Back to Journals » ClinicoEconomics and Outcomes Research » Volume 14
Budget Impact Analysis of Mepolizumab for Eligible Patients in the Setting of a Severe Asthma Clinic Within Dubai Health Authority (DHA)
Authors Mahboub B , Mohy A, El-Amir I, Lukić T , Gouhar R, Noibi S
Received 21 December 2021
Accepted for publication 5 April 2022
Published 26 April 2022 Volume 2022:14 Pages 265—279
DOI https://doi.org/10.2147/CEOR.S343249
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Giorgio Colombo
Bassam Mahboub,1 Ahmed Mohy,2 Islam El-Amir,2 Tamara Lukić,3 Raef Gouhar,4 Saeed Noibi5
1Pulmonary Medicine Department, Rashid Hospital, Dubai Health Authority, Dubai, United Arab Emirates; 2Medical Affairs Department, GSK, Jeddah, Kingdom of Saudi Arabia; 3GSK Research and Development, Dubai, United Arab Emirates; 4Medical Affairs Department, GSK, Dubai, United Arab Emirates; 5Medical Excellence and Value Access, Emerging Markets Region, GSK, Jeddah, Kingdom of Saudi Arabia
Correspondence: Raef Gouhar, Medical Affairs Department, GSK, Dubai, United Arab Emirates, Email [email protected]
Purpose: To estimate 5-years budgetary impact of introducing mepolizumab to eligible patients with uncontrolled severe eosinophilic asthma treated at a tertiary care hospital within Dubai Health Authority (DHA).
Patients and Methods: A budget impact analysis (BIA) model was adapted to the setting of Rashid Hospital, DHA to estimate the budgetary implications of introducing first-in-class anti-IL5 (mepolizumab) as add-on therapy for eligible patients with severe eosinophilic asthma. The eligible patient population (n=60) was estimated from aggregate data provided by the clinic. Patients were eligible to treatment with mepolizumab if they had ≥ 2 exacerbation in the previous year and eosinophil count ≥ 150 cell/μL. The analysis compared the cost of treating patients in two alternative scenarios; a scenario where patients are treated with optimized usual care or with available biologic as add-on therapy, and a second scenario where mepolizumab is fully accessible to eligible patients.
Results: Administration of mepolizumab to eligible patients at Rashid Hospital is predicted to result in overall savings estimated at £ 270,545 over a 5-year time horizon. Exacerbation rates could not be indirectly compared for mepolizumab and omalizumab, since treatment continuation rules were defined differently. Therefore, these parameters were directly taken from the clinical trials for mepolizumab and omalizumab. The savings were estimated due to drug acquisition costs (£ 269,900) and estimated reduction in exacerbation (n=15). One-way sensitivity analysis showed that the model results was most sensitive to changing the method of calculating omalizumab dose and varying the drug acquisition cost of omalizumab by ± 20%.
Conclusion: The BIA showed that full accessibility of mepolizumab to eligible severe asthma patients is predicted to be budget saving in the Dubai Health Authority. This evaluation is relevant to healthcare decision making as it demonstrates that mepolizumab is budget saving for eligible patients, while reducing burden by improving their control and symptoms.
Keywords: asthma phenotypes, eosinophil count, exacerbations, anti-IL5, treatment cost
Introduction
Severe asthma is defined as asthma that requires treatment with high dose inhaled corticosteroids plus a second controller and/or systemic corticosteroids to prevent it from becoming “uncontrolled” or that remains “uncontrolled” despite this therapy. Severe asthma accounts for 5–10% of all asthma cases.1 About 50% of healthcare resource utilization due to asthma has been reported to be attributable to severe asthma, owing to frequent hospital admissions, use of emergency services and drug consumption.2
In recent years the heterogeneity of asthma has increasingly been recognized, leading to the concept of multiple subgroups of patients, based on clinical characteristics, termed phenotypes.3 With increasing understanding of the immunological mechanisms driving asthma presentations, asthma phenotypes are evolving to also reflect the underlying pathophysiology ie endotypes.4 These advances provide the potential of targeting therapy to a specific phenotype. Severe eosinophilic asthma have emerged as a prevalent type of severe asthma.5,6
Mepolizumab is a first-in-class humanized monoclonal antibody targeted towards interleukin-5 (IL-5), a cytokine that plays an important role in the growth, differentiation, recruitment, activation, and survival of eosinophils. Interleukin-5 (IL-5) is a target in the inflammatory pathways of asthma, given that eosinophil levels have been linked to greater airway remodeling, increased asthma severity, and exacerbations.7
Healthcare in the United Arab Emirates (UAE) comprises of government-funded health services and the private health sector. Healthcare is regulated at both the Federal and Emirate levels. Public healthcare services are administered by different regulatory authorities including the Ministry of Health and Prevention, Health Authority-Abu Dhabi (HAAD) and, the Dubai Health Authority (DHA).8
According to Asthma Insights and Reality in the Gulf and Near East (AIRGNE) study, this study is based on information from 1000 patients with asthma in five Gulf countries including children and adults, 23% of hospitalization was due to asthma whilst 52% of emergency room (ER) visits was also due to asthma.9
Over eight thousand asthma patients were reported to be registered with DHA in 2019, from clinic records analysis. This population of asthma patients represents those from Dubai and Northern Emirates which constitute less than 20% of the total base population of the UAE. Rashid Hospital is a 762 bed specialized tertiary care center within DHA.10
With increasing constrains on healthcare resources, Budget Impact Analysis (BIA) provide a framework for estimating the financial consequences of adopting a healthcare intervention for a specific patient population within a specific healthcare setting.11
Optimized usual care involves the addition of controller therapy on top of high dose inhaled corticosteroid (ICS). This may include oral corticosteroids (OCS), Long Acting Muscarinic Antagonists (LAMA) and to lesser extent theophylline.
