Back to Journals » ClinicoEconomics and Outcomes Research » Volume 7
Budget impact analysis of botulinum toxin A therapy for upper limb spasticity in the United Kingdom
Authors Abogunrin S, Hortobagyi L, Remak E, Dinet J , Gabriel S, Bakheit A
Received 21 October 2014
Accepted for publication 23 January 2015
Published 1 April 2015 Volume 2015:7 Pages 185—193
DOI https://doi.org/10.2147/CEOR.S76141
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Giorgio L Colombo
Seye Abogunrin,1 Linda Hortobagyi,2 Edit Remak,3 Jerome Dinet,4 Sylvie Gabriel,5 Abdel Magid O Bakheit6
1Meta Research, 2Health Economics, Evidera, London, UK; 3Health Economics, Evidera, Budapest, Hungary; 4Health Economics and Outcomes Research (Global), 5Global Market Access and Pricing, Ipsen Pharma, Boulogne-Billancourt, France; 6Neurological Rehabilitation, Moseley Hall Hospital, Birmingham, UK
Background: Botulinum toxin A (BoNT-A) is an effective treatment for patients with upper limb spasticity (ULS), which is a debilitating feature of upper motor neuron lesions. BoNT-A preparations available in the UK are associated with different costs.
Methods: We developed a budget impact model to assess the effect of changing market shares of different BoNT-A formulations – abobotulinumtoxinA, onabotulinumtoxinA, and incobotulinumtoxinA – and best supportive care, from the UK payer perspective, over a 5-year time horizon. Epidemiological and resource use data were derived from published literature and clinical expert opinion. One-way sensitivity analyses were performed to determine parameters most influential on budget impact.
Results: Base-case assumptions showed that an increased uptake of abobotulinumtoxinA resulted in a 5-year savings of £6,283,829. Treatment with BoNT-A costs less than best supportive care per patient per year, although treating a patient with onabotulinumtoxinA (£20,861) and incobotulinumtoxinA (£20,717) cost more per patient annually than with abobotulinumtoxinA (£19,800). Sensitivity analyses showed that the most influential parameters on budget were percentage of cerebral palsy and stroke patients developing ULS, and the prevalence of stroke.
Conclusion: Study findings suggest that increased use of abobotulinumtoxinA for ULS in the UK could potentially reduce total ULS cost for the health system and society.
Keywords: stroke, cerebral palsy, multiple sclerosis, traumatic brain injury
Introduction
Upper limb spasticity (ULS) is an important debilitating characteristic of conditions featuring upper motor neuron lesions, including stroke, multiple sclerosis,1–4 cerebral palsy, and traumatic brain, and spinal cord injury. Although data regarding the prevalence of ULS in the UK are sparse, the occurrence of ULS in stroke patients, who comprise the majority of cases, is estimated to be up to 40%.4 ULS is defined as involuntary hyperkinetic movements of the muscles controlling the upper limb,5 in which patients are unable to control the initiation of muscle reflexes, resulting in abnormal postures, deformity, and pain. This inadvertently influences the activities of daily living and, consequently, predisposes patients and their caregivers alike to a significant burden of care,6 making delivery of care to these patients unduly difficult and tasking.7
In addition to this considerable negative quality of life effect on both patients and their caregivers, the costs of treating patients with upper motor neuron lesions and spasticity are estimated to be four times as great as those without spasticity.8 Evidence suggests that spasticity-related costs generally comprise costs of conventional treatment, including, but not limited to, hospitalization, rehabilitative therapy, and pharmacotherapy costs.9
Guidelines in the UK recommend the use of botulinum toxin A (BoNT-A),10 which is an effective11–17 and potentially cost-effective9,18,19 antispastic pharmaceutical treatment, as adjunct to conventional treatment in instances where ULS significantly hinders patient care and hygiene, impedes the use of fine motor functions, causes postural discomfort or pain, or cosmetic concern to the affected individual.10,20 There are, however, variations in the costs and pharmacodynamic properties of the three BoNT-As being used in the management of ULS in the UK – abobotulinumtoxinA (Dysport®, Ipsen Biopharm SAS, Boulogne-Billancourt Cedex, France), onabotulinumtoxinA (Botox®, Allergan Inc., Irvine, CA, USA), and incobotulinumtoxinA (Xeomin®, Merz Pharma GmbH & Co. KGaA, Frankfurt am Main, Germany). The differences in either BoNT-A cost or, for example, duration of effect, can impact the frequency of use of health care resources and overall budget to treat ULS patients.
