 arms: i) BLV monotherapy followed by pegIFN for 48 weeks, ii) BLV combined with pegIFN for 24 weeks followed by pegIFN monotherapy for 24 weeks, and iii) pegIFN monotherapy for 48 weeks. While ALT normalized in 75% of patients, there was no significant decline in serum HBsAg in any arm. Serum HDV-RNA declined in all patients treated with BLV. The combination of pegIFN and BLV resulted in undetectable HDV-RNA at the end-of-therapy (EOT) in 5 of 7 patients. Side effects included asymptomatic serum bile acid elevations and mild hematologic abnormalities. The results supported an enhanced antiviral effect adding BLV to pegIFN.
In the multicentre Phase II MYR-202 study, 118 tenofovir-treated patients were randomized to different BLV doses (2, 5 or 10 mg/day) or tenofovir monotherapy for 24 weeks followed by 24 weeks of tenofovir alone.19 Approximately half of patients had baseline compensated cirrhosis. A dose-dependent reduction in HDV-RNA and ALT was noticed. In contrast, HBsAg titers did not change under BLV therapy. The virological response at EOT was 50%, 44% and 73% in groups treated with BLV at doses of 2, 5 and 10 mg. However, all relapsed within the next 6 months.
The MYR-203 trial was a multicenter, open-label randomized study that examined the efficacy and safety of BLV as monotherapy or in combination with pegIFN or tenofovir.20 Initially, 60 patients with chronic hepatitis delta were randomized into 4 treatment arms to receive pegIFN monotherapy, BLV 2 mg monotherapy, BLV 2 mg plus pegIFN, or BLV 5 mg plus pegIFN, for 48 weeks with additional 24 weeks of follow-up. The primary endpoint was undetectable HDV-RNA at week 72. A good tolerability profile was noticed for BLV, regardless dosing. Serum HDV-RNA declined in all treatment arms, but undetectable HDV-RNA at week 72 was seen in only the low-dose BLV+pegIFN (8/15 patients) and high-dose BLV+pegIFN (4/15 patients) groups. While neither pegIFN or BLV monotherapies resulted in significant HBsAg declines, HBsAg loss occurred in 3/15 patients in the low-dose BLV+pegIFN arm at week 48, and unexpectedly in 4/15 patients at week 72. ALT normalization was more frequent in patients receiving BLV monotherapy. Hepatic fibrosis went down in 4/8 paired biopsies in patients on BLV monotherapy. Reductions in necroinflammation were noticed in 6/8 patients. Given the evidence of functional HBV cure alongside sustained HDV-RNA suppression, the MYR-203 trial was the first to demonstrate the curative potential of BLV in combination with pegIFN for hepatitis delta.
The MYR-204 trial is a multicenter randomized Phase 2b study that examines the safety of BLV with or without pegIFN over 48 weeks.21,22 It includes monotherapy arms, along with 2 and 10 mg dose arms of BLV. The primary endpoint was defined as undetectable HDV-RNA at week 24 of treatment. EOT response was 13%, 24%, 34% and 4% in the pegIFN monotherapy, 2 mg BLV+pegIFN, 10 mg BLV+pegIFN, and BLV 10 mg monotherapy arms, respectively. While BLV alone produced the highest rate of ALT normalization, the combination therapy arms and BLV 10 mg monotherapy resulted in higher rates of HDV-RNA decline.
The MYR-301 trial is the first multicenter randomized, Phase 3 study testing BLV.23 The trial assesses the efficacy and safety of delayed BLV therapy compared to 2 mg or 10 mg daily dosing. A total of 150 patients with chronic hepatitis delta were randomized into 3 arms: i) controls (10 mg BLV for 48 weeks after 48 weeks no treatment), ii) BLV 2 mg (144 weeks followed by 96 weeks off-treatment), iii) BLV 10 mg (144 weeks followed by 96 weeks off treatment). All patients were followed for 240 weeks. Undetectable HDV-RNA at week 48 was achieved in 0%, 6% and 8% of patients, respectively. Similar to MYR-202, there was no clear dose effect of BLV. Furthermore, HBsAg levels did not change with BLV monotherapy.
End-Points for HDV Therapies
To date, the combination of a >2 log decline in HDV-RNA and ALT normalization (combined response) has been used as a primary endpoint in most clinical studies. However, a significant viral load decline without reaching undetectability should better be considered a partial virologic response to BLV, only being complete when there is a sustained HDV-RNA clearance. The interpretation of persistent elimination should only be applied when HDV-RNA undetectability is seen for at least 24 weeks (SVR24). On the other hand, ALT is an unreliable marker of liver damage in this population. Firstly, HBV may still cause hepatic harm after removal of HDV. Secondly, some HDV patients with advanced chronic liver disease may depict ALT values within the normal range.24
Given the dependence of HDV for HBsAg, some authors have defended that HDV cure would ultimately depends on clearing circulating HBsAg. However, some patients treated for HDV have kept on undetectable HDV-RNA for long periods (years) without eliminating HBsAg. Certainly, others have experienced late relapses. Thus, the term sustained virological response (used for HCV infection) should be avoided for HDV. Instead, the durable response would be more appropriate (when HDV RNA remains undetectable).25 On the other hand, there are two different situations for HBsAg elimination, known as HBsAg seroreversion, meaning clearance of circulating HBsAg without developing anti-HBs; and HBsAg seroconversion, referring to development of anti-HBs along with disappearance of circulating HBsAg.