A Budget Impact Analysis (BIA) was developed to estimate potential budgetary implications of mepolizumab for the treatment of eligible severe eosinophilic asthma patients from the United Kingdom (UK) National Health Services (NHS) perspective. The current analysis is an adaptation of the BIA model to the setting of a severe asthma clinic within DHA. The objective of the analysis was to estimate the budgetary implications over a period of 5 years, when mepolizumab is fully accessible to eligible severe eosinophilic asthma patients presenting in the severe asthma clinic at Rashid Hospital, While the approach taken to obtain anonymized and aggregated data inputs and clinical assumptions for this BIA adaptation does not fall within GSK’s definition of Human Subject Research (HSR), confirmation was still obtained from the relevant Ethics Committee (EC) of the Dubai Health Authority (DHA) that prior ethics approval was not required for the BIA adaptation except a notification prior to external publication of the analysis.
Materials and Methods
Model Structure
This model is a budget impact analysis that estimates the budgetary implications when mepolizumab is reimbursed and introduced in a healthcare setting for patients with severe eosinophilic asthma. These implications were calculated over a time horizon that could be set to a maximum of 5 years to accommodate a generally acceptable time frame for future adaptations to other healthcare systems.
The analysis compared budget estimations of two different healthcare situations: one where mepolizumab is not available versus a situation where mepolizumab is introduced to treat patients with severe eosinophilic asthma (see Figure 1).
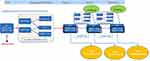 |
Figure 1 Model overview See attached figure to the manuscript. |
Mepolizumab-eligible patients are allocated in the model to each of the available treatments based on insights from the clinic. These treatments are mepolizumab with restricted access; where only specific pulmonologists can prescribe it; omalizumab, and optimized care (henceforth referred to as Standard of Care - SoC).
Treatment continuation criteria for the alternative biologics (mepolizumab and omalizumab) is applied respectively in the model, in line with the respective local prescribing information. Patients on biologic treatment either meet the continuation criteria and continue biologic treatment or, fail and revert to SoC alone. At the end of each year, the probability of discontinuing biologic drug treatment is applied in the model. The model also considers the entrance of newly eligible patients each year.
The cost components in the model were drug acquisition costs and exacerbation costs. While the base model also includes the option to include drug monitoring costs, this was determined to be negligible from the perspective of DHA. In addition to costs, the current analysis predicted the number of exacerbations in each of the BIA scenarios. Consequently, results were expressed as total and incremental costs and, total and incremental exacerbations, comparing a situation with and without the full accessibility of mepolizumab to eligible patients.
Patient Population
Severe asthma patients treated at Rashid Hospital were typically administered oral corticosteroids more than once per year and, have had hospital admission due to asthma in the previous year. About 40% of these patients are optimized on their current therapies. This included the addition of a Long Acting Muscarinic Antagonist (LAMA) monotherapy and to a much lesser extent, theophylline. Considering that they are on high dose Inhaled Corticosteroids (ICS) and Long Acting Beta Agonists (LABA), these patients may be on up to four concurrent asthma medications ie ICS, LABA, LAMA and others. The remaining 60% are treated with available biologic therapy (omalizumab) in addition to their standard of care (SoC).
