Back to Journals » ClinicoEconomics and Outcomes Research » Volume 8
Budget impact analysis of sofosbuvir-based regimens for the treatment of HIV/HCV-coinfected patients in northern Italy: a multicenter regional simulation
Authors Cenderello G, Artioli S, Viscoli C, Pasa A, Giacomini M, Giannini B, Dentone C , Nicolini LA, Cassola G, Di biagio A
Received 3 August 2015
Accepted for publication 3 November 2015
Published 31 December 2015 Volume 2016:8 Pages 15—21
DOI https://doi.org/10.2147/CEOR.S93641
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Giorgio Colombo
Giovanni Cenderello,1 Stefania Artioli,2 Claudio Viscoli,3 Ambra Pasa,4 Mauro Giacomini,5 Barbara Giannini,5 Chiara Dentone,6 Laura Ambra Nicolini,3 Giovanni Cassola,1 Antonio Di Biagio3
1Infectious Diseases Unit EO, Ospedali Galliera, Genoa, 2Infectious Diseases Unit, ASL-5 Spezzina, La Spezia, 3Infectious Diseases Unit, AOU San Martino, IST, Genoa University, Genoa, 4IT Unit, Ospedali Galliera, Genoa, 5Department of Informatics, Bioengineering, Robotics and System Engineering (DIBRIS), University of Genoa, Genova, 6Infectious Diseases Unit, ASL-1 Imperiese, Sanremo, Imperia, Italy
Objectives: Chronic hepatitis C virus (HCV) is a leading cause of hospitalization and death in populations coinfected with human immunodeficiency virus (HIV). Sofosbuvir (SOF) is a pan-genotypic drug that should be combined with other agents as an oral treatment for HCV. We performed a 5-year horizon budget impact analysis of SOF-based regimens for the management of HIV/HCV-coinfected patients.
Methods: A multicenter, prospective evaluation was conducted, involving four Italian Infectious Diseases Departments (Galliera, San Martino, Sanremo, and La Spezia). All 1,005 genotype-coinfected patients (30% cirrhotics) under observation were considered (patients in all disease-stages were considered: chronic hepatitis C, cirrhosis, transplant, hepatocellular carcinoma). Disease stage costs per patient were collected; the expected disease progression in the absence of treatment and sustained virological response (SVR) success rate for SOF-based regimens were calculated based on the literature and expert opinion. Drug prices were based on what the National Health Service paid for them. The comparison of "no treatment" disease progression costs versus the economic impact of SOF-based regimens was investigated.
Results: Over the following 5 years, the disease progression scenario resulted in direct costs of approximately €54 million. Assuming an SVR success rate of 90%, average SOF-based regimens cost up to €50,000 per person, resulting in a final cost of more than €56 million, so this option is not economically viable. At the average price of €12,000, SOF-based regimens, expense was €17 million, saving 68%. At this price level, the economic resources invested in treating mild to moderate fibrosis stage patients would be equal to the amount of direct costs of disease management in this stage, resulting in a valid return of investment in the short-term.
Conclusion: Given the high rates of SVR, in the Italian Healthcare System, SOF-based regimens, price is a determinant and a predictor of the overall cost for the Hepatitis C patient's management. At the average price per therapy of €12,000 over the next 5 years, SOF-based regimens are becoming highly sustainable.
