Back to Journals » OncoTargets and Therapy » Volume 13
Bruton’s Tyrosine Kinase (BTK) Inhibitor (Ibrutinib)-Suppressed Migration and Invasion of Prostate Cancer
Authors Zhu Z, Ling L, Qi L, Chong Y, Xue L
Received 13 January 2020
Accepted for publication 14 April 2020
Published 13 May 2020 Volume 2020:13 Pages 4113—4122
DOI https://doi.org/10.2147/OTT.S245848
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Sanjeev K. Srivastava
Zhen Zhu,1,* Lanlan Ling,2,* Lezhong Qi,1 Yue Chong,3 Li Xue4
1Department of Urology, Affiliated Hospital of Yangzhou University, Yangzhou, Jiangsu, People’s Republic of China; 2Health Management Center, Affiliated Hospital of Yangzhou University, Yangzhou, Jiangsu, People’s Republic of China; 3Department of Urology, First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, Shaanxi, People’s Republic of China; 4Department of Urology, Second Affiliated Hospital of Xi’an Jiaotong University, Xi’an, Shaanxi, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Li Xue
Department of Urology, Second Affiliated Hospital of Xi’an Jiaotong University, No. 157 Xiwulu Road, Xi’an, Shaanxi 710004, People’s Republic of China
Tel/Fax +86-29-87679533
Email [email protected]
Introduction: Bruton’s tyrosine kinase (BTK) inhibitors have long been known in the treatment of B-cell malignancies. Recently, BTK inhibitors have also become promising novel treatment reagents for prostate cancer. The current study was designed to investigate expression of BTK in prostate cancer tissues in comparison with benign hyperplasia and effect of BTK inhibitor on prostate cancer cell proliferation, migration and invasion.
Methods: BTK expression was assessed by immunohistochemistry; migration and invasion prostate cancer cell lines (DU145 and PC3) were assessed by Transwell migration and wound-healing assay; cancer cell proliferation was assessed using MTT assay kit; expression of matrix metalloproteinases-2 and -9 (MMP-2 and MMP-9) was assessed by immunoblotting.
Results: Strong expression of BTK was detected in the prostate cancer tissues, especially in the tumors from prostate cancer patients with bone metastasis. BTK inhibitor (Ibrutinib) significantly inhibited cell proliferation, migration and invasion of prostate cancer cells as well as protein synthesis of MMP-2 and MMP-9 by the tumor cells. Overexpressing BTK could partially but significantly block the inhibitory effect of Ibrutinib on cell proliferation, migration and invasion, and protein synthesis of MMP-2 and MMP-9 of the cancer cells.
Conclusion: These findings suggested that BTK could serve as not only a biomarker but also a therapeutic target for the prostate cancer and that Ibrutinib may be applied as a therapeutic drug for the prostate cancer.
Keywords: Bruton’s tyrosine kinase, BTK, prostate cancer, matrix metalloproteinase, MMP
Introduction
Prostate cancer is a common male cancer with high lethality in the world, especially in the population of 65-year-oldsters with three-quarters prevalence.1 In the United States of America, prostate cancer is the most commonly diagnosed malignancy in male population.2 In 2008, over half of newly diagnosed primary cancers were originated from prostate, lung and bronchus, or colon and rectum, and of them, prostate cancer alone accounted for one-quarter of incident cases in men in the US.2 In China, prostate cancer has become the most frequently diagnosed tumor in male urinary malignancies since 2008.3 The incident rate was 9.80/100,000 in 2014 and ranked the sixth common malignancy in male malignant tumors.3 While there are many choices for the treatment of prostate cancer such as surgery, radiotherapy, chemotherapy, and immunotherapy,3 currently, tyrosine kinase inhibitors (TKIs) become potentially promised therapeutic drug for prostate cancer and most of which are directed against receptor tyrosine kinases.