The target population consists of eligible severe eosinophilic asthma patients that present at the severe asthma clinic. The model allows to estimate the size of eligible population using funneling approach where successive epidemiological filters are applied starting from the country population. For the purpose of this adaptation, the funneling approach was not used. Rather, the size of the eligible population was provided by the clinic as aggregate estimate and included biologic -naïve and prevalent biologic administered patients. Actual patient-level data was not used. A total of 60 severe asthma patients presenting at the severe asthma clinic were estimated to be eligible for mepolizumab at the time of conducting the analysis. Patient eligibility was defined by the following criteria: severe asthma patients from 6 years old not controlled on SoC, had 2 or more exacerbations in the past year and, have blood eosinophilic level ≥ 150 cell/µL.
At the time of conducting the analysis, all patients who were reported to be eligible for mepolizumab were treated with the existing biologic at the clinic (omalizumab). The total target population was assumed to grow at a rate of 5% each year.
Severe Asthma Biologics Included in the Analysis
Anti-IL-5
Mepolizumab (Nucala, GlaxoSmithKline) is a humanized monoclonal antibody (IgG1, kappa), which targets human interleukin-5 (IL-5) with high affinity and specificity. IL-5 is the major cytokine responsible for the growth and differentiation, recruitment, activation and survival of eosinophils. Mepolizumab inhibits the bioactivity of IL-5 with nanomolar potency by blocking the binding of IL-5 to the alpha chain of the IL-5 receptor complex expressed on the eosinophil cell surface, thereby inhibiting IL-5 signaling and reducing the production and survival of eosinophils. Mepolizumab is indicated as an add-on treatment for severe refractory eosinophilic asthma in adults, adolescents and children aged 6 years and older. In Adults and adolescents aged 12 years and over, the recommended dose of mepolizumab is 100 mg administered subcutaneously once every 4 weeks. In children aged 6 to 11 years old, the recommended dose of mepolizumab is 40mg administered subcutaneously once every 4 weeks.12
Anti-IgE
Omalizumab (Xolair, Genentech USA, Inc. and Novartis Pharmaceuticals Corporation) binds to Immunoglobulin E (IgE) and prevents binding of IgE to FcɛRI (high-affinity IgE receptor) on basophils and mast cells, thereby reducing the amount of free IgE that is available to trigger the allergic cascade. Treatment of atopic subjects with omalizumab resulted in a marked down-regulation of FcɛRI receptors on basophils. Treatment with omalizumab inhibits IgE-mediated inflammation, as evidenced by reduced blood and tissue eosinophils and reduced inflammatory mediators, including IL4, IL-5, and IL-13 by innate, adaptive and non-immune cell. Omalizumab is indicated in adults, adolescents and children (6 to <12 years of age). Omalizumab treatment should only be considered for patients with convincing IgE (immunoglobulin E) mediated asthma. Omalizumab is indicated as add-on therapy to improve asthma control in patients with severe persistent allergic asthma who have a positive skin test or in vitro reactivity to a perennial aeroallergen and frequent daytime symptoms or night-time awakenings and who have had multiple documented severe asthma exacerbations despite daily high-dose inhaled corticosteroids, plus a long-acting inhaled beta2- agonist. For Adults and adolescents (12 years of age and older) have reduced lung function (FEV1 <80%) in addition to the above-mentioned criteria. Omalizumab dose is not fixed and depends on patient’s weight and serum IgE levels.13
Standard of Care
Standard of Care (SoC) was defined as regular treatment with high-dose ICS or equivalent with or without maintenance Oral Corticosteroid (OCS) and required additional controller medication besides ICS, eg LABA, leukotriene receptor antagonist or theophylline.
Market Shares of Different Treatments
The market shares distributions adopted in the model for the scenario without full mepolizumab accessibility and the scenario with full mepolizumab access are presented in Table 1. The Market shares were informed by insights of the study sponsor.
 |
Table 1 Market Share Distribution for A) Scenario Without Mepolizumab and B) Scenario with Mepolizumab |
Total Number of Patients at the Start of Each Year
The number of patients at the start of each year on each treatment in both the scenario with and without mepolizumab was determined by the combination of a) the total target population eligible for treatment with mepolizumab, b) the expected growth rate of the target population and c) the market share distributions, (Table 2). Patient numbers projected over the period of the analysis were rounded-up.
 |
Table 2 Total Number of Patients Each Year in A) Scenario Without Mepolizumab and B) Scenario with Mepolizumab |
Clinical Parameters
Clinical parameters that were included in the BIA were derived from mepolizumab and omalizumab clinical trials (vs SoC). In the base case model, both published and unpublished data from the Mepolizumab Treatment in Patients with Severe Eosinophilic Asthma (MENSA) study14 was used in the analysis at the time. Furthermore, the mepolizumab data of the 75mg IV formulation arm was combined with 100mg SC arm from MENSA. When comparing mepolizumab added to SoC versus SoC alone, evidence for exacerbation rates and potential continuation criteria is available from MENSA. Mortality was not included in the budget impact analysis due to the short time horizon (5 years) and infrequent number of asthma-related deaths expected. The total target population was adjusted accordingly when one of the subgroups was selected. Nevertheless, the base-case of the model, and the current adaptation uses the ITT population for analysis ie the entire eligible population.
