Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 14
Bronchodilators for hyperinflation in COPD associated with biomass smoke: clinical trial
Authors Ramírez-Venegas A , Velázquez-Uncal M , Aranda-Chávez A , Guzmán-Bouilloud NE , Mayar-Maya ME , Pérez Lara-Albisua JL , Hernández-Zenteno RJ , Flores-Trujillo F, Sansores RH
Received 12 January 2019
Accepted for publication 12 April 2019
Published 6 August 2019 Volume 2019:14 Pages 1753—1762
DOI https://doi.org/10.2147/COPD.S201314
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Alejandra Ramírez-Venegas,1 Mónica Velázquez-Uncal,1 Adrián Aranda-Chávez,1 Nicolás Eduardo Guzmán-Bouilloud,1 María Eugenia Mayar-Maya,2 José Luis Pérez Lara-Albisua,1 Rafael de Jesus Hernández-Zenteno,3 Fernando Flores-Trujillo,3 Raúl H Sansores4
1Department of Tobacco Smoking and COPD Research, Instituto Nacional de Enfermedades Respiratorias “Ismael Cosió Villegas”, Mexico City, Mexico; 2Department of Medical Attention, Instituto Nacional de Enfermedades Respiratorias “Ismael Cosió Villegas”, Mexico City, Mexico; 3Obstructive Disease Ward, Pulmonary Obstructive Diseases Clinical Service, Instituto Nacional de Enfermedades Respiratorias “Ismael Cosío Villegas”, Mexico City, Mexico; 4Department of Respiratory Medicine, Medica Sur Clinic & Foundation, Mexico City, Mexico
Introduction: The efficacy of long-acting bronchodilators for COPD associated with biomass (BE-COPD) has not been properly evaluated.
Objective: To determine the acute effect of indacaterol (IND) 150 μg q.d and tiotropium (TIO) 18 μg q.d. on lung hyperinflation, walking distance (WD) and dyspnea during the six-minute walking test (6MWT) in moderate BE-COPD at 30, 60 and 240 mins post-drug administration.
Design: Randomized, controlled, open-level, crossover noninferiority clinical trial. Forty-two women with BE-COPD were randomly assigned to a bronchodilator sequence: IND–TIO or vice versa.
Results: There were statistically significant changes over time in inspiratory capacity (IC) (p<0.0001), FEV1 (p<0.0001) and FVC (p<0.0001) when IND was used. When TIO was administered, an increase over all time periods was observed only for FEV1 (p<0.0001) and FVC (p<0.0001), whereas for IC an increase was observed only at 30 mins and 24 hrs after TIO administration. We did not find clinically significant increases in WD and dyspnea after the administration of both bronchodilators.
Conclusion: Both IND and TIO showed significant and fast onset improvement in hyperinflation. Therefore, either of them may be recommended as a first line of treatment for COPD associated with BE-COPD.
Keywords: indacaterol, tiotropium, biomass exposure, inspiratory capacity, spirometry, six-minute walking test
Introduction
The WHO estimates a total of 2.7 million deaths due to COPD and approximately 844,000 due to solid fuel exposure.1 COPD causes approximately 4.7% of global deaths and accounts for 4.3% of the world’s disability-adjusted life-years, and exposure to biofuel smoke accounts for approximately one-third of these cases.2 Most deaths occur in developing countries, especially the poorest ones, and mostly among women. The majority of these women came from a rural environment, with limited access to basic urbanization services (electricity, drinkable water, gas and sewer system), and some came from marginal urban areas. In the setting of these women's daily living, and because of their limited access to basic services, including electricity and gas, they have the need to use biomass fuel for cooking and heating purposes.3 Most of these women were exposed to biomass during their childhood and adult life; on average, they had an exposure to biomass smoke 6–8 hrs per day.4 The material that is generally used for burning consists of wood, crop residues and dung. Their combustion is an important source of exposure to a variety of toxins.5 The combustion produced by the burning of products of wood include varius gases (nitrogen oxides, carbon monoxide (CO) and carbon dioxide), particulate matter including those with median aerodynamic diameter ≤2.5 µm (PM2.5) that consists of hydrocarbons, inorganic particles and semivolatile organic compounds which are able to penetrate deeply into the lung, causing irritation and oxidative stress (additive to other compounds), and producing lung and airway inflammation, hyperresponsiveness, and after long-term exposures airway remodeling and emphysema.6,7
Research on COPD associated with biomass exposure is scant, and there are no clinical trials including patients with COPD due to biomass (BE-COPD).