Bruton’s tyrosine kinase (BTK) is known to cause the most significant reduction in cancer cell survival when it is knockdown,4 especially, in B-cell malignancy.5 The implication of BTK in B-cell malignancies has been extensively studied.6,7 In this regard, BTK has long been known to be involved in modulating survival, activation, proliferation, and differentiation of B-lymphocytes.8,9 Recently, BTK has also become a novel target in solid tumors including prostate cancer.4,10-12 BTK inhibitors including PCI-32765 (Ibrutinib) were originally developed as immunosuppressants and chemotherapeutic drug for B-cell malignancies in the clinical trials13 and has already been approved for the treatment of hematopoietic malignancies, mantle cell lymphoma and multiple myeloma.8
Given the novel therapeutic role of BTK inhibitors in prostate cancer survival,11,12 in the current study, we examined the expression of BTK in prostate cancer tissues with or without bone metastasis. We showed that BTK expression was increased in prostate cancer tissues with strongest in the tumors with bone metastasis. In vitro studies with two cell lines (DU145 and PC3) of prostate cancers demonstrated that suppression of BTK with Ibrutinib resulted in inhibition of proliferation, migration and invasion of the cells. In addition, MMP-2 and MMP-9 proteins were highly expressed in the prostate cancer cell lines, which were significantly inhibited by Ibrutinib.
Materials and Methods
Ethics
This work protocol was approved by the Ethics Committee of Second Affiliated Hospital of Xi’an Jiaotong University and the study was in accordance with the Declaration of Helsinki. Written informed consent had been collected from all patients.
Cell Culture and Treatment
Two prostate cancer cell lines, PC3 and DU145, were provided from the Institute of Urology, the First Affiliated Hospital of Xi’an Jiao Tong University. Cells were cultured with RPMI-1640 medium supplemented with 10% fetal calf serum (FCS), 1% penicillin and streptomycin. The use of cancer cell lines was approved by the Ethics Committee of Second Affiliated Hospital of Xi’an Jiaotong University.
MTT Assay
Cells were trypsinized and plated into 96-well plates at a density of 5 x 103 cells/well/100µL medium. Next day, cells were treated with 0, 10, 20, 30, 40, 50, 60, and 70 µM BTK inhibitor, Ibrutinib (purchased from Cell Signaling Technology), for 0, 24, 48, and 72 h. Twenty microliters of 0.5% MTT was then added into each well and further cultured for 4 h. After brief washing, 150µL/well of DMSO was then added and shaken for 10min. OD value was then measured with ELISA reader at 490 nm.
Wound-Healing Assay
PC3 and DU145 cells were pre-treated with 0, 5, 10µM Ibrutinib for 24 h. Cells were then plated into 6-well plate at a density of 5x105 cells/well and cultured till 90% confluence. A wound in each well was created with pipette tip. Cells were then further cultured with serum-free medium for 0, 12, 18, and 24 h. Wound-healing process was photographed at each time point. Wounded area was quantified using Image-pro software.
Cell Migration Assay
PC3 and DU145 cells were pre-treated with 0, 5, 10µM Ibrutinib for 24 h. Cells were then trypsinized and plated into the upper well of the 24-well size Transwell plate at a density 2 x 104/well, 200µL/well, and 800µL medium containing serum were plated into the bottom well of the Transwell. Cells were allowed to migrate for 24 h. Non-migrated cells were then wiped off with cotton stick followed by washing three times with PBS. Migrated cells were fixed with 4% formaldehyde for 15 min at room temperature, and stained with 0.1% crystal violet staining solution for 10 min. After washing 3 times with PBS, migrated cells in three randomized high-power fields (x 200) were counted under microscope.
Tumor Cell Invasion Assay
Membrane (8µm) of the Transwell top well was pre-coated with Matrigel (1:9 dilution of Matrigel with serum-free medium). PC3 and DU145 cells were pre-treated with 0, 5, 10µM Ibrutinib for 24 h. Cells were then trypsinized and plated into the Matrigel-pre-coated top well of the 24-well size Transwell plate at a density 5 x 104/well, 200µL/well, and 800µL medium containing serum were plated into the bottom well of the Transwell. Cells were allowed to migrate through the Matrigel-pre-coated membrane for 24 h. Non-migrated cells were wiped off with cotton stick followed by washing three times with PBS. Migrated cells were fixed with 4% formaldehyde for 15 min at room temperature, and stained with 0.1% crystal violet staining solution for 10 min. After washing 3 times with PBS, migrated cells in three randomized high-power fields (x 200) were counted under microscope.