Clinically Significant Exacerbations
Three subtypes of clinically significant exacerbations were distinguished in the BIA. There were exacerbations treated with a burst of OCS; those that require an emergency department (ED) visit, and events that require hospitalization.
Mepolizumab vs SoC
Studies comparing mepolizumab add-on therapy with placebo were used as inputs on exacerbation rates for mepolizumab + SoC and SoC alone. A post hoc analysis was conducted to calculate annualised exacerbation rates using a negative binomial model with covariates of treatment group, baseline maintenance OCS use, exacerbations in year prior to study as an ordinal variable, and baseline percent predicted FEV1 with the logarithm of time on treatment as an offset variable. Up to the time point of a potential treatment continuation rule, patients on mepolizumab experience the treatment effect observed for all patients randomised to mepolizumab in the trials. Continuation rule of mepolizumab was defined by end-of-trial exacerbation reduction at week 52.
After the assessment according to the criteria for treatment continuation, the cohort was separated into patients meeting and those not meeting these continuation criteria. The proportion of patients meeting the continuation criteria was isolated from the MENSA clinical trial and post-hoc data analyses informed their corresponding exacerbation rate. Patients not meeting the continuation rule revert to standard therapy alone and experience the exacerbation rates of the SoC group.
Omalizumab vs SoC
The INNOVATE study15 was chosen for the base-case population of adults and adolescents for omalizumab as it was, to the date of model development, the only double-blind RCT in which the Global Evaluation of Treatment Effect (GETE) has been used to assess response to treatment and where a responder analysis is available.
Treatment efficacy by response status was also available from EXALT study,16 however, the open-label design of EXALT makes the trial more susceptible to a number of potential biases with knowledge of treatment allocation on post-randomized treatment decisions and on reporting of outcomes.
The rates of exacerbation reduction used for each treatment option are presented in (Tables 3 and 4).
 |
Table 3 Exacerbation Rates of Treatment Options Used in the BIA Model for Mepolizumab + SoC and SoC |
 |
Table 4 Exacerbation Rates of Treatment Options Used in the BIA Model for Omalizumab + SoC |
Drug Discontinuation
The model allows for a user-adjustable input (set at 10% for mepolizumab and omalizumab in the base case) for drug discontinuation.17 This is to accommodate the possibility that not every patient will be 100% compliant to biologic treatment. The reported discontinuation rate of 20% at the clinic was applied in the model for this adaptation.
Healthcare Resource Use and Costs
Costs used within the model consisted of drug acquisition costs and exacerbation costs. An additional cost in the model was drug monitoring cost. Treatment monitoring cost in the model accounts for medication (biologics and SoC) administration time (assumed to be 10 minutes) based on the hourly tariff of the consultant and nurse (nurse hourly tariff is assumed to be half that of the consultant).
As at the time of this adaptation, the cost of biologics administration was considered not to be significant enough to include in the BIA adaptation from DHA perspective.
Drug Acquisition Costs
Drug costs were based on public prices published by the Ministry of health, UAE. Unit costs of biologics included in the model are presented in Table 5.
 |
Table 5 Drug Acquisition Costs (Biologics) |
The unit cost of mepolizumab reflects the cost per 4-weeks, as it is administered once every four weeks for all patients. Omalizumab is administered as a subcutaneous injection every 2–4 weeks and the exact dose depends on the patient’s serum IgE and weight. To estimate omalizumab dose per 4 weeks period, the average vial use per month was directly input in the model. The latter method was used for the base-case of this model adaptation. Based on the observed prescription patterns at the clinic, the average omalizumab dosage over 4-weekly period was estimated to be 450mg (three 150mg vials) per patient.18
Unit costs of different SoC therapies were obtained from Dubai Drug Code (DDC) list.19 Proportion of patients (%) of each therapy was based on treatment patterns within the severe asthma clinic at Rashid Hospital and obtained through the principle investigator (Table 6).
 |
Table 6 Standard of Care Use and Costs per 4 Weeks |
Exacerbation Event Costs
Healthcare resource use costs were obtained from a previous cost analysis conducted independently by the principle investigator. The analysis which included the cost of patient exacerbation leading to ER visit and hospitalisation adopted a bottom up approach at estimating associated costs (Table 7). The figures from the cost analysis which was conducted in 2015 was used in the BIA adaptation with the express permission of the principle investigator.