As a result, we investigated the importance of differences in BoNT-A cost and health care resource use to overall budget in a UK population with ULS and conducted a budget impact assessment of changing the market share of abobotulinumtoxinA, onabotulinumtoxinA, or incobotulinumtoxinA.
Methods
Overview
A budget impact model was developed in Microsoft Excel® 2007 to determine the budgetary impact of changing market share of abobotulinumtoxinA relative to other BoNT-As (onabotulinumtoxinA and incobotulinumtoxinA) and standard of care in the management of ULS in the UK, from a National Health Service (NHS) and personal social services perspective, over a 5-year time horizon. The budget impact model measures the net cumulative cost of treatment with a therapy for an eligible patient population to be treated, in order to help payers understand the impact of the new drug on spending; therefore this type of analysis does not assess the cost-effectiveness or effectiveness of treatment.21 However, there is evidence that BoNT-A is more effective compared with standard of care.11,12,22,23
The model evaluated two scenarios – the status quo, which is the current mix of available competing BoNT-A treatments according to their prevailing market shares, compared with a new market share scenario, which assumed an increased uptake of abobotulinumtoxinA relative to the other two BoNT-As. In both scenarios, the number of patients receiving best supportive care without BoNT-A treatment remained the same throughout the time horizon.
The model required epidemiological, resource use, unit cost, and market share proportions data. These data were retrieved from a variety of sources, including previously published literature, the British National Formulary (BNF),24 the Personal Social Services Research Unit (PSSRU),25 NHS reference costs,26 and interviews with two practicing senior neurologists in the UK with extensive clinical experience in the management of patients with spasticity.
For each of the scenarios, the interventions examined were abobotulinumtoxinA, onabotulinumtoxinA, incobotulinumtoxinA, and best supportive care without BoNT-A treatment. Best supportive care in our budget impact assessment comprised the use of analgesics, skeletal muscle relaxants, hospital admission, and rehabilitative therapy, including health care professional visits, laboratory tests, splinting, and transportation.
Model inputs
Patient population
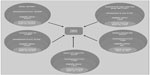
The number of patients considered eligible to receive BoNT-A treatment was determined on clinical grounds and calculated using a top-down, prevalence-based approach, as shown in Figure 1. The model determined the proportion of patients within each original disease area who develop ULS (based on epidemiological studies),2–4,31–34 proportion of patients with problematic spasticity (spasticity was considered problematic if it interfered with motor function and/or caused distressing symptoms, such as painful muscle spasms) requiring treatment, and those who are offered BoNT-A treatment (Ipsen Biopharm Ltd, unpublished data, October 2012).
Market share
The status quo and new market share scenarios were based on the proportion of patients who are treated with BoNT-A identified by a UK market based research conducted by Ipsen Biopharm Ltd (Ipsen Biopharm Ltd, unpublished data, October 2012). Each scenario was defined as:
- Status quo: only 7% of ULS patients receive BoNT-A; and abobotulinumtoxinA, onabotulinumtoxinA, and incobotulinumtoxinA are used in 33%, 52%, and 15% of patients with ULS receiving BoNT-A in the UK, respectively.
- New projections: the market share of BoNT-A remains 7%, but the use of abobotulinumtoxinA relative to other BoNT-As increases by 10% annually (up to 73% in year 5) in patients with ULS receiving BoNT-As.
Table 1 provides more details of the market shares for the status quo and new projections scenarios.
  | Table 1 Market shares for the status quo and the new projections scenarios |
Direct costs of BoNT-A treatments
Three major types of resource use items were considered in the model; BoNT-A treatments, concomitant medications, and other medical or nonmedical resource use items. Items included as resource use were identified from previous economic evaluations9,18,19 and key opinion leader interviews. Dosages of BoNT-A injections were based on summaries of product characteristics (SPCs). In the base case, patients receiving abobotulinumtoxinA, onabotulinumtoxinA, or incobotulinumtoxinA were assumed to receive injections at 12-weekly intervals.27–29 No vial-sharing was assumed. Given that BoNT-A injection vials come in different sizes, we assumed that the specialist administering the BoNT-A treatment would be rational and use the smallest available vial combination. Table 2 shows the doses per injection, vial costs, and available vial sizes.