Immunoblotting
PC3 and DU145 cells were lysed with RIPA solution containing PMSF. Protein concentration was quantified by BCA method. Samples were diluted with 6x concentrated loading buffer and heated at 95°C for 5 min followed by cooling on ice. Total of 20 µg/lane proteins were loaded and separated with 10% SDS-PAGE electrophoresis. Proteins were transferred to PVDF membrane and blocked with 5% BSA-PBS solution. Primary antibodies (anti-BTK antibody from Santa Cruz @ 1:1000; MMP-2 from Abcam @ 1:2000; MMP-9 from Abcam @ 1:2000, and GAPDH from CWBIO, China @ 1:1000 dilution) were incubated at 4°C overnight. Next day, after washing, appropriate 2nd antibodies (Invitrogen) were allowed to react for 1 h at room temperature. After washing, protein bands were visualized with ECL reagent and ChemiDocTMXRS Imaging system.
Immunohistochemistry
Prostate tissues were collected from 12 prostate cancer patients and 8 patients with benign prostatic hyperplasia (IRB#: 2019075). The cancer tissues or benign prostatic hyperplasia tissues were fixed with 4% formaldehyde and sectioned after being embedded with paraffin. Samples were deparaffinized with xylene, rehydrated in a graded alcohol series, and incubated in a 0.3% hydrogen peroxide solution for 15 min to neutralize endogenous peroxidase activity. The sections were then microwaved in citrate buffer for antigen retrieval. The tissues were incubated with primary antibodies (1:50) at 4°C overnight. Next day, HRP-conjugated secondary antibodies were then allowed to react for 1 h at room temperature. DAB substrate was used to reveal peroxidase activity. Sections were counterstained with Hematoxylin. Intensity of the immunostaining was semi-quantitatively analyzed using Image-Pro Plus 6.0 software.
Plasmid Construction and Stable Transfection
As previously reported, the plasmid containing negative control sequence or BTK overexpressing sequence was propagated into Escherichia coli and purified by the Omega Plasmid Extraction Kit (Promega). The plasmid was then transfected into the cells following the manufacturer’s instruction of Lipofectamine 2000 transfection reagent (Life Technologies). The extent of gene knockdown was determined by immunoblot.
Statistical Analysis
All experiments were repeated at least three times. Results were presented as the mean ± SD. Statistical analysis was performed by one-way analysis of variance (ANOVA) and Student’s t test using SPSS software (SPSS Inc., Chicago, IL, USA). P < 0.05 was considered as significantly different.
Results
BTK Expression in the Prostate Cancer
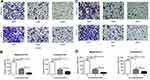
In order to investigate the role of BTK in the development and metastasis of prostate cancer, BTK protein expression in the prostate cancer tissue was assessed by immunohistochemical staining in 12 prostate cancer tissues in comparison with 8 tissues of benign prostatic hyperplasia. As shown in Figure 1, BTK protein expression was dramatically up-regulated in the prostate cancer tissues (B–F) compared to that in the benign prostatic hyperplasia (A). In addition, the higher Gleason Score in the prostate cancer was, the stronger expression of BTK was found in the tissue (B vs E in the tissues without bone metastasis, or C vs D vs F in the tissues with bone metastasis). Tissues with bone metastasis also had stronger BTK expression (C, D and F) compared to the tissues without bone metastasis (B and E). Furthermore, semi-quantitative analysis of the immunostaining gray intensity showed that prostate cancer with bone metastasis had strongest staining intensity (0.1434 ± 0.0138) than that of prostate cancer without bone metastasis (0.0130 ± 0.0019, P = 0.004) or benign prostate hyperplasia (0.0001 ± 0.00001, G).