European Post-Approval Studies
Outside clinical trials, experiences using BLV at distinct European countries have been released following BLV conditional approval on July 2020. Table 2 records the main findings in these real-world studies.
 |
Table 2 Bulevirtide Real-World Studies |
France
The early access program for BLV in France began in late 2019. Results for the first 146 patients were recently reported.26,27 Overall, 77 patients received BLV 2 mg/day monotherapy and 56 BLV 2 mg/day plus PegIFN over 12 months. Most patients were men (70%), mean age 41 years, and 64% had advanced fibrosis or cirrhosis. At 48 weeks, 39% and 85% of patients, respectively, achieved undetectable HDV-RNA. ALT normalized in 49% and 36%, respectively. Mild side effects (headache, asthenia, etc.) were reported in patients treated with BLV but none discontinued treatment due to adverse events. Almost all patients showed asymptomatic increases of bile acids. This first real-world cohort showed that daily BLV 2 mg monotherapy or combined with pegIFN was well tolerated over 12 months. Significant antiviral responses were noticed in real-life, confirming the results from the MYR clinical trials.
BuleDelta
BuleDelta is an ongoing observational study planned to recruit 400 patients with chronic hepatitis delta treated with BLV, under the auspices of the French early access program. Until May 2022, the cohort had been enrolled 173 patients (69% males; mean age 41 years-old; 46% sub-Saharan Africans; 55% cirrhosis).28 BLV had been given for a mean of 16 months, in 41% associated with pegIFN. In a preliminary analysis, HDV-RNA was undetectable in 23/115 patients at week 24 (44% of those with BLV plus pegIFN vs 8% of those on BLV monotherapy). Severe adverse events occurred in 22% of patients (nearly half represented by bile acid elevations).
Italy
The results from a single center were reported for 18 patients with hepatitis delta and compensated cirrhosis, all with portal hypertension and/or liver cancer.29,30 At 24 weeks of BLV 2 mg/day monotherapy, 83% of patients achieved negative HDV-RNA or >2 log IU/mL reductions. However, 11% of patients show virological non-response, defined as <1 log IU/mL decline at week 24. HBsAg levels did not change significantly. In this difficult-to-treat population, BLV was well tolerated with only asymptomatic increases of bile acids.
Austria
Results recorded from 15 patients (mean age, 50 years-old; 9 with compensated cirrhosis) that received BLV have been released.31 During BLV monotherapy, 4 achieved undetectable viremia. HBsAg levels did not change significantly. Bile acid levels increased but there was no pruritus. BLV was discontinued in two patients with undetectable HDV-RNA treated for more than 6 months. One persisted with undetectable viremia at least for 20 weeks off therapy. The second experienced viral rebound.
Germany
Data were reported for 8 patients with chronic hepatitis delta, all being treated with nucleos(t)ide analogues, that were treated with BLV 2 mg/day for 16 weeks.32 Mean HDV-RNA levels dropped by 0.5 log IU/mL. BLV was discontinued in 1 patient who did not show significant ALT or HDV-RNA responses. No side effects apart from asymptomatic elevations of bile acids were reported.
More recently, clinical experiences and anecdotal observations have appeared that provide new insights into the spectrum of clinical responses to BLV.33,34 A German study of 7 patients with compensated HDV cirrhosis showed that BLV 2 mg plus tenofovir provided virological response in most patients. However, one experienced viral breakthrough at week 48 for unclear reasons.35 Similarly, another patient from France experienced viral rebound after initial response to BLV plus pegIFN, although interferon dosing had to be reduced due to poor tolerance.36
As shown in Box 1, a subset of patients seems to be primarily resistant to BLV. Others respond initially but exhibit viral breakthrough under BLV, perhaps due to the development of an adaptive immune response to the drug. A third group responds to BLV and even achieves undetectable viremia under prolonged treatment but relapses upon drug discontinuation. Finally, a group of BLV treated patients suppress viremia on treatment and keep undetectable viral replication after stopping the drug. Some of the latest HDV cured patients show HBsAg seroreversion or seroconversion, whereas the majority remain positive for HBsAg.
 |
Box 1 Spectrum of Responses to Bulevirtide |
Long-Term BLV Monotherapy
Anecdotal cases of BLV monotherapy have been released. In two patients with compensated cirrhosis, ALT normalization occurred before 6 months and both achieved undetectable HDV-RNA before 1 year. Responses persisted for over 3 years, even after reducing BLV dosing from 10 to 5 and 2 mg/day.37 In one of the patients, with compensated cirrhosis and portal hypertension, esophageal varices disappeared, histological damage ameliorated, and platelets and albumin levels significantly improved. Overall, BLV was well tolerated, with a clinically silent dose-dependent increase of total bile acids.