Keywords: HCV treatment, sofosbuvir, HIV, budget impact, HIV/HCV coinfection, cirrhosis
Introduction
Human immunodeficiency virus (HIV) and hepatitis C virus share the same mode of transmission (through direct blood to blood contact), and therefore, the incidence of coinfection in Italy is extremely high, reaching a peak of 45% as reported by Italian Cohort Naive Antiretrovirals foundation in 2002.1 This issue is largely due to the high percentage of former drug users among Italian people living with HIV.1
The most recent guidelines from the European Liver Association do not make any distinction between mono or coinfected patients2 for the treatment with anti-HCV directly active antivirals. On the other hand, it is generally accepted that in HIV/HCV patients there is faster progression toward cirrhosis and hepatocellular carcinoma (HCC) than in mono-infected.3–11
Several study cohorts have shown the natural history of end-stage liver disease in HIV/HCV-coinfected patients.12–14 In addition, the natural history of compensated cirrhosis in coinfected patients has been described in smaller cohorts of less than 200 patients.15,16 Moreover in HIV/HCV coinfection, SVR may also decrease the progression of HIV infection and mortality not related to liver disease.17,18
The clinical benefits associated with the eradication of HCV have been well characterized in patients with advanced fibrosis or cirrhosis but not in patients with less advanced stages of liver fibrosis. Also, a comprehensive costs–opportunity evaluation in patients with a less severe disease is missing. This is a relevant question, particularly in HIV/HCV-coinfected patients, for whom the delivery of effective HCV treatment could be a priority even in mild to moderate stages of liver fibrosis, in order to prevent a more rapid disease progression. Based on hospital databases, in Liguria region one out of three HIV patients is coinfected with HCV; in addition, Liguria has the highest prevalence of AIDS among other regions in Italy.19 Chronic HCV in Italy is also a leading cause of infectious admissions in hospital for HIV patients.20 Therefore, earlier treatment in this population is crucial in terms of clinical benefits, prevention of disease progression and HCV-related health care direct costs limitation.21 Sofosbuvir (SOF) is a pan-genotypic drug which can be combined with other agents like ribavirin, simeprevir, daclatasvir or, in single-tablet regimen, together with ledipasvir as all oral treatment for HCV, with different entry price levels paid and reimbursed by the National Health Service.
Also, SOF and sofosbuvir/ledipasvir are linked to a price/volume payback scheme covered by a secrecy agreement22 that, in practice, affects regimen’s cost variations. Due to budget constraints, as of year 2015, the national registration in terms of reimbursement of such regimens has been restricted to patients with more severe stages of disease, according to ethical and clinical considerations.23–26 But, we may expect in the near future costs declining, according to new price negotiations that try to include patients with less severe conditions to be eligible to reimbursement by National Health Authorities.
Therefore, we tried to assess a 5-year horizon budget impact (ie, the Regional Health System Management’s term) and cost saving analysis related to the effects of SVR after treatment with SOF regimens on mortality and liver related complications in HIV/HCV-coinfected patients in any disease stage.
Patients and methods
Model structure
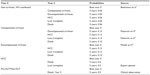
A Markov model has been applied to conduct a multicenter, 5-year horizon prospective costs evaluation, involving four Italian Infectious Diseases Departments (Galliera, San Martino, Sanremo, and La Spezia) in Liguria region, Italy. In all, 1,005 all genotypes adult HIV/HCV patients, under observation by clinicians as of May 2015, were included in the analysis. The patients’ characteristics in the group of pooled data were classified according to their current disease stage: non cirrhotics, cirrhotics, HCC, and transplants (Table 1).
On completion of treatment (at 12 or 24 weeks), patients who achieve SVR are considered to be permanently cured of the infection (SVR at 12 weeks has been set as an appropriate endpoint for regulatory approval and is accepted by most clinical and regulatory authorities).27 Patients who do not achieve SVR progress to more advanced stages of the disease. In this case, transition probabilities and disease progression rates are based both on literature and clinical experts’ opinion. All cause mortality rates were applied to all health states in the model.
The cycle length was 3 months to account for the 12-week treatment duration for some SOF-based regimens, costs and outcomes were discounted at 3% as recommended by the Italian Association of Health Economics.28 All data were anonymously processed and analyzed. The comparison of 1) “no treatment” disease progression costs vs 2) the economic impact of SOF-based regimens was investigated.
This study was developed with the data available inside the IANUA study (Indagine sull’appropiatezza prescrittiva degli antiretrovirali antiretrovirali ) which was approved by Comitato Etico Regione Liguria, with provision number PR 032 REG2014, obtained on 08 April, 2014.
Efficacy: SVR rates
The SVR rates of SOF-based regimens for each patient disease stage were derived from trials and literature29–33 and validated by expert opinion. The average SVR success rate for SOF-based regimens included in the model was 90%.
Transition probabilities
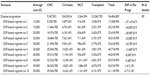
Transition probabilities in the analysis related to HCV progression from one disease stage to another were taken from the literature17,34–37 (Table 2); where this was not possible, we obtained them from expert opinion. These estimates were converted to annual probabilities for the analysis.