 |
Table 7 Unit Costs of Healthcare Resource Use Due to Asthma Exacerbations Provided by the Clinic |
OCS Sparing Effect
The Phase III SIRIUS study20 was designed to demonstrate the OCS sparing effect of mepolizumab. Based on the results of this study, the OCS sparing potential of mepolizumab in terms of reduced SoC treatment (ie reduced OCS dose within SoC treatment) was included in the BIA. The OCS sparing potential in terms of a possible reduction in OCS related adverse events was however not included in the BIA. Data from SIRIUS showed that a median 50% of OCS dose reduction benefit was achieved for mepolizumab compared to SoC. As a result, the daily OCS dose of patients after 24 weeks on mepolizumab was 9.24mg. It was assumed that patient that discontinue mepolizumab return to the OCS dose within the SoC arm (13.2mg). A similar OCS sparing effect was assumed for omalizumab.
Analysis
Base-Case Model Settings
An overview of base case model settings is presented in Table 8
 |
Table 8 Base-Case Model Settings |
Main Model Assumptions
Number of Incident Patients
The number of incident patients each year and the substitution rates were estimated by the predefined model inputs for size of the total eligible population, expected population growth, and the expected market share inputs for each drug each year.
Patients Movement to SoC
Due to the inclusion of continuation rules for mepolizumab and omalizumab, a substantial proportion of patients may move to SoC within the first year after treatment initiation. When it is expected that the patient population treated with mepolizumab and omalizumab stabilizes over the years, the percentage of patients that moved to SoC after the continuation rule were supplemented at the start of each subsequent year.
Treatment Continuation
It was assumed that the proportion of patients passing the continuation rule (measured at a fixed time point) can be applied to each variable time point in the BIA model. Drug discontinuation was assumed to take place at the end of each year, this may vary from clinical practice elsewhere.
Mortality
Mortality was not included in the model. Typical of BIA, the model employed a short time horizon of a maximum period of 5 years. As such, cause-specific mortality was expected to be infrequent.
OCS Related Adverse Events
The impact of reduced dose of OCS on incidence of OCS related adverse events was not incorporated.
Sensitivity Analysis
One-way sensitivity analysis was conducted on the budget impact analysis adaptation to evaluate the effect of varying selected input parameters, including: mepolizumab continuation criteria, no continuation rule, source of clinical evidence of omalizumab + SoC EXALT study, omalizumab average vial use dosing schedule and average weight IgE, and mepolizumab drug cost ±20%.
Results
Base-Case Analysis
Exacerbations
The cumulative reduction in exacerbations over 5 years was estimated to be 15 when mepolizumab will be fully accessible to the eligible patient population per the pre-defined market share distribution (Table 9).
 |
Table 9 Predicated Budget Impact When Mepolizumab is Fully Accessible |
Budget Impact
The predicted budgets in the scenario with and without mepolizumab, disintegrated per cost component, and the budgetary implications are presented in Table 10
 |
Table 10 The Predicted Budgets in the Scenario with, Without Mepolizumab and Overall Budget Impact |
Sensitivity Analysis
One-way sensitivity analysis showed that the evaluation was most sensitive to changing the method of calculating omalizumab vial use and, cost. Within the parameters used to test the sensitivity, the most significant change in the model results was due to using average weight and IgE levels to calculate the average vial use of omalizumab as opposed to the use of average vial use (3 vials) used in the base case of this model adaptation (see Table 11). Following that, the model was also sensitive to increasing omalizumab cost by 20%. Within the tested parameters, the only case where there was an increase in budget rather than budget savings as a result of full accessibility of mepolizumab, was when the cost of omalizumab was reduced by 20%.
 |
Table 11 Omalizumab Dosing Based on Weight/IgE Level |
Discussion
Given the peculiarities of individual healthcare systems and decision makers’ varying perspectives, a Budget Impact Analysis (BIA) is not intended to provide a single budget impact estimate for all decision makers.11 Hence, this BIA adaptation to the setting of a healthcare organisation within Dubai Health Authority (DHA) provides a useful framework to estimate the plausible budgetary impact of a first-in-class phenotype guided therapy in severe asthma management – if made fully accessible.