Patient-Reported Outcomes Under BLV
An exploratory analysis using the EuroQol 5D visual analog scale (EQ-5D VAS) scores was conducted in patients with chronic hepatitis delta enrolled in the phase MYR-301 trial.38 A total of 150 patients were assigned to three arms (BLV 2 mg or 10 mg or controls). The EQ-5D VAS is a self-completed rating of the patients’ health state (or quality of life) with a range of 0–100 (100 = Best health state). The quality-of-life analysis was based on EQ-5D VAS scores at baseline and week 48. Baseline characteristics were well balanced. Patients treated with BLV 2 mg reported statistically significant improvement in quality of life at week 48 and also showed statistically significantly better EQ-5D VAS change from baseline scores as compared to controls. BLV 10 mg EQ-5D VAS scores did not reach the statistical significance levels at week 48. There were similar improvement trends among patients with and without cirrhosis.
Histological Response to BLV
First acknowledged virological and biochemical responses to BLV should provide histological and clinical benefits to be considered as therapeutic. Paired liver biopsies were collected at baseline and at week 48 from 79 patients enrolled in the Phase III clinical trial MYR-301.39
At week 48, intrahepatic HDV-RNA strongly declined with median reductions from baseline of 2.2 log IU/mL in the BLV 2 mg group (n = 21) and 2.5 log IU/mL in the BLV 10 mg group (n = 27), with undetectable HDV-RNA in 33% and 52% of cases, respectively. Intrahepatic HDV-RNA levels did not change in untreated controls.
The number of HDAg+ cells significantly decreased in patients treated with BLV, with strong correlations with serum HDV-RNA levels. Transcriptional levels of several inflammatory chemokines (eg, CXCL10) and interferon-stimulated genes (eg, ISG15) simultaneously declined. Importantly, these reductions strongly correlated with HDV-RNA declines, suggesting that HDV replication is the major driver of intrahepatic damage. By contrast, BLV treatment did not reduce intrahepatic HBV-DNA or HBV-RNA levels, further confirming that BLV does not influence long-term HBV-infected cells. This result is noteworthy given that most patients in the MYR-301 trial received nucleos(t)ide analogues as HBV treatment.23
BLV Resistance
In the MYR-204 trial, virological non-response (HDV-RNA decline <1 log IU/mL at week 24 of treatment) was recognized in 5 patients treated with BLV.20,21 Alike, there were 15 patients in the MYR-301 trial.23 Drug susceptibility studies were carried out in vitro on samples from 3 and 13 of these patients, respectively.40 No differences in BLV susceptibility were recognized comparing baseline and week 24 specimens. Furthermore, plasma concentration of BLV was recognized in all cases, exceeding the in vitro EC50 by more than 2-fold. These results point out that virological non-response to BLV is a real phenomenon, occurring in roughly 10–20% of patients; it does not seem to result from the selection of HDV resistance.
BLV Safety
Both phase II–III clinical trials and real-world studies have provided strong confidence about the good tolerability of BLV. The MYR-202 trial reported safety data for 24 weeks of BLV 2, 5 and 10 mg monotherapy.18 Likewise, the MYR-203 study reported tolerance for 48 weeks of BLV 2 and 10 mg monotherapy.20 There was no recording of serious adverse events nor side effects leading to drug discontinuation.
Mild symptoms/signs such as fatigue, nausea, headache, dizziness, leukopenia and thrombocytopenia occurred in 5%–8% of patients. In the MYR-301 study, injection site reactions were recorded by 6% and 26% of patients, treated with BLV 2 mg and 10 mg, respectively.23 Local skin reactions were generally mild and short lasting.
A dose-dependent increase of bile acids was uniformly reported across studies, but increases were generally asymptomatic. Upon BLV discontinuation, bile acids returned to baseline values.
In the MYR-202 trial, relapses in serum HDV-RNA upon BLV discontinuation were generally associated with moderate ALT flares, none with clinical consequences.18 Of note, favourable safety outcomes were noticed in the subset of patients with advanced compensated cirrhosis.19 In patients that combined BLV with pegIFN, side effects were generally those usually associated with the latest. In the MYR-204 trial, the rate of any treatment-emergent grade 3 or 4 adverse events was greater in patients treated with pegIFN than in those receiving BLV monotherapy.21,22
A recent pooled safety analysis of BLV treated patients was performed using data from the phase II MYR-203 and MYR-204 along with phase III MYR-301 trials.41 A total of 179 patients treated with BLV were examined. By week 48, the overall incidence of participants experiencing adverse events was 86% in both BLV 2 mg and 10 mg groups compared to rates of 90% in the pegIFN group. No serious adverse events potentially related to BLV were recorded and none led to BLV discontinuation. Asymptomatic dose-dependent increases in serum bile acids were frequent.