Resource consumption and costs
In this study, cost data were analyzed from the Regional Health Service point of view. Costs associated with each health state were obtained from the White Paper by the Italian Association of the Study of the Liver38 and the COME Study,21 which are aligned with the costs for each health state resulting from the Ospedale Galliera budgeting system (which is a component of Italian Network for Standard Costs in Healthcare). As part of the reimbursement agreement with the Italian reimbursement agency, we assumed that 24 weeks of treatment with SOF had the same price as 12 weeks of treatment. Drug prices (VAT included) for all the regimens used in the analysis are those applied to the Italian NHS hospital pharmacies (Table 3).
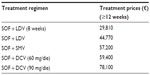  | Table 3 SOF-based regimens’ treatment prices (VAT included) |
Sensitivity analysis
The sensitivity analysis verified the impact of a series of variations of the base case with a large influence on the obtained results. A series of univariate analyses was carried out on some parameters of the simulation model, such as SVR rates, anti-HCV cost regime, and mortality rate. Each parameter was varied with ±10% with respect to the base-case scenario on the basis of Italian guidelines on economic evaluation.24
Results
Table 4 shows the main results. In the “disease progression” scenario, over the next 5 years, the disease progression without treatments resulted in €54 million ca costs and 89 deaths. On the other hand, treatment containing SOF would save 68 people from HCV-related deaths. Overall, the SOF-based regimens with an average price >€50,000 do not offer a favorable cost saving profile, as this cost generates more than €56 million expense. Moreover, when the average price of an SOF regimen varies from €45,000 to €15,000 the budget impact ranges from €50 to €20 million ca, ie, saving from 6% to 53% of liver-related disease management costs, respectively (min: 3.5€M–max: 34.1€ M ca). At the average price of €12,000, SOF-based regimens’ expense was €17 million ca, saving 68% (€37 million ca) of costs compared with the disease progression scenario.
This result indicates that at this price level, the economic resources invested to treat from mild to moderate fibrosis stage patients are equal to the amount of direct costs of disease management in this stage, resulting in a convenient return of investment in the short term. The sensitivity analysis carried out on the main variables does not highlight significant variations with respect to the base case (Table 5).
Discussion
The use of SOF regimens substantially reduces the clinical burden of HCV disease. We decided to focus on SOF-based regimens because, today, it is the only pan-genotypic drug (backbone) available. Although several studies reported that SOF therapies are cost-effective,39–44 the resources needed to treat a large number of patients with HCV/HIV infection could be challenging for the Regional Healthcare System. On the other hand, an average SOF-based regimen price that starts descending progressively from the level of €45,000 would save direct costs optimizing economic resources and bringing a return on investments in the mid-term, at the same time. SOF and sofosbuvir/ledipasvir joint reimbursed agreement is affected by a price/volume scheme leading to incremental economic payback when patient thresholds are achieved.45 In fact as stated in the National Drug Agency (AIFA) resolution published,46 Liguria region received its first amount of payback related to the usage of SOF-based regimens of €0.7 million starting to show benefit from the cost reduction. Therefore, it is reasonable to assume that in the near future the real average cost of SOF-based regimens will be lower than the initial entry prices. Finally, the agreement signed between the company and the AIFA lasts 18 months47,48 as a consequence it will expire by mid-2016 leading to a new price negotiation. To our knowledge, this is the first study that evaluates the budget impact of SOF-based regimens for HIV/HCV-coinfected patients in Italy.
Our study presents some limitations. First, clinical disease progression and SVR rates included in our analysis by clinicians may differ in clinical practice; therefore, our study used only the best available evidence on treatment efficacy for this population. Moreover, this analysis assumed that the achievement of SVR was equivalent to a permanent cure of patients, which could overestimate the benefits of therapies. In addition, additional potential costs of failures related to adverse events and interactions were not calculated. We also did not include the future possibility of re-treatment with next generation of antiviral agents because of a lack of data at the time of this evaluation. A much more comprehensive evaluation may consider budget impact including new unknown mono-infected patients that may emerge through awareness campaigns aiming to eradicate the disease. This data showing the reduction of coinfection of HIV/HCV is a clear sign that in a five year period the target population could not increase.49 Finally, this analysis was focused just on the HCV issues of such patients; additional HIV drugs expense and related direct costs management were not considered but should be included by the Regional Healthcare System at the time of a comprehensive economic budget planning.