This budget impact analysis showed that full accessibility of mepolizumab to eligible patients treated in the severe asthma clinic at Rashid Hospital within DHA, will result in budgetary savings mainly due to drug acquisition costs substituting Omalizumab with some attendant reduction in clinically significant exacerbations. Based on the assumptions employed in the model, and the input data used, a point estimate of a cumulative budget saving of GBP 270,545 over 5 years was predicted. Most of the budgetary savings predicted was due to drug acquisition cost savings (GBP 269,900). Based on the available clinical data at the time of the evaluation a much smaller contribution to the saving was due to reduction in exacerbation event cost (GBP 645). A published data from South Italy, switching patients from Omalizumab to Mepolizumab significantly decrease the number of exacerbations per year and significantly decrease the percentage of patients who were dependent on corticosteroids.21
This Budget Impact Analysis (BIA) adaptation is relevant to healthcare decision making as it does not entail only a financial calculation, but also includes clinical efficacy assumptions of alternative severe asthma therapeutic interventions. This therefore provides comprehensive costs-of-illness in severe asthma management due to alternative interventions for a target population eligible for mepolizumab. To be meaningful to healthcare decision makers, this predicted budgetary impact should be put in context of the healthcare budget spent on asthma or severe asthma management and, the opportunity cost.
As at the time of the evaluation, mepolizumab-eligible patients at the severe asthma clinic at Rashid Hospital consisted of biologic-naïve and prevalent biologic users. The input market share data suggested that budgetary savings will be realized due to increasing use of mepolizumab among the prevalent biologic users whilst the proportion of patients on SoC remained the same. This informs of two key insights from the existing practice at the severe asthma clinic at Rashid hospital. Firstly, all patients currently on existing biologic (omalizumab) have been reported to be mepolizumab-eligible patients, this is different than IDEAL study where only a small proportion of patients on omalizumab are eligible for mepolizumab.22 This represents a significant opportunity to maximise patient outcomes - with improvement in quality of life and decrease in OCS use and its associated adverse outcomes; a more targeted phenotype-guided therapy for severe eosinophilic asthma patients. Secondly, the major factor for non-accessibility of mepolizumab to eligible biologic-naïve patients has been reported to be due to costs of co-payments for patients. While most patients presenting at the clinic are nationals (approximately 80%), the remaining patients (20%) contribute 20% of the cost of their treatment as co-payment.
Savings demonstrated in drug acquisition cost was based on the average vial usage of omalizumab per month (3 vials) which incurred incremental cost to the 4-weekly dose of mepolizumab. Based on the reported prescription patterns at the clinic, the average monthly omalizumab dosage is around 450mg per patient. This is consistent with evidence from real world studies, National Center of Pharmacoeconomics (NCPE) evaluation of omalizumab,23 and the WHO omalizumab Defined Daily Doses (DDD).24
As shown in the cost input parameters, cost of exacerbation (ED visit and hospitalisation) was significant (AED 1616 and up to AED 20,144 respectively), while this BIA predicted minimal exacerbation reduction over 5 years (- 15 exacerbations) (see Table 12). Given the overlap population of mepolizumab and omalizumab, more relevant comparative data have emerged which compares alternative anti-IL5 therapies to demonstrate significant difference in exacerbation reduction and quality of life.25–28 These data become more important when considering an updated BIA that compares new entrants of alternative anti-IL therapies for eligible severe eosinophilic asthma patients.
 |
Table 12 Predicted Exacerbation Events in the Scenario Without and with Mepolizumab |
Two of the key model assumptions were of significant implication to the clinical practice and experience at Rashid Hospital Severe Asthma clinic. Typically, patients were assessed 4-monthly within the clinic, up to a maximum of 6 months. The default setting of the model was to assess patients’ continuation of mepolizumab at 12-months which is in line with local prescribing information of mepolizumab in the United Arab Emirates. This difference between the label and practice was deemed not to have significant implication on the outcome of the analysis as the reported discontinuation rate for eligible patients of 20% was applied in the model.
Secondly, the non-incorporation of the impact of reduced dose of OCS on incidence of OCS-related adverse event may be a significant under-representation of the budgetary savings as a result of the OCS-sparing effect of mepolizumab.29 Such analysis would have required a more detailed study of the Electronic Medical Records (EMR) and may go beyond the scope of the current adaptation. However, future empirical study is required to estimate the cost of OCS-related adverse events as this is considered to represent significant unmet medical need for these patients.30
This Budget Impact Analysis (BIA) provides useful insights for decision making on the need for full accessibility of mepolizumab to eligible patients within the severe asthma clinic at Rashid Hospital within Dubai Health Authority (DHA). Though this BIA was limited by the fact that the data inputs and clinical assumptions were based on aggregate data, the assumption therein were validated in clinical practice.
A subsequent adaptation with patient-level data that includes the entire DHA will further reinforce the generalisability of the budget impact analysis results.