Despite the favorable experience in the MYR studies, two cases of immediate-type hypersensitivity reactions and allergic skin reaction were recently reported in patients treated with BLV.42,43 Anyway, both were able to continue BLV treatment.
Future Prospects
Given the strong dependency of HDV from HBV, it has been claimed that any curative strategy for hepatitis delta will require a simultaneous “eradication” of HBV24,44 or at least disappearance of HBsAg from the bloodstream. However, the experience collected to date support that sustained clearance of HDV-RNA despite persistence of serum HBsAg may occur using pegIFN alone or in combination with BLV or lonafarnib. Anyway, the high rate of HBsAg loss observed in 4 out of 15 HDV/HBV-coinfected patients in the MYR-203 phase 2 trial20 is hard to explain and should not be generalizable. More rarely, HBsAg seroreversion or seroconversion may be seen using pegIFN but almost never with BLV monotherapy.
The achievement of HDV cure might hypothetically be obtained using two therapeutic strategies, one focused on HBsAg clearance and another pursuing dual HBV and HDV replication blocking. In the first group will be the new promising anti-HBV gene therapies, such as bepirovirsen,45 AT-2173, VIR-2218, JNJ-3989, REP-2139, etc.46 In the second group will be agents with dual anti-HBV and anti-HDV activity, such as pegIFN or BLV (Table 3). Adding specific anti-HDV agents, such as lonafarnib, will hypothetically enhance the chances of HDV-RNA clearance. Lonafarnib inhibits the host enzyme farnesyl-transferase which is required for modifying the delta antigen precursor and promote viral assembly. Blocking the latest step of the HDV life cycle with lonafarnib might provide a synergistic effect with BLV.47
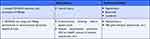 |
Table 3 Hepatitis Delta Cure Modalities |
Late HDV-RNA relapses off therapy may occur after sustained virological responses, defined as HDV-RNA clearance 24 weeks after treatment completion. In a retrospective analysis of the HIDIT-1 trial that tested adefovir plus pegIFN in chronic hepatitis delta patients, 9/16 with undetectable HDV-RNA 24 weeks after the end of therapy, experienced viral rebound during a 5-year follow-up.48,49 Likewise, late relapses were recognized in the HIDIT-II trial that tested tenofovir plus pegIFN.50 In both studies, however, a few patients sustained undetectable serum HDV-RNA indefinitely, occasionally despite remaining positive for HBsAg.
Outside clinical trials, deferred HDV-RNA rebounds have been seen in other studies, mostly using pegIFN monotherapy. In a long-term follow-up conducted at one single centre in Belgium, SVR24 was seen in 12/23 (52%) of patients treated with pegIFN. However, 4 relapsed later within 3 years. Hence, definitive SVR was seen in only 8/23 (35%).51 Therefore, undetectable HDV-RNA at SVR24 may not represent true HDV clearance and longer-term follow-up could be required. Hence, some authors defend that the ideal endpoint for HDV cure should be HBsAg loss. Since HDV needs HBsAg as its envelope for entry into hepatocytes, only a “functional cure” of chronic hepatitis B will ensure a sustained stop of HDV replication.
The reasons why deferred viral rebounds may occur in hepatitis delta should discharge false-negative HDV-RNA results when a low sensitivity assay is used.52 Alternatively, significant HDV-RNA fluctuations could be more frequent in HDV carriers than in other chronic viral infections.6 Finally, spontaneous HDV clearance has been noticed occasionally, often accompanying episodes of immune imbalance or immune restoration.53
At the MYR-202 trial, the combination of BLV plus tenofovir did not modify serum HBsAg concentrations.18 Thus, a rebound in serum HDV-RNA upon BLV discontinuation was expected. Rather than extending treatment duration, for achieving HDV elimination, adding new anti-HDV agents seems to be the path for success. A promising drug, lonafarnib, is completing phase 3 trials as HDV therapy.54,55 It specifically blocks the assembly of HDV virions within hepatocytes. Peginterferon lambda is another antiviral drug currently been tested in phase 3 studies as hepatitis D therapy.56
Since there is no stable cell reservoir for the HDV-RNA genome, viral clearance might hypothetically be achieved if complete blocking of viral replication occurs using antivirals for a minimum timeframe.57 The combination of several specific anti-HDV agents will be required. This is what happens in hepatitis C combining direct-acting antivirals, with cure of nearly all patients treated for 3 months. At this time, the dynamics of HDV and infected hepatocytes have estimated a half-life for viral particles of 1.3 days,58 which is somewhat longer than for HBV and HCV (Table 4).