Conclusion
Given the high rates of SVR these results suggested that, in the Italian Healthcare System, SOF-based regimens’ price is a determinant and a predictor of the overall cost for the HCV management. At the average price per therapy of €12,000 over the next 5 years, SOF-based regimens are highly sustainable for the Healthcare System saving several million Euro of economic resource money needed to manage HCV in the HIV-coinfected population.
Disclosure
GC received an educational unrestricted grant from Gilead and speaking fees from ABBVIE, BMS, Gilead, and Janssen. AdB received speaking fees from ABBVIE, BMS, Gilead, and Janssen. The authors report no other conflicts of interest in this work.
References
De Luca A, Bugarini R, Lepri AC, et al. Coinfection with hepatitis viruses and outcome of initial antiretroviral regimens in previously naive HIV-infected subjects. Arch Intern Med. 2002;162(18):2125–2132. | |
European Association for the Study of Liver. EASL recommendations on treatment of hepatitis C 2015. J Hepatol. 2015;63(1):199–236. | |
Graham CS, Baden LR, Yu E, et al. Influence of human immunodeficiency virus infection on the course of hepatitis C virus infection: a meta-analysis. Clin Infect Dis. 2001;33(4):562–569. | |
Martin-Carbonero L, de Ledinghen V, Moreno A, et al. Liver fibrosis in patients with chronic hepatitis C and persistently normal liver enzymes: influence of HIV infection. J Viral Hepat. 2009;16(11):790–795. | |
Rockstroh JK, Spengler U. HIV and hepatitis C virus co-infection. Lancet Infect Dis. 2004;4(7):437–444. | |
Thomas DL, Astemborski J, Rai RM, et al. The natural history of hepatitis C virus infection: host, viral, and environmental factors. JAMA. 2000;284(4):450–456. | |
Benhamou Y, Bochet M, Di Martino V, et al. Liver fibrosis progression in human immunodeficiency virus and hepatitis C virus coinfected patients. The Multivirc Group. Hepatology. 1999;30(4):1054–1058. | |
Fattovich G, Giustina G, Degos F, et al. Morbidity and mortality in compensated cirrhosis type C: a retrospective follow-up study of 384 patients. Gastroenterology. 1997;112(2):463–472. | |
Fattovich G, Pantalena M, Zagni I, Realdi G, Schalm SW, Christensen E. Effect of hepatitis B and C virus infections on the natural history of compensated cirrhosis: a cohort study of 297 patients. Am J Gastroenterol. 2002;97(11):2886–2895. | |
Ragni MV, Belle SH. Impact of human immunodeficiency virus infection on progression to end-stage liver disease in individuals with hemophilia and hepatitis C virus infection. J Infect Dis. 2001;183(7):1112–1115. | |
Schwarcz SK, Vu A, Hsu LC, Hessol N. Changes in causes of death among persons with AIDS: San Francisco, California, 1996–2011. AIDS Patient Care STD. 2014;28(10):517–523. | |
Pineda JA, Romero-Gómez M, Díaz-García F, et al. HIV coinfection shortens the survival of patients with hepatitis C virus-related decompensated cirrhosis. Hepatology. 2005;41(4):779–789. | |
Merchante N, Girón-González JA, González-Serrano M, et al. Survival and prognostic factors of HIV-infected patients with HCV-related end-stage liver disease. AIDS. 2006;20(1):49–57. | |
Giron-Gonzalez JA, Brun F, Terron A, Vergara A, Arizcorreta A. Natural history of compensated and decompensated HCV related cirrhosis in HIV-infected patients: a prospective multicenter study. Antivir Ther. 2007;12(6):899–907. | |
Pineda JA, Aguilar-Guisado M, Rivero A, et al. Natural history of compensated hepatitis C virus-related cirrhosis in HIV-infected patients. Clin Infect Dis. 2009;49(8):1274–1282. | |