Ibrutinib Inhibited Prostate Cancer Cell Proliferation
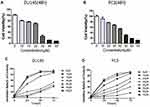
Cell proliferation and effect of Ibrutinib on cell proliferation were assessed using MTT assay. As shown in Figure 2, Ibrutinib, the BTK inhibitor, significantly inhibited proliferation of the prostate cancer cell lines, DU145 (Figure 2A) and PC3 (Figure 2B), in a concentration-dependent and time-dependent manner (Figure 2C and D). IC50 of Ibrutinib on PC3 cell at 24, 48, and 72 h treatment was 53µM, 34µM, and 22µM, respectively; on DU145 was 32µM, 21µM, and 16µM, respectively.
Ibrutinib Inhibited Migration and Invasion of Prostate Cancer Cells
Next effect of Ibrutinib on migration and invasion of prostate cancer cell lines, DU145 and PC3, were assessed using wound-healing assay and Transwell assay methods. As shown in Figure 3, after 24 h treatment, 2.5µM and 5µM Ibrutinib significantly inhibited wound close of DU145 (A and C) and PC3 (B and C), P < 0.05. Furthermore, as shown in Figure 4 Transwell assay results, 5 and 10µM Ibrutinib significantly inhibited not only migration of DU145 cells (top row of Figure 4A and left bar graph of Figure 4B, P < 0.05) but also invasion of DU145 cells into Matrigel (bottom row of Figure 4A and right bar graph of Figure 4B, P < 0.05). Similar effect of Ibrutinib on PC3 cell migration and invasion was found (Figure 4C and D, P < 0.05).
Effect of Ibrutinib on BTK and MMP Protein Synthesis
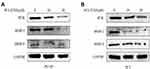
To investigate potential mechanism of Ibrutinib inhibition on prostate cancer cell migration and invasion, effect of Ibrutinib on the protein synthesis of BTK, MMP-2 and MMP-9 was assessed by immunoblotting. As shown in Figure 5A, 10µM and 20µM Ibrutinib dramatically inhibited protein synthesis of BTK, MMP-2, and MMP-9. Similar effect of Ibrutinib on BTK and MMP synthesis was also observed in PC3 cell line (Figure 5B).
Overexpression of BTK Partially Blocked Inhibitory Effect of Ibrutinib
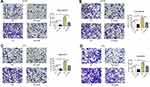
To further investigate role of BTK in modulating prostate cancer cell migration and invasion, plasmid of negative control or overexpressing BTK was transfected into the prostate cancer cell lines, DU145 and PC3. Cells were then pre-treated with 5µM Ibrutinib for 24 h followed by migration and invasion using Transwell assay method. As shown in Figure 6, migration (Figure 6A) and invasion (Figure 6B) capability of the DU145 cells overexpressing BTK increased significantly compared to the cells transfected with negative control plasmid (P < 0.05), and overexpressing BTK could partially but significantly blocked the inhibitory effect of Ibrutinib on cell migration and invasion (P < 0.05). Similar effect was observed in the PC3 cells (Figure 6C and D, P < 0.05).
In addition, overexpression of BTK in the cells resulted in significant up-regulation of MMP-2 and MMP-9 compared to the cells transfected with negative control plasmid (Figure 7), and overexpression of BTK could partially but significantly blocked the inhibitory effect of Ibrutinib on MMP synthesis (Figure 7).
Discussion
BTK has been known as a novel treatment target in hematopoietic malignancies and autoimmune diseases.6,11 In the current study, by immunohistochemical staining, we first demonstrated that BTK expression was significantly increased in the invasive prostate cancers (bone metastasis) compared to that of non-invasive prostate cancer (without bone metastasis) or benign prostate hyperplasia. Next, by in vitro studies of prostate cancer cell lines, we further demonstrated that the BTK inhibitor, Ibrutinib, significantly inhibited prostate cancer cell proliferation, wound healing, migration and invasion of the prostate cancer cells; and inhibited MMP-2 and MMP-9 protein synthesis in the tumor cells. We also demonstrated that overexpressing BTK in the prostate cancer cells partially antagonized the inhibitory effect of Ibrutinib on migration and invasion of the prostate cancer cells as well as expression of MMP-2 and MMP-9. These findings suggested that BTK related signaling play an important role in the cellular proliferation, migration and invasion of prostate cancer.