As aforementioned, the outcomes of a BIA adaptation reflect the use of specific assumptions and data inputs that are relevant to the decision maker rather than a single base case intended to be generally applicable.11 An initial model adaptation to the same setting was conducted in 2019 and the number of eligible populations was estimated to range between 40 and 50.31 The increase in mepolizumab-eligible patients since the period of the previous adaptation resulted in increase in budget savings. Other inputs that, if altered, can change the results include market share of each intervention and drug acquisition cost. Hence, varying these key input parameters may predict varying magnitudes of the expected savings to Dubai Health Authority (DHA) as a result of introducing mepolizumab to eligible patients. Using weight and IgE assumptions to calculate Omalizumab treatment costs led to more savings (-£813,488) while using dosing frequency distribution les to less savings (-£109,016).
Results of one-way sensitivity analysis was consistent with the findings of the base-case analysis where most cost savings were driven by difference in drug acquisition cost. Based on available omalizumab dosing charts in allergic asthma,32 the appropriate dose for patients weighing 60kg with IgE level of 450 IU/mL is 300mg every 2 weeks, ie four 150mg vials. The use of dosing frequency distribution (Table 13) based on INNOVATE study reduced the estimated cost savings of mepolizumab.15 However, these dosing schedules may underestimate the actual use of omalizumab as the dosing tables have been expanded after INNOVATE study was conducted.33 Similarly, changing the drug cost of omalizumab had a considerable impact on the model results. Increasing omalizumab cost by 20% increased the estimated savings from £270,545 to £591,469 due to full accessibility of mepolizumab. However, reducing omalizumab cost changed the direction of the model results in which full accessibility and use of mepolizumab would be associated with increased cost.
 |
Table 13 Omalizumab Dosing Frequency Distribution |
Conclusion
This Budget Impact Analysis showed that full accessibility of mepolizumab to eligible severe asthma patients is predicted to be budget saving to Dubai Health Authority (DHA). This evaluation is relevant to healthcare decision making as it demonstrates that mepolizumab is budget saving for eligible patients, while reducing burden to patients and improving their control and symptoms. To be meaningful to healthcare decision makers, this predicted budgetary saving should be put in context of the healthcare budget spent on asthma or severe asthma management and, the opportunity cost.
Acknowledgments
The original Budget Impact Analysis model and the associated technical reports were created by Pharmerit BV (Marten Meesweg 107, 3068 AV Rotterdam, Netherlands) and funded by GlaxoSmithKline plc (GSK). The Authors would like to acknowledge the efforts of Mohamed Hamouda, Value Evidence and Outcomes Manager, GSK Gulf countries, for providing review and editorial suggestions to draft versions of this paper.
Funding
This study was sponsored and funded by GlaxoSmithKline plc. [Study Reference: HO-19-19855]. The adaptation of the model was conducted by the sponsor.
Disclosure
BM has no potential or competing conflicts of interest, AM, IEA, TL, RG & SN are full-time employees of GSK, SN is also shareholder in GSK. The authors report no other conflicts of interest in this work.
References
1. Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43(2):343–373. doi:10.1183/09031936.00202013
2. Louis R. Severe asthma: how can we differentiate phenotypes? Swiss Med Wkly. 2009;139(19–20):247–277.
3. Wenzel SE. Asthma phenotypes: the evolution from clinical to molecular approaches. Nat Med. 2012;18(5):716. doi:10.1038/nm.2678
4. Kuruvilla ME, Lee FE, Lee GB. Understanding asthma phenotypes, endotypes, and mechanisms of disease. Clin Rev Allergy Immunol. 2019;56(2):219–233. doi:10.1007/s12016-018-8712-1
5. Pavlidis STakahashi K, Kwong FN, et al. “T2-high” in severe asthma related to blood eosinophilexhaled nitric oxide and serum periostin. Eur Respir J. 2019;3553(1).
6. Bakakos A, Loukides S, Bakakos P. Severe eosinophilic asthma. J Clin Med. 2019;8(9):1375. doi:10.3390/jcm8091375
7. Pelaia C, Paoletti G, Puggioni F, et al. Interleukin-5 in the pathophysiology of severe asthma. Front Physiol. 2019;10:1514. doi:10.3389/fphys.2019.01514
8. Latham and Watkins. Healthcare regulation in the UAE; 2011 [cited September 15, 2020]. Available from: https://www.lw.com/upload/pubContent/_pdf/pub3951_1.pdf.
9. Khadadah M, Mahboub B, Al-Busaidi NH, et al. Asthma insights and reality in the gulf and the Near East. Int J Tuberc Lung Dis. 2009;13(8):1015–1022.
10. Government of Dubai. About Rashid Hospital; 2020 [cited October 6, 2020]. Available from: https://www.dha.gov.ae/en/RashidHospital/pages/aboutrashidhospital.aspx.