Tuma P, Jarrin I, Del Amo J, et al. Survival of HIV-infected patients with compensated liver cirrhosis. AIDS. 2010;24(5):745–753. | |
Berenguer J, Rodriguez E, Miralles P, et al. Sustained virological response to interferon plus ribavirin reduces non-liver-related mortality in patients coinfected with HIV and hepatitis C virus. Clin Infect Dis. 2012;55(5):728–736. | |
Casado JL, Banon S, Quereda C, Moreno A, Perez Elias MJ, Moreno S. Liver fibrosis regression after anti HCV therapy and the rate of death, liver-related death, liver-related complications, and hospital admissions in HIV/HCV co-infected patients with cirrhosis. IAS 2015. In: 8th Conference on HIV Pathogenesis, Treatment and Prevention; July 19–22, 2015; Vancouver, British Columbia. Abstract TUAB0204. | |
Camoni, L, Boros S, Regine V, Santaquilani M. Notiziario Istituto Superiore Sanità [Update of HIV and AIDS diagnosis in Italy. Italian Minister of Health Bulletin]. 2014;27(9 Suppl 1):3–47. | |
Cenderello G, Tittle V, Pasa A, et al. Inpatient admissions of patients living with HIV in two European centres (UK and Italy); comparisons and contrasts. J Infect. 2015;70(6):690–694. | |
Scalone L, Fagiuoli S, Ciampichini R, et al. The societal burden of chronic liver diseases: results from the COME study. BMJ Open Gastroenterol. 2015;2:e000025. | |
Minerva D, Epatite C. Il farmaco c’è ma costa troppo. Available from: http://espresso.repubblica.it/visioni/scienze/2015/02/27/news/quel-farmaco-costa-troppo-1.201536. Accessed June 27, 2015. | |
Etzion O, Ghany MG. A cure for the high cost of hepatitis C virus treatment. Ann Intern Med. 2015;162(9):660–661. | |
Bagcchi S. Campaigners challenge patent applications for hepatitis C drug in five countries. BMJ. 2015;350:h2938. | |
Cartabellotta A. Position statement GIMBE Efficacia e costo-efficacia del sofosbuvir nel trattamento dell’epatite C [Position paper of Evidence-Based Medicine Italian Group. Efficacy and cost effectiveness of sofosbuvir in Hepatitis C treatment]. Evidence. 2015;7(4):e1000111. | |
Pani L. AIFA Focus Epatite C: verso un approccio terapeutico clinicamente appropriato ed economicamente sostenibile. Website of AIFA (Italian Medicines Agency). Available from: www.agenziafarmaco.gov.it/it/content/epatite-c-verso-un-approccio-terapeutico-clinicamente-appropriato-ed-economicamente-sostenib. Accessed May 29, 2015. | |
Chen J, Florian J, Carter W, et al. Earlier sustained virologic response end points for regulatory approval and dose selection of hepatitis C therapies. Gastroenterology. 2013;144(7):1450–1455. | |
Italian Health Economics Association (AIES). Italian guidelines proposal on how to conduct economic evaluation studies of health programs. Pharmacoecon Ital Res Artic. 2009;11:83–93. | |
Osinusi A, Townsend K, Kohli A, et al. Virologic response following combined ledipasvir and sofosbuvir administration in patients with HCV genotype 1 and HIV co-infection. JAMA. 2015;313(12):1232–1239. | |
Wyles D, Ruane P, Sulkowski M, et al. Daclatasvir in combination with sofosbuvir for HIV/HCV coinfection: ALLY-2 study. N Engl J Med. 2015;373(8):714–725. | |
Naggie S, Cooper C, Saag M, et al. Ledipasvir-sofosbuvir in GT1 or GT4 and HIV coinfection ION-4. N Engl J Med. 2015;378:705–713. | |
Sulkowski M, Rodriguez-Torres M, Lalezari JP, et al. All oral therapy with Sofosbuvir plus ribavirin for the treatment of HCV genotype 1, 2 and 3 infection in patients co-infected with HIV (PHOTON-1). In: 64rd Annual Meeting of the American Association for the Study of Liver Diseases; November 1–5, 2013; Washington, DC. Abstract #212 AASLD. | |