Tyrosine-protein kinases induce protein phosphorylation and modulate activity of their substrates, and by which mechanism, these kinases involve in intracellular signal transduction that regulate various kinds of cellular function including proliferation and migration. Aberrant activation of tyrosine protein kinases leads to alteration of cellular behaviors, including cell proliferation and differentiation, survival and death, migration and invasion, and neoplastic formation.14,15 In this content, Bruton’s tyrosine kinase (BTK) is known to play a critical role in cell survival of B-cell type malignancies. BTK was found to be mutated in the primary immunodeficiency X-linked agammaglobulinemia (XLA) and crucial for B lymphocyte development.16,17 Recently, however, studies have also indicated that BTK expressed and activated in various kinds of solid tumors including prostate cancer, lung cancer, and ovarian cancer.10,11,18,19 Thus, BTK inhibitors have become as potential therapeutic drugs not only for B-cell malignancies but also for solid tumors including prostate cancer.11,20,21 In this regard, Ibrutinib (PCI-32,765), an orally bio-available and highly potent inhibitor of BTK,22 specifically binds to Cys481 of BTK, and by which mechanism, it irreversibly blocks the kinase activity.23 Here, we demonstrated that BTK is highly expressed in the tissues of prostate cancer, especially in the cancers with bone metastasis. Furthermore, Ibrutinib significantly inhibited cellular proliferation, migration and invasion of prostate cancer. Findings of the current study were consistent with studies that showed BTK signal transduction was critical for proliferation and migration of prostate cancer cells and suggested that BTK could serve as not only a potential biomarker for diagnosis and prognosis but also a novel therapeutic target for prostate cancer.
A special feature of malignant tumor is its ability to destroy matrix barriers, and by which mechanism, cancer cells migrate and invade into the surrounding connective tissues as well as transfer to distant organs.24,25 Cell migration and invasion require proteolytic action of the enzymes that can hydrolyze structural components of the extracellular matrix (ECM) so that cancer cells initiate migration and disseminate into the surrounding tissues. In this regard, matrix metalloproteinases (MMPs) are known to play crucial role in breaking ECM and regulating functions of various kinds of cancer cells including migration and angiogenesis, and thus, MMPs have been the targets of anticancer studies.15,26,27 In this content, by multivariate analyses after adjusting for the Gleason score, tumor-node-metastasis stage, and initial serum prostate-specific antigen, Trudel et al28 reported that MMP-2 expression by >50% of malignant epithelial cells was associated with decreased disease-free survival (hazard ratio, 4.267; P < 0.0012). Oguic et al29 immunohistochemically evaluated MMP-2 and MMP-9 expression in the tissue microarrays of 120 archival prostate carcinoma samples and compared with clinic pathological parameters. They found that MMP-9 expression was significantly elevated in tumors from patients who had biochemical recurrence and that MMP-9 was a good predictor of biochemical recurrence as analyzed by multivariate analysis.29 In addition, expression of MMP-2 in tumor cells was significantly higher at the positive margins than in the main tumor mass.29 A meta-analysis, including 8 studies with 675 patients, showed that MMP-2 expression in the prostate cancer group was significantly higher than that in the benign prostatic hyperplasia (BPH) group and that MMP-2 expression was significantly associated with Gleason Score and clinical stages.30 Consistent with these previous reports, the current study demonstrated that MMP-2 and MMP-9 were highly expressed in the prostate cancer cell lines, which was significantly suppressed by BTK inhibitor, Ibrutinib. Furthermore, overexpressing BTK in the prostate cancer cell lines resulted in blockade of the inhibitory effect of Ibrutinib, suggesting BTK may play a critical role in modulating MMP-2 and MMP-9 expression in the prostate cancer cells.