Medical or nonmedical resource use
Costs for other forms of resource use were considered in the model. These included:
- Health care professional contact (physiatrist, neurologist, physiotherapist, occupational therapist, orthotist, practice nurse, general practitioner [GP])
- Analgesics, gabapentin, benzodiazepines
- Skeletal muscle relaxants, such as oral baclofen, tizanidine, and oral dantrolene
- Laboratory tests (liver function tests for monitoring skeletal muscle relaxants)
- Procedures (eg, splinting or ultrasound)
- Other medical/social services costs (hospital admission days)
- Nonmedical costs (transportation).
Data on the proportion of patients using each service and the number of visits/services per year were mainly provided by Ward et al19 and clinical experts, while the unit cost of visits/services were extracted from the NHS reference costs26 (laboratory tests, hospital admission days, day hospital) and PSSRU (medical staff visits, emergency department visits, day center, home care services, caregiver time, meals on wheels). The frequency of physiatrist/rehabilitation medicine specialist, neurologist visits, and ultrasound procedures, and transportation to the clinic are directly associated with the administration of each BoNT-A injection and, therefore, their associated costs are incurred at each treatment session.
The major differences in resource use between the BoNT-A treatment and best supportive care were based on the frequency and length of health care professional visits, the frequency of laboratory test, the use of skeletal muscle relaxants, and need for transportation. Generally, patients in the best supportive care arm were assumed to have more frequent visits to the physiatrist, physiotherapist, practice nurse, and the GP but fewer visits to neurologists. Their visits to the physiatrist, neurologist, and neurosurgeon were also shorter compared with patients receiving BoNT-A, lasting only half an hour19 compared with 1 hour for the latter group. Additionally, patients on best supportive care received a higher proportion of skeletal muscle relaxants (oral baclofen, tizanidine, and oral dantrolene)19 compared with those on BoNT-A treatment. In the case of those treated with tizanidine, routine laboratory tests were required on a regular basis (every 3 months). Lastly, 13% of patients receiving BoNT-A treatment were assumed to have an ultrasound before injection administration (key opinion leader opinion). Table 3 provides more detail on the differences in resource use between BoNT-A and best supportive care. It should be noted that cost in the best supportive care arm did not affect budget impact since market share evolution concerns changed among BoNT-As only, while the percentage of patients receiving best supportive care was unchanged. However, comparisons in costs between patients receiving best supportive care and BoNT-A can be made.
Analysis/impact calculation
The budget impact model first accounted for the number of patients eligible for the different types of treatments each year. Second, annual treatment costs were calculated based on the unit costs and the monthly/yearly utilization of the different resource use categories described above. Third, BoNT-A-eligible patients were distributed to receive each type of treatment, based on the market shares in the status quo and new market share scenarios. The difference between the total costs for both scenarios was the net budget impact of an increased market share for abobotulinumtoxinA.
Sensitivity analysis
The treated patient population is very heterogeneous; they may differ in the required dose, number of injections per year, and in all other medical and nonmedical resource use. To capture these differences, one-way sensitivity analyses were conducted to determine which parameters were most influential on budget impact. Model parameter values were varied between two extremes based on ranges identified from the literature, when data were available. For parameters whose range could not be specified, a predefined ±20% range from the base-case values were used. Results are presented as a tornado diagram.
Results
The calculated eligible patient number for year 1 is presented in Figure 1. Table 1 shows that 6,033 patients were being treated in year 1 with one of the three BoNT-A formulations, amongst a total of 86,187 patients with ULS, rising to 6,281 treated patients by year 5. A 4% increase was expected in the patient number at the end of the fifth year due to the growing population size in the UK. Under base-case assumptions (5-year time horizon, no vial-sharing), the total expected cost of treating ULS patients with BoNT-A injections was £10,375,314,465, while under the new market share scenario, it was £10,369,030,636. These costs correspond to the entire 5-year budget of treating ULS patients (including the 93% not receiving BoNT-A) for all drugs and medical resource use. Amongst the 7% who receive BoNT-A, the 5-year budget was £630 million. Of this total, BoNT-A represented less than 7% of this cost, while health care professional contacts and other medical costs represented 70% and 20%, respectively. An increased uptake of abobotulinumtoxinA resulted in a total of £6,283,829 savings at the end of year 5, as shown in Table 4. The main cost drivers were costs associated with contact with health care professionals and use of concomitant medications, when comparing BoNT-A treatment with best supportive care.
  | Table 4 Net budget impact |
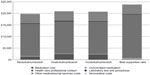
Annual total costs decreased by between £602,616 in year 2 and by £2,574,557 in year 5 by increasing the market share of abobotulinumtoxinA constantly over the 5-year period. In the base-case scenarios, treatment with BoNT-A (range £19,800–£20,861) cost less than best supportive care (£23,829) per patient per year. However, treating a patient with onabotulinumtoxinA (£20,861) and incobotulinumtoxinA (£20,717) cost more than treatment with abobotulinumtoxinA (£19,800) per patient per year. BoNT-A medication costs accounted for the differences in annual treatment with BoNT-A, as all other resource use were assumed to be equal across BoNT-As. Figure 2 shows a breakdown of the summary cost components for each treatment per patient per year.