 |
Table 4 Estimated Half-Life of Virions |
Hepatitis delta is a unique condition, and clearance of HDV-RNA genomes might occur despite HBV persistence as cccDNA or integrated HBV-DNA within hepatocytes (Figure 1). Supporting this concept are cases of HDV elimination despite persistence of serum HBsAg following treatment with BLV or lonafarnib generally along with pegIFN.20,28,59–61
Although the extent of HDV genetic variability has been considered a potential caveat as a source of differences in susceptibility among distinct HDV genotypes,62 more recent findings do not support this concern.60 Indeed, BLV susceptibility seems to be quite similar across all eight HDV genotypes.63
Conclusion
Investigations about the mechanism of viral entry for HBV and HDV led to the identification of NTCP as the hepatocyte cell surface receptor. The synthesis of a modified peptide that mimics the HBsAg pre-S1 region led to produce myrcludex, a potent inhibitory competitor of HBV/HDV entry. The drug has been developed clinically as BLV. Phase II/III trials have demonstrated its potent antiviral activity along with good safety profile.64 Significant reductions in serum HDV-RNA are seen in most but not all patients at 24–48 weeks. However, there is no effect on serum HBsAg levels. In combination with pegIFN, a synergistic effect is seen with BLV. However, viral rebound occurs in most patients upon drug discontinuation.
Rare cases of hypersensitivity reactions have been reported using BLV. In some real-world studies, the addition of pegIFN and/or using higher BLV doses have resulted in greater virological response rates. The market authorization has only been obtained for the 2 mg/day dose of BLV. The optimal dose as monotherapy (2 vs 10 mg per day), the duration of therapy, stopping rules, and long-term results after stopping BLV treatment should be better characterized.
The advent of new specific HDV antivirals, such as lonafarnib, will allow to explore the efficacy of combination therapy for hepatitis delta. Given the biological mechanisms of viral persistence within infected cells, attempts to eradicate HDV and cure hepatitis delta should be explored. The combination of several antiviral drugs most likely will be needed. At this time, cocktails with BLV, lonafarnib, pegIFN and/or tenofovir are being investigated in vitro and in animal models. Alike in the treatment of hepatitis C, we envision that combination therapies for a limited time frame (ie, 3–6 months) will cure hepatitis delta.56
Abbreviations
BLV, bulevirtide; HBV, hepatitis B virus; HDV, hepatitis delta virus; HCV, hepatitis C virus; LNF, lonafarnib.
Data Sharing Statement
The data supporting the findings of the article are all available upon request.
Disclosure
The authors declare no conflicts of interest, financial or otherwise for this study.
References
1. Stockdale A, Kreuels B, Henrion M., et al. The global prevalence of hepatitis D virus infection: systematic review and meta-analysis. J Hepatol. 2020;73:523–532. doi:10.1016/j.jhep.2020.04.008
2. Giersch K, Bhadra O, Volz T, et al. Hepatitis delta virus persists during liver regeneration and is amplified through cell division both in vitro and in vivo. Gut. 2019;68:150–157. doi:10.1136/gutjnl-2017-314713
3. Zhang Z, Ni Y, Lempp F, et al. Hepatitis D virus-induced interferon response and administered interferons control cell division-mediated virus spread. J Hepatol. 2022;77:957–966. doi:10.1016/j.jhep.2022.05.023
4. Urban S, Neumann-Haefelin C, Lampertico P. Hepatitis D virus in 2021: virology, immunology and new treatment approaches for a difficult-to treat disease. Gut. 2021;70:1782–1794. doi:10.1136/gutjnl-2020-323888
5. Farci P, Niro G. Clinical features of hepatitis delta. Semin Liver Dis. 2012;32:228–236. doi:10.1055/s-0032-1323628
6. Buti M, Homs M, Rodriguez-Frias F, et al. Clinical outcome of acute and chronic hepatitis delta over time: a long-term follow-up study. J Viral Hepat. 2011;18:434–442. doi:10.1111/j.1365-2893.2010.01324.x
7. Salpini R, D’Anna S, Piermatteo L, Svicher V. Novel concepts on mechanisms underlying hepatitis delta virus persistence and related pathogenesis. J Viral Hepat. 2022;29(12):1038–1047. doi:10.1111/jvh.13755