Bone is the most common metastatic site for prostate cancer, and metastasis of prostate cancer cells contributes to the prostate cancer-related mortality.31 In the current study, therefore, BTK expression was examined in the prostate cancer tissues and compared the expression level in the patients with or without bone metastasis. We found that expression of BTK was higher in the prostate cancer with bone metastasis than that without bone metastasis, suggesting BTK may be involved in process of cancer cell distant metastasis through modulating MMPs.
Taken together, the current study demonstrated that BTK was aberrantly expressed in the prostate cancer, especially in the prostate cancer with bone metastasis. Ibrutinib significantly inhibited proliferation, migration and invasion of the prostate cancer cells as well as expression of MMP-2 and MMP-9 proteins in the tumor cells. These data suggest that BTK could be not only a biomarker of prostate cancer but also a therapeutic target of prostate cancer and that Ibrutinib maybe use as an adjuvant therapeutic drug for prostate cancer.
Funding
This study was supported by the Key Research and Development Foundation of Shaanxi Province (No. 2018YBXM-SF-13-5).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Epidemiology. Epidemic rates of cancer incidence in Latin America. Nat Rev Clin Oncol. 2013;10(6):304. doi:10.1038/nrclinonc.2013.77
2. Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58(2):71–96. doi:10.3322/CA.2007.0010
3. China. Chinese guidelines for diagnosis and treatment of prostate cancer 2018 (English version). Chin J Cancer Res. 2019;31(1):67–83. doi:10.21147/j.issn.1000-9604.2019.01.04
4. Eifert C, Wang X, Kokabee L, et al. A novel isoform of the B cell tyrosine kinase BTK protects breast cancer cells from apoptosis. Genes Chromosomes Cancer. 2013;52(10):961–975. doi:10.1002/gcc.22091
5. de Weers M, Verschuren MC, Kraakman ME, et al. The Bruton’s tyrosine kinase gene is expressed throughout B cell differentiation, from early precursor B cell stages preceding immunoglobulin gene rearrangement up to mature B cell stages. Eur J Immunol. 1993;23(12):3109–3114. doi:10.1002/eji.1830231210
6. Buggy JJ, Elias L. Bruton tyrosine kinase (BTK) and its role in B-cell malignancy. Int Rev Immunol. 2012;31(2):119–132. doi:10.3109/08830185.2012.664797
7. Kurosaki T. Regulation of B-cell signal transduction by adaptor proteins. Nat Rev Immunol. 2002;2(5):354–363. doi:10.1038/nri801
8. Herman SE, Gordon AL, Hertlein E, et al. Bruton tyrosine kinase represents a promising therapeutic target for treatment of chronic lymphocytic leukemia and is effectively targeted by PCI-32765. Blood. 2011;117(23):6287–6296. doi:10.1182/blood-2011-01-328484
9. Hendriks RW, Yuvaraj S, Kil LP. Targeting Bruton’s tyrosine kinase in B cell malignancies. Nat Rev Cancer. 2014;14(4):219–232. doi:10.1038/nrc3702
10. Zucha MA, Wu AT, Lee WH, et al. Bruton’s tyrosine kinase (Btk) inhibitor ibrutinib suppresses stem-like traits in ovarian cancer. Oncotarget. 2015;6(15):13255–13268. doi:10.18632/oncotarget.3658
11. Kokabee L, Wang X, Sevinsky CJ, et al. Bruton’s tyrosine kinase is a potential therapeutic target in prostate cancer. Cancer Biol Ther. 2015;16(11):1604–1615. doi:10.1080/15384047.2015.1078023
12. Guo W, Liu R, Bhardwaj G, et al. Targeting Btk/Etk of prostate cancer cells by a novel dual inhibitor. Cell Death Dis. 2014;5:e1409. doi:10.1038/cddis.2014.343