Deterministic sensitivity analyses showed that the percentage of cerebral palsy and stroke patients developing ULS, and the prevalence of stroke, respectively, were the most influential parameters on the total budget impact of ULS treatment, impacting both drug acquisition costs and physician costs. Figure 3 shows the tornado diagram of factors influencing the base-case findings (Table 5 provides definition for the abbreviations used).
  | Figure 3 Tornado diagram of factors influencing the base-case analysis. |
Discussion
We examined the budget impact of altering the market share of BoNT-A treatments in a UK population with ULS. To our knowledge, this is the first study examining the budget impact of the use of BoNT-As in ULS patients in the UK. Our results suggest there could be savings of up to £4,029 per patient per year with the use of BoNT-A compared with best supportive care. In particular, our model showed that a gradual and incremental use of abobotulinumtoxinA in comparison with other BoNT-A formulations and best supportive care resulted in savings of £6,283,829 over a period of 5 years. There are several limitations of the model, as discussed below.
Economic evaluations involve the extrapolation of information from various sources and therefore require assumptions to reflect the complexity of the reality of treating patients, in a more simple and comprehensive way. Thus the economic methodology is often less well-defined than in clinical studies.30 In anticipation of the deviation from real-life scenarios by the assumptions of the economic model, we conducted extensive sensitivity analyses to determine the most influential variables on the base-case analysis findings in the budget impact analysis.
Generally it was difficult to clarify the number of patients diagnosed with ULS and those receiving treatment for the condition. The number of patients who would eventually receive BoNT-A treatment for symptoms and signs of ULS may have been underestimated. Data on the number of patients with ULS secondary to stroke, multiple sclerosis, cerebral palsy, and traumatic brain and spinal cord injury was based on the assumption that the number of patients who developed ULS would be constant over the 5-year horizon modeled. In reality, however, this number could vary increasingly or decreasingly.
In addition, the frequency and dose of BoNT-A injections, and other resource use types differ from SPCs or guidelines, in clinical practice. We assumed constant doses for BoNT-A treatment and explored possibilities of having different reinjection times for abobotulinumtoxinA. Most of the resource use data incorporated into our model was based on expert opinion or Ward et al.19 Further research is required to investigate resource use in the UK as it is a big cost driver of BoNT-A treatment compared with best supportive care. However, some real-world data is available from a large, international, observational cohort study to describe the real-life practice and outcomes in the treatment of poststroke ULS.31 In this study, the total dose range for abobotulinumtoxinA, onabotulinumtoxinA, and incobotulinumtoxinA was 40–1900 units, 50–500 units, 100–600 units, respectively, and the median (range) reinjection time was 14 (2.6–32.3) weeks.31 Notably, the proportion of patients on antispasmodic medication fell from 46% to 28.5%, from baseline to second visit. Further analysis of this data also showed abobotulinumtoxinA to be less costly per patient annually compared with other BoNT-As, based on real-world dosing of the three BoNT-As. More specifically, a homogeneous sample of patients with the same injected limb segments, such as “upper arm and lower arm only” was analyzed for the dose injected for each BoNT-A. In this case, the mean (standard deviation) dose injected for abobotulinumtoxinA, onabotulinumtoxinA, and incobotulinumtoxinA was 665 (280) units, 183 (99) units, and 235 (108) units, respectively. Under assumptions of equal reinjection cycles and no vial-sharing, the annual BoNT-A cost per patient in the UK was modeled as £1,068, £1,198, and £1,399 for abobotulinumtoxinA, onabotulinumtoxinA, and incobotulinumtoxinA, respectively. These findings from real-world settings strengthen the results of our base-case analysis, which showed that the use of abobotulinumtoxinA would reduce the annual BoNT-A cost of treating each patient by 11% compared with onabotulinumtoxinA and by 24% compared with incobotulinumtoxinA.32
Finally, there are variations in vial-sharing practices, where vials are shared in some cases and they are not in other cases. However, our model did not allow for sharing of vials of BoNT-A injections compared with best supportive care. This conservative approach was chosen in order not to overestimate the savings that might be associated with sharing vials of BoNT-A injections.34,35
In addition to the effectiveness of BoNT-A treatment and the reduction of patient and caregiver burden stemming from functional impairments due to ULS, an increased use of abobotulinumtoxinA, compared with onabotulinumtoxinA and incobotulinumtoxinA, for ULS in the UK could potentially reduce total ULS cost, representing cost-savings to the health system and society.