8. Alfaiate D, Clément S, Gomes D, Goossens N, Negro F. Chronic hepatitis D and hepatocellular carcinoma: a systematic review and meta-analysis of observational studies. J Hepatol. 2020;73:533–539. doi:10.1016/j.jhep.2020.02.030
9. Sandmann L, Wedemeyer H. Interferon-based treatment of chronic hepatitis D. Liv Int. 2022. doi:10.1111/liv.15410
10. Yan H, Zhong G, Xu G, et al. Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus. Elife. 2012;1:e00049. doi:10.7554/eLife.00049
11. Petersen J, Dandri M, Mier W, et al. Prevention of hepatitis B virus infection in vivo by entry inhibitors derived from the large envelope protein. Nat Biotechnol. 2008;26:335–341. doi:10.1038/nbt1389
12. Ni Y, Zhang Z, Engelskircher L, et al. Generation and characterization of a stable cell line persistently replicating and secreting the human hepatitis delta virus. Sci Rep. 2019;9:10021. doi:10.1038/s41598-019-46493-1
13. Lempp F, Schlund F, Rieble L, et al. Recapitulation of HDV infection in a fully permissive hepatoma cell line allows efficient drug evaluation. Nat Commun. 2019;10:2265. doi:10.1038/s41467-019-10211-2
14. Burwitz BJ, Zhou Z, Li W. Animal models for the study of human hepatitis B and D virus infection: new insights and progress. Antiviral Res. 2020;182:104898. doi:10.1016/j.antiviral.2020.104898
15. Giersch K, Dandri M. In vivo models of HDV infection: is humanizing NTCP enough? Viruses. 2021;13:588. doi:10.3390/v13040588
16. Blank A, Markert C, Hohmann N, et al. First-in-human application of the first-in-class hepatitis B and hepatitis D virus entry inhibitor myrcludex B. J Hepatol. 2016;65:483–489. doi:10.1016/j.jhep.2016.04.013
17. Sauter M, Blank A, Stoli F, Lutz N, Haefeli W, Burhenne J. Intact plasma quantification of the large therapeutic bulevirtide lipopeptide. Analyt Bioanalyt Chem. 2021;413:5645–5654. doi:10.1007/s00216-021-03384-7
18. Bogomolov P, Alexandrov A, Voronkova N, et al. Treatment of chronic hepatitis D with the entry inhibitor myrcludex B: first results of a phase Ib/IIa study. J Hepatol. 2016;65:490–498. doi:10.1016/j.jhep.2016.04.016
19. Wedemeyer H, Schöneweis K, Bogomolov P, et al. Safety and efficacy of bulevirtide in combination with tenofovir disoproxil fumarate in patients with hepatitis B virus and hepatitis D virus coinfection (MYR-202): a multicentre, randomised, parallel-group, open-label, phase 2 trial. Lancet Infect Dis. 2022;23(1):117–129. doi:10.1016/S1473-3099(22)00318-8
20. Wedemeyer H, Schöneweis K, Bogomolov P, et al. 48 weeks of high dose (10 mg) bulevirtide as monotherapy or with peginterferon alfa-2a in patients with chronic HBV/HDV co-infection. J Hepatol. 2020;73(suppl 1):S52–3. doi:10.1016/S0168-8278(20)30651-6
21. Asselah T, Arama S, Bogomolov P, et al. Safety and efficacy of bulevirtide monotherapy and in combination with peginterferon alfa-2a in patients with chronic hepatitis delta: 24 weeks interim data of MYR-204 phase 2b study. International Liver Congress. 2021;1;54.
22. Asif B, Koh C. Hepatitis D virus (HDV): investigational therapeutic agents in clinical trials. Exp Op Invest Drugs. 2022;31:905–920. doi:10.1080/13543784.2021.1977795
23. Wedemeyer H, Aleman S, Brunetto M, et al. Efficacy and safety of bulevirtide monotherapy given at 2 mg or 10 mg dose level once daily for treatment of chronic hepatitis delta: week 48 primary end point results from a phase 3 randomized, multicenter, parallel design study. J Hepatol. 2022;77(suppl 1):4–5. doi:10.1016/S0168-8278(22)00433-0
24. Yurdaydin C, Abbas Z, Buti M, et al. Treating chronic hepatitis delta: the need for surrogate markers of treatment efficacy. J Hepatol. 2019;70:1008–1015. doi:10.1016/j.jhep.2018.12.022
25. Khalfi P, Kennedy P, Majzoub K, Asselah T. Hepatitis D virus: improving virological knowledge to develop new treatments. Antivir Res. 2023;209:105461. doi:10.1016/j.antiviral.2022.105461
26. Asselah T, Loureiro D, Le Gal F, et al. Early virological response in six patients with hepatitis D virus infection and compensated cirrhosis treated with Bulevirtide in real-life. Liver Int. 2021;41:1509–1517. doi:10.1111/liv.14950
27. De Ledinghen V, Hermabessiere P, Metivier S, et al. Bulevirtide with or without peginterferon in HDV infected patients in a real-life setting: two-year results from the French multicenter early access program. Hepatology. 2022;76(Abstract):28.