Younossi ZM, Stepanova M, Sulkowski M, et al. Sofosbuvir and ribavirin for treatment of chronic hepatitis C in patients coinfected with hepatitis C virus and HIV: the impact on patient-reported outcomes. J Infect Dis. 2015;212(3):367–377. | |
Lopez-Dieguez M, Montes ML, Pascual-Pareja JF, et al. The natural history of liver cirrhosis in HIV–hepatitis C virus-coinfected patients. AIDS. 2011;25(7):899–904. | |
Berenguer J, Zamora FX, Carrero A, et al. Effects of sustained viral response in patients with HIV and chronic hepatitis C and nonadvanced liver fibrosis. J Acquir Immune Defic Syndr. 2014;66(3):280–287. | |
Macias J, Mancebo M, Marquez M, et al. Low risk of liver decompensation among human immunodeficiency virus/hepatitis C virus-coinfected patients with mild fibrosis in the short term. Hepatology. 2015;61(5):1503–1511. | |
Mira J, Rivero-Juarez A, Lopez-Cortes LF, et al. Benefits from sustained virologic response to pegylated interferon plus ribavirin in HIV/hepatitis C virus-coinfected patients with compensated cirrhosis. Clin Infect Dis. 2013;56(11):1646–1653. | |
Associazione italiana per lo studio del fegato. 2011. Available from: http://www.webaisf.org/. Accessed June 28, 2015. | |
Messori A, Maratea D, Fadda V, Trippoli S. Letter: estimating the cost-neutral price of sofosbuvir-based triple therapy for the treatment of naíve patients with genotype 1 HCV infection in Italy. Aliment Pharmacol Ther. 2014;40 (2):213–220. | |
Messori A, Maratea D, Fadda V, Gatto R, Trippoli S. An Italian perspective: studying the cost-effectiveness of sofosbuvir before completion of national price negotiations. Eur J Gastroenterol Hepatol. 2014;26(7):813–814. | |
Younossi ZM, Park H, Saab S, Ahmed A, Dieterich D, Gordon SC. Cost-effectiveness of all-oral ledipasvir/sofosbuvir regimens in patients with chronic hepatitis C virus genotype 1 infection. Aliment Pharmacol Ther. 2015;41(6):544–563. | |
Najafzadeh M, Andersson K, Shrank WH. Cost-effectiveness of novel regimens for the treatment of hepatitis C virus. Ann Intern Med. 2015; 162(6):407–419. | |
Chhatwal J, Kanwal F, Roberts MS, Dunn MA. Cost-effectiveness and budget impact of hepatitis C virus treatment with sofosbuvir and ledipasvir in the United States. Ann Intern Med. 2015;162(6):397–406. | |
Cure S, Guerra I, Cammà C, Craxì A, Carosi G. Cost-effectiveness of sofosbuvir plus ribavirin with or without pegylated interferon for the treatment of chronic hepatitis C in Italy. J Med Econ. 2015; 18(9):678–690. | |
Pani L. AIFA. Available from: http://www.rainews.it/dl/rainews/articoli/Luca-Pani-direttore-AIFA-Le-criticita-ci-sono-ma-solo-in-alcune-Regioni-7ad958b2-2174-4a4e-a339-7b0b959964da.html. Accessed April 16, 2015. | |
Official Italian Gazette. GU n 169- 23/07/2015. AIFA resolution n 982/2015. | |
Official Italian Gazette. General Series n 283 – 05/12/2014. DETERMINA 12 novembre 2014. Regime di rimborsabilità e prezzo del medicinale per uso umano «Sovaldi (sofosbuvir)» [Price and reimbursement process of drug for human use named “Sovaldi”(sofosbuvir)]. (Determina 1353/2014). | |
Official Italian Gazette. General Series n 109 – 13/05/2015. DETERMINA 8 maggio 2015. Regime di rimborsabilità e prezzo del medicinale per uso umano «Harvoni (ledipasvir/sofosbuvir)» [Price and reimbursement process of drug for human use named “Harvoni”(ledipasvir/sofosbuvir)]. (Determina 544/2015). | |
Casari S, Suligoi B, Camoni L, et al. Epidemiological and clinical characteristics and behaviours of individuals with newly diagnosed HIV infection: a multicentre study in north Italy. J Prev Med Hyg. 2012;53(4):190–194. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.