Disclosure
Seye Abogunrin, Linda Hortobagyi, and Edit Remak are employees of Evidera Inc., which received consulting fees from Ipsen Pharma to conduct the research. Jerome Dinet and Sylvie Gabriel are full-time employees of Ipsen Pharma. Professor Bakheit has received research and educational grants and consultancy fees from Ipsen Pharma, Allergan Inc., and Merz Pharmaceutical.
References
Bandi S, Ward AB. 2010. Spasticity. In: JH Stone, M Blouin, editors. International Encyclopedia of Rehabilitation. Available online: http://cirrie.buffalo.edu/encyclopedia/en/article/32/. Accessed February 18, 2015. | |
Buchanan RJ, Chakravorty BJ, Tyry T, Hatcher W, Vollmer T. Age-related comparisons of people with multiple sclerosis: demographic, disease, and treatment characteristics. NeuroRehabilitation. 2009;25(4):271–278. | |
Lawrenson R, Wyndaele J-J, Vlachonikolis I, Farmer C, Glickman S. Renal failure in patients with neurogenic lower urinary tract dysfunction. Neuroepidemiology. 2001;20(2):138–143. | |
Watkins CL, Leathley MJ, Gregson JM, Moore AP, Smith TL, Sharma AK. Prevalence of spasticity post stroke. Clin Rehabil. 2002;16(5):515–522. | |
Ivanhoe CB, Reistetter TA. Spasticity: the misunderstood part of the upper motor neuron syndrome. American Journal of Physical Medicine & Rehabilitation. 2004;83(10):S3–S9. | |
Doan QV, Brashear A, Gillard PJ, et al. Relationship between disability and health-related quality of life and caregiver burden in patients with upper limb poststroke spasticity. PM R. 2012;4(1):4–10. | |
Esquenazi A. The human and economic burden of poststroke spasticity and muscle overactivity. J Clin Outcomes Manag. 2011;18(1):607–614. | |
Lundström E, Smits A, Borg J, Terént A. Four-fold increase in direct costs of stroke survivors with spasticity compared with stroke survivors without spasticity: the first year after the event. Stroke. 2010;41(2):319–324. | |
Doan QV, Gillard P, Brashear A, et al. Cost-effectiveness of onabotulinumtoxinA for the treatment of wrist and hand disability due to upper-limb post-stroke spasticity in Scotland. Eur J Neurol. 2013;20(5):773–780. | |
National Institute for Health and Care Excellence. Spasticity in Children and Young People with Nonprogressive Brain Disorders: Management of Spasticity and Co-existing Motor Disorders and their Early Musculoskeletal Complications (CG145). Manchester: National Institute for Health and Care Excellence; 2012. | |
Bakheit AM, Thilmann AF, Ward AB, et al. A randomized, double-blind, placebo-controlled, dose-ranging study to compare the efficacy and safety of three doses of botulinum toxin type A (Dysport) with placebo in upper limb spasticity after stroke. Stroke. 2000;31(10):2402–2406. | |
Bhakta BB, Cozens JA, Bamford JM, Chamberlain MA. Use of botulinum toxin in stroke patients with severe upper limb spasticity. J Neurol Neurosurg Psychiatry. 1996;61(1):30–35. | |
Brashear A, Gordon MF, Elovic E, et al; Botox Post-Stroke Spasticity Study Group. Intramuscular injection of botulinum toxin for the treatment of wrist and finger spasticity after a stroke. N Engl J Med. 2002;347(6):395–400. | |
Caty GD, Detrembleur C, Bleyenheuft C, Deltombe T, Lejeune TM. Effect of upper limb botulinum toxin injections on impairment, activity, participation, and quality of life among stroke patients. Stroke. 2009;40(7):2589–2591. | |
Francis HP, Wade DT, Turner-Stokes L, Kingswell RS, Dott CS, Coxon EA. Does reducing spasticity translate into functional benefit? An exploratory meta-analysis. J Neurol Neurosurg Psychiatry. 2004; 75(11):1547–1551. | |
Lukban MB, Rosales RL, Dressler D. Effectiveness of botulinum toxin A for upper and lower limb spasticity in children with cerebral palsy: a summary of evidence. J Neural Transm. 2009;116(3):319–331. | |
Ozcakir S, Sivrioglu K. Botulinum toxin in poststroke spasticity. Clin Med Res. 2007;5(2):132–138. | |