28. Zoulim F, Fougerou C, Roulot D, et al. Efficacy and safety of treatment with bulevirtide in chronic hepatitis delta: preliminary results of the real-life ANRS HD EP01 Buledelta cohort. Hepatology. 2022;76(Abstract):1017.
29. Degasperi E, Anolli MP, Uceda-Renteria SC, et al. Improvement of clinical parameters in HDV patients with advanced compensated cirrhosis treated with bulevirtide monotherapy for 48 weeks. Hepatology. 2022;76(Abstract):1011.
30. Degasperi E, Anolli M, Renteria S, et al. Bulevirtide monotherapy for 48 weeks in patients with HDV-related compensated cirrhosis and clinically significant portal hypertension. J Hepatol. 2022;77(6):1525–1531.
31. Jachs M, Schwarz C, Panzer M, et al. Response-guided long-term treatment of chronic hepatitis D patients with bulevirtide-results of a “real world” study. Aliment Pharmacol Ther. 2022;56:144–154. doi:10.1111/apt.16945
32. Zöllner C, Hofmann J, Lutz K, Tacke F, Demir M. Real-life experiences with bulevirtide for the treatment of hepatitis delta-48 weeks data from a German centre. Liver Int. 2022;42:2403–2407. doi:10.1111/liv.15408
33. Herta T, Hahn M, Maier M, et al. Efficacy and safety of bulevirtide plus tenofovir disoproxil fumarate in real-world patients with chronic hepatitis B and D co-Infection. Pathogens. 2022;11:517. doi:10.3390/pathogens11050517
34. Giordan M, Bengone-Abogourin J, Aherfi S, Allemand I, Colson P. Hepatitis delta treatment with bulevirtide in real life: a case report. Int J Antimicrob Agents. 2022;59:106562. doi:10.1016/j.ijantimicag.2022.106562
35. Degasperi E, Anolli MP, Lampertico P. Bulevirtide for patients with compensated chronic hepatitis delta: a review. Liver Int. 2022. doi:10.1111/liv.15389
36. Lampertico P, Roulot D, Wedemeyer H. Bulevirtide with or without pegIFNα for patients with compensated chronic hepatitis delta: from clinical trials to real-world studies. J Hepatol. 2022;77(5):1422–1430. doi:10.1016/j.jhep.2022.06.010
37. Loglio A, Ferenci P, Uceda-Renteria S, et al. Safety and effectiveness of up to 3 years’ bulevirtide monotherapy in patients with HDV-related cirrhosis. J Hepatol. 2022;76:464–469. doi:10.1016/j.jhep.2021.10.012
38. Buti M, Wedemeyer H, Aleman S, et al. Bulevirtide improves health related quality of life measured by EQ-5D VAS in patients with chronic hepatitis delta: an exploratory analysis of a phase 3 trial at 48 weeks. Hepatology. 2022;76:1019.
39. Allweiss L, Volmari A, Ladiges Y, et al. Strong intrahepatic decline of hepatitis D virus RNA and antigen after 48 weeks of treatment with bulevirtide in chronic HBV/HDV confected patients: interim results from a multicenter, open-label, randomized phase 3 clinical trial (MYR-301). Hepatology. 2021;74:S148A.
40. Hollnberger J, Schlund F, Schöneweis K, Zehnder B, Urban S. Rare cases of non-response in bulevirtide (BLV) treated patients from the MYR-204/301 studies are not associated with the development of BLV resistance. Hepatology. 2021;74:S426A.
41. Asselah T, Lampertico P, Aleman S, et al. Bulevirtide monotherapy is safe and well tolerated in patients with chronic hepatitis D: an integrated safety analysis of 48-week data. Hepatology. 2022;76(Abstract):1016.