13. Vassilev AO, Uckun FM. Therapeutic potential of inhibiting Bruton’s tyrosine kinase, (BTK). Curr Pharm Des. 2004;10(15):1757–1766. doi:10.2174/1381612043384475
14. Gross S, Rahal R, Stransky N, Lengauer C, Hoeflich KP. Targeting cancer with kinase inhibitors. J Clin Invest. 2015;125(5):1780–1789. doi:10.1172/JCI76094
15. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi:10.1016/j.cell.2011.02.013
16. Vetrie D, Vorechovsky I, Sideras P, et al. The gene involved in X-linked agammaglobulinaemia is a member of the src family of protein-tyrosine kinases. Nature. 1993;361(6409):226–233. doi:10.1038/361226a0
17. Tsukada S, Saffran DC, Rawlings DJ, et al. Deficient expression of a B cell cytoplasmic tyrosine kinase in human X-linked agammaglobulinemia. Cell. 1993;72(2):279–290. doi:10.1016/0092-8674(93)90667-F
18. Gao W, Wang M, Wang L, et al. Selective antitumor activity of ibrutinib in EGFR-mutant non-small cell lung cancer cells. J Natl Cancer Inst. 2014;106:9. doi:10.1093/jnci/dju204
19. Campbell R, Chong G, Hawkes EA. Novel indications for Bruton’s tyrosine kinase inhibitors, beyond hematological malignancies. J Clin Med. 2018;7(4). doi:10.3390/jcm7040062
20. Burger JA, Buggy JJ. Bruton tyrosine kinase inhibitor ibrutinib (PCI-32765). Leuk Lymphoma. 2013;54(11):2385–2391. doi:10.3109/10428194.2013.777837
21. Hutcheson J, Vanarsa K, Bashmakov A, et al. Modulating proximal cell signaling by targeting Btk ameliorates humoral autoimmunity and end-organ disease in murine lupus. Arthritis Res Ther. 2012;14(6):R243. doi:10.1186/ar4086
22. Honigberg LA, Smith AM, Sirisawad M, et al. The Bruton tyrosine kinase inhibitor PCI-32765 blocks B-cell activation and is efficacious in models of autoimmune disease and B-cell malignancy. Proc Natl Acad Sci U S A. 2010;107(29):13075–13080. doi:10.1073/pnas.1004594107
23. Pan Z, Scheerens H, Li SJ, et al. Discovery of selective irreversible inhibitors for Bruton’s tyrosine kinase. ChemMedChem. 2007;2(1):58–61. doi:10.1002/cmdc.200600221
24. Gong Y, Chippada-Venkata UD, Oh WK. Roles of matrix metalloproteinases and their natural inhibitors in prostate cancer progression. Cancers (Basel). 2014;6(3):1298–1327. doi:10.3390/cancers6031298
25. Liotta LA, Kohn EC. The microenvironment of the tumour-host interface. Nature. 2001;411(6835):375–379. doi:10.1038/35077241
26. Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141(1):52–67. doi:10.1016/j.cell.2010.03.015
27. Overall CM, Lopez-Otin C. Strategies for MMP inhibition in cancer: innovations for the post-trial era. Nat Rev Cancer. 2002;2(9):657–672. doi:10.1038/nrc884
28. Trudel D, Fradet Y, Meyer F, Harel F, Tetu B. Significance of MMP-2 expression in prostate cancer: an immunohistochemical study. Cancer Res. 2003;63(23):8511–8515.
29. Oguic R, Mozetic V, Cini Tesar E, Fuckar Cupic D, Mustac E, Dordevic G. Matrix metalloproteinases 2 and 9 immunoexpression in prostate carcinoma at the positive margin of radical prostatectomy specimens. Patholog Res Int. 2014;2014:262195.
30. Xie T, Dong B, Yan Y, Hu G, Xu Y. Association between MMP-2 expression and prostate cancer: A meta-analysis. Biomed Rep. 2016;4(2):241–245. doi:10.3892/br.2015.553
31. Gartrell BA, Saad F. Managing bone metastases and reducing skeletal related events in prostate cancer. Nat Rev Clin Oncol. 2014;11(6):335–345. doi:10.1038/nrclinonc.2014.70
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.