11. Sullivan SD, Mauskopf JA, Augustovski F, et al. Budget impact analysis-principles of good practice: report of the ISPOR 2012 budget impact analysis good practice II Task Force. Value Health. 2014;17(1):5–14. doi:10.1016/j.jval.2013.08.2291
12. Nucala: EPAR-product information; 2019.
13. Xolair-EPAR: product Information; 2020.
14. Ortega HG, Liu MC, Pavord ID, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014;371(13):1198–1207. doi:10.1056/NEJMoa1403290
15. Humbert M, Beasley R, Ayres J, et al. Benefits of omalizumab as add‐on therapy in patients with severe persistent asthma who are inadequately controlled despite best available therapy (GINA 2002 step 4 treatment): INNOVATE. Allergy. 2005;60(3):309–316. doi:10.1111/j.1398-9995.2004.00772.x
16. Bousquet J, Siergiejko Z, Świebocka E, et al. Persistency of response to omalizumab therapy in severe allergic (IgE‐mediated) asthma. Allergy. 2011;66(5):671–678. doi:10.1111/j.1398-9995.2010.02522.x
17. Chang H-L, Hsu J-F, Tsai Y-M, Lin S-Y, Kuo H-F, Yang C-J. Drop-out rate among patients treated with omalizumab for severe asthma: literature review and real life experience. BMC Pulm Med. 2016;16:1–9. doi:10.1186/s12890-015-0163-3
18. WHO collaborating center for drug statistics methodology Norwegian Institute of Public Health; 2021.
19. DHA. eClaimLink; 2012 [cited September 21, 2019]; Available from: https://www.eclaimlink.ae/NoneRegisteredCodingSets.aspx. .
20. Bel EH, Wenzel SE, Thompson PJ, et al. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med. 2014;371(13):1189–1197. doi:10.1056/NEJMoa1403291
21. Carpagnano GE, Pelaia C, D’Amato M, et al. Switching from omalizumab to mepolizumab: real-life experience from Southern Italy. Ther Adv Respir Dis. 2020;14:175346662092923. doi:10.1177/1753466620929231
22. Albers FC, Necdet HM, Gunsoy B, et al. Biologic treatment eligibility for real-world patients with severe asthma: the IDEAL study. J Asthma. 2018;55:152–160. doi:10.1080/02770903.2017.1322611
23. NCPE. Cost-Effectiveness of Omalizumab (Xolair®) for the Treatment of Severe Allergic Asthma. Ireland: National Centre for Pharmacoeconomics; 2015.
24. World Health Organization. Omalizumab Defined Daily Dose (DDD); 2019 [cited September 24, 2020]. Available from: https://www.whocc.no/atc_ddd_index/?code=R03DX05&showdescription=no.
25. Benralizumab for treating severe eosinophilic asthma; 2018 [cited September 24, 2020]. Available from: https://www.nice.org.uk/guidance/ta565/documents/final-appraisal-determination-document.
26. Assessment report: fasenra; 2018 [cited September 24, 2020]. Available from: https://www.ema.europa.eu/en/documents/assessment-report/fasenra-epar-public-assessment-report_en.pdf.
27. Biologic therapies for treatment of asthma associated with type 2 inflammation: effectiveness, value, and value-based price benchmarks; 2018. [cited September 24, 2020]; Available from: https://icer-review.org/wp-content/uploads/2018/04/Asthma-Revised-Report-FOR-PUBLICATION-11.13.2018.pdf.
28. Busse W, Chupp G, Nagase H, et al. Anti–IL-5 treatments in patients with severe asthma by blood eosinophil thresholds: indirect treatment comparison. J Allergy Clin Immunol. 2019;143(1):190–200. doi:10.1016/j.jaci.2018.08.031
29. Carpagnano GE, Resta E, Povero M, et al. Clinical and economic consequences of switching from omalizumab to mepolizumab in uncontrolled severe eosinophilic asthma. Sci rep2021;11(5453).
30. Lefebvre P, Duh MS, Lafeuille MH, et al. Acute and chronic systemic corticosteroid–related complications in patients with severe asthma. J Allergy Clin Immunol. 2015;136(6):1488–1495. doi:10.1016/j.jaci.2015.07.046
31. Mahboub B. Budget impact analysis of mepolizumab for eligible patients in the setting of a severe asthma clinic within Dubai health authority (DHA).
32. Genentech USA Inc. and Novartis Pharmaceuticals Corporation. XOLAIR dosing for allergic asthma; 2020 [cited October 5, 2020]. Available from: https://www.xolairhcp.com/starting-treatment/dosing.html.
33. Lowe PJ, Janice Canvin PG. Revision of omalizumab dosing table for dosing every 4 instead of 2 weeks for specific ranges of bodyweight and baseline IgE. Regulatory Toxicol Pharmacol. 2015;71(1);71
34. medications. M.o.H.a.P.M.p.l.o.r; 2021.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