Shaw L, Rodgers H, Price C, et al; BoTULS investigators. BoTULS: a multicentre randomised controlled trial to evaluate the clinical effectiveness and cost-effectiveness of treating upper limb spasticity due to stroke with botulinum toxin type A. Health Technol Assess. 2010;14(26):1–113, iii. | |
Ward A, Roberts G, Warner J, Gillard S. Cost-effectiveness of botulinum toxin type a in the treatment of post-stroke spasticity. J Rehabil Med. 2005;37(4):252–257. | |
Royal College of Physicians; British Society of Rehabilitation Medicine; Chartered Society of Physiotherapy; Association of Chartered Physiotherapists Interested in Neurology. Spasticity in Adults: Management Using Botulinum Toxin. National Guidelines. London: Royal College of Physicians; 2009. | |
Timothy W, Smith BA, Tierce JC. Designing and developing budget impact models suited for global adaptation. ISPOR Connections. 2006; 12(4)7–10. | |
Hesse S, Reiter F, Konrad M, Jahnke MT. Botulinum toxin type A and short-term electrical stimulation in the treatment of upper limb flexor spasticity after stroke: a randomized, double-blind, placebo-controlled trial. Clin Rehabil. 1998;12(5):381–388. | |
Simpson DM, Alexander DN, O’Brien CF, et al. Botulinum toxin type A in the treatment of upper extremity spasticity: a randomized, double-blind, placebo-controlled trial. Neurology. 1996;46(5):1306–1310. | |
BNF: Joint Formulary Committee. British National Formulary (online) London: BMJ Group and Pharmaceutical Press http://www.medicinescomplete.com. Accessed December 2012. | |
PSSRU: Unit Costs of Health and Social Care 2011. Compiled by L Curtis. PSSRU. http://www.pssru.ac.uk/project-pages/unit-costs/2011/index.php. Accessed December 2012. | |
NHS reference costs: NHS reference costs – 2010–2011. http://www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_131140. Accessed December 2012. | |
Botox 100 units [Summary of Product Characteristics]. Datapharm; 2013 [updated May 22, 2104]. Available from: http://www.medicines.org.uk/emc/medicine/112/SPC/BOTOX+100+Units. Accessed January 26, 2015. | |
Dysport 300 units, Dysport 500 units [Summary of Product Characteristics]. Datapharm; 2012 [updated December 23, 2013]. Available from: http://www.medicines.org.uk/emc/medicine/870. Accessed January 26, 2015. | |
Xeomin 100 units, [Summary of Product Characteristics]. Datapharm; 2012 [updated June 30, 2014]. Available from: http://www.medicines.org.uk/emc/medicine/20666/SPC/Xeomin+100+Units. Accessed 26, 2015. | |
Buxton MJ, Drummond MF, Van Hout BA, et al. Modelling in economic evaluation: an unavoidable fact of life. Health Econ. 1997;6(3):217–227. | |
Turner-Stokes L, Fheodoroff K, Jacinto J, Maisonobe P. Results from the Upper Limb International Spasticity Study-II (ULISII): a large, international, prospective cohort study investigating practice and goal attainment following treatment with botulinum toxin A in real-life clinical management. BMJ Open. 2013;3(6):e002771. | |
Dinet J, Lambrelli D, Balcaitiene J. Economic modeling of the use of botulinum toxin A in a homogenous patient population based on real-life clinical practice: Ulis-Ii (the Upper Limb International Spasticity Study). Value Health. 2014;17(7):A377. | |
Beecham J, Perkins M, Snell T, Knapp M. Treatment paths and costs for young adults with acquired brain injury in the United Kingdom. Brain Inj. 2009;23(1):30–38. | |
World Health Organization. Atlas: Multiple Sclerosis Resources in the World 2008. Geneva: World Health Organization; 2008. | |
Yeargin-Allsopp M, Van Naarden Braun K, Doernberg NS, Benedict RE, Kirby RS, Durkin MS. Prevalence of cerebral palsy in 8-year-old children in three areas of the United States in 2002: a multisite collaboration. Pediatrics. 2008;121(3):547–554. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.