42. Schwarz C, Chromy D, Bangert C, et al. Immediate-type hypersensitivity reaction to bulevirtide and successful desensitization in a patient with HBV/HDV-associated compensated cirrhosis. J Hepatol. 2022;77:254–255. doi:10.1016/j.jhep.2022.03.004
43. Behrendt P, Traidl S, Böker K, Wedemeyer H, Deterding K. T-cell driven allergic cutaneous reaction complicating treatment of hepatitis delta virus infection with bulevirtide. Liver Int. 2022;42:1770–1771. doi:10.1111/liv.15330
44. Jachs M, Reiberger T, Ferenci P. Reply to: pegylated interferon combined with bulevirtide for chronic hepatitis delta – new life for an old timer? Aliment Pharmacol Ther. 2022;56(5):914. doi:10.1111/apt.17138
45. Yuen M, Lim S, Plesniak R, et al.; B-Clear Study Group. Efficacy and safety of bepirovirsen in chronic hepatitis B infection. N Engl J Med. 387;2022:1957–1968. doi:10.1056/NEJMoa2210027
46. Asselah T. Beyond bulevirtide: alternative therapeutic options for the management of HDV. J Viral Hepat. 2022. doi:10.1111/jvh.13789
47. Brancaccio G, Gaeta G. Treatment of chronic hepatitis delta due to hepatitis B and hepatitis delta virus coinfection. Int J Antimicrob Agents. 2019;54:697–701. doi:10.1016/j.ijantimicag.2019.09.012
48. Heidrich B, Yurdaydin C, Kabacam G, et al. Late HDV RNA relapse after peginterferon alpha-based therapy of chronic hepatitis delta. Hepatology. 2014;60:87–97. doi:10.1002/hep.27102
49. Wranke A, Hardtke S, Heidrich B, et al. Ten-year follow-up of a randomized controlled clinical trial in chronic hepatitis delta. J Viral Hepat. 2020;27:1359–1368. doi:10.1111/jvh.13366
50. Sandmann L, Yurdaydin C, Deterding K, et al.; HIDIT-II Study Group. HBcrAg levels are associated with virological response to treatment with interferon in patients with hepatitis delta. Hepatol Commun. 2022;6:480–495. doi:10.1002/hep4.1821
51. Kilic Z, Koksal A, Kalkan IH, Suna N, Yildiz H, Kacar S. Long term efficacy of pegylated interferon in the treatment of delta hepatitis: a single center experience. Acta Gastroenterol Belg. 2016;79:329–335.
52. Bremer B, Anastasiou O, Hardtke S, et al. Residual low HDV viremia is associated HDV RNA relapse after PEG-IFNa-based antiviral treatment of hepatitis delta: results from the HIDIT-II study. Liver Int. 2021;41:295–299. doi:10.1111/liv.14740
53. Kapuria D, Ben Yakov G, Koh C, Heller T. Spontaneous clearance of chronic delta hepatitis. Hepatology. 2020;71:873–875. doi:10.1002/hep.31073
54. Koh C, Canini L, Dahari H, et al. Oral prenylation inhibition with lonafarnib in chronic hepatitis D infection: a proof-of-concept randomised, double-blind, placebo-controlled phase 2A trial. Lancet Infect Dis. 2015;15:1167–1174. doi:10.1016/S1473-3099(15)00074-2
55. Yurdaydin C, Keskin O, Yurdcu E, et al. A phase 2 dose-finding study of lonafarnib and ritonavir with or without interferon alpha for chronic delta hepatitis. Hepatology. 2022;75:1551–1565. doi:10.1002/hep.32259
56. Etzion O, Hamid S, Lurie Y, et al. PS-052-End of study results from LIMT HDV study: 36% durable virologic response at 24 weeks post-treatment with peginterferon lambda mono-therapy in patients with chronic hepatitis delta virus infection. J Hepatol. 2019;70:e32. doi:10.1016/S0618-8278(19)30058-1
57. Soriano V, Mendoza C, Barreiro P, Treviño A, Corral O. Envisioning a hepatitis delta cure with new antivirals. Future Microbiol. 2021;16:927–930. doi:10.2217/fmb-2021-0177
58. Shekhtman L, Cotler S, Hershkovich L, et al. Modelling hepatitis D virus RNA and HBsAg dynamics during nucleic acid polymer monotherapy suggest rapid turnover of HBsAg. Sci Rep. 2020;10:7837. doi:10.1038/s41598-020-64122-0
59. Anolli MP, Degasperi E, Allweiss L, et al. Cure of hepatitis delta infection following 3 years of bulevirtide monotherapy in a patient with compensated advanced cirrhosis. Hepatology. 2022;76:1012.
60. Yurdaydin C, Keskin O, Kalkan C, et al. Optimizing lonafarnib treatment for the management of chronic delta hepatitis: the LOWR HDV-1 study. Hepatology. 2018;67:1224–1236. doi:10.1002/hep.29658
61. Dassetto C, Borentain P, Gérolami R, Colson P. Bulevirtide 2mg-based real-life treatment of hepatitis Delta with genotypes 1 and 5 viruses at more than 20 months of follow-up. Res Hepatol Gastroenterol. 2022;46:102035. doi:10.1016/j.clinre.2022.102035
62. Wang W, Lempp F, Schlund F, et al. Assembly and infection efficacy of hepatitis B virus surface protein exchanges in 8 hepatitis D virus genotype isolates. J Hepatol. 2021;75:311–323. doi:10.1016/j.jhep.2021.03.025
63. Manhas S, Han B, Xu S, et al. Bulevirtide is broadly active against all HDV genotypes expressing envelopes from HBV genotypes A-H and a large panel of clinical isolates. J Hepatol. 2022;77(suppl 1):S244. doi:10.1016/S0168-8278(22)00858-3
64. Kang C, Syed Y. Bulevirtide: first approval. Drugs. 2020;80:1601–1605. doi:10.1007/s40265-020-01400-1
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