Regarding treatment for BE-COPD, different guidelines on COPD have focused only on COPD associated with tobacco smoking (TE-COPD). Therefore, treatment for BE-COPD is given empirically following the recommendations for TE-COPD.8,9 The advent of long-acting bronchodilators has revolutionized the treatment of COPD, as they are now considered the first line in COPD treatment.8–11 The first long-acting bronchodilator with proven effectiveness within the first 24 hrs was tiotropium (TIO), a long-acting muscarinic agent (LAMA).12,13 Ten years later, indacaterol (IND), the first 24-hr beta-two agonist, was launched for COPD and has also a rapid onset effect on bronchodilation.14,15 Both drugs have proven to be effective for improving lung function, dyspnea and exacerbations, and both are also effective for improving exercise tolerance in patients with COPD.8 An additional potential benefit of both bronchodilators is a decrease in hyperinflation, as demonstrated by the improvement in inspiratory capacity.16,17
Unfortunately, the benefits of these bronchodilators have been demonstrated in different clinical trials only for smoking-induced COPD, whereas its use for women with BE-COPD is unknown, not just in terms of their potential impact on lung function but also with regard to functional exercise capacity for daily physical activities, for instance, as measured by the six-minute walking test (6MWT). In this sense, both TIO and IND have been examined in comparison to placebo18–21 and by using open-label control medication with different bronchodilators. Hyperinflation, one of the causes of dyspnea in patients with COPD, has been targeted in order to show the impact of long-acting bronchodilators in COPD.22,23
Therefore, this study was designed to determine the impact of IND 150 μg q.d. and TIO 18 μg q.d. on inspiratory capacity (IC), forced expiratory volume in one second (FEV1)and forced vital capacity (FVC), as surrogates of lung hyperinflation and on dyspnea and walking distance (WD) during the 6MWT in patients with moderate COPD associated with biomass.
Setting and subjects
This study was performed in the COPD Clinic at the National Institute of Respiratory Disease, Mexico City, Mexico, a tertiary care hospital that is the referral hospital for respiratory diseases that mainly provides service to economically deprived populations in Mexico. Mexico City’s mean altitude is 2,240 m above sea level; mean PaO2 and PaCO2 values in young subjects are about 66–72 and 28–32 mm Hg, respectively.24
Inclusion criteria
This study included only women coming from a low-income social environment, aged 50–80 years old, who were exposed to biomass smoke, mainly wood smoke. For the purpose of this study, biomass exposure was defined as patients who cooked with a biomass stove for at least 6 months and/or had a cumulative exposure of more than 150 hr-years, which is the product of the number of years of cooking with biomass multiplied by the average number of hours spent daily in the kitchen.25 This index (biomass exposure) has been validated in different longitudinal studies of COPD associated with biomass that have already been published by our group and others.26,27 The included women who had only been exposed to biomass never were exposed to tobacco smoke.
COPD was diagnosed according to 2014 GOLD guidelines8 with FEV1/FVC ratio less than 70% and FEV1 less than 80% of the expected value (GOLD stage II, III). Women had to be available to attend all study visits and perform pulmonary function tests correctly. They had to have a stable disease with no exacerbations in the last six weeks prior to inclusion. They also had to have stability with regard to any associated chronic degenerative diseases. Exclusion criteria included a) pregnancy, b) medical history of asthma, c) bronchiectasis, d) tuberculosis, e) suspected cancer and f) patients whose treatment included inhaled or oral corticosteroids of recent onset (less than 30 days) and if present, neuromuscular or locomotive impairment affecting the ability to walk were also excluded.
Study design and methods
A randomized, controlled, open-label, crossover, noninferiority clinical trial was designed. To prevent biases induced by open-label drugs and to be able to introduce blindness in the drug administration, both technicians who performed lung function tests and 6MWT and the attending physicians for each patient at each interval (VUM, ACA, GBN, PAJL, HZR, FTF) were unaware of the administered bronchodilator at the day of the test.
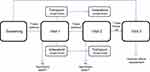
Forty-two BE-COPD women were randomly assigned to a bronchodilator sequence: IND 150 mcg– TIO 18 mcg or vice versa (Figure 1).
The baseline visit included signing the informed consent form and assessment of inclusion and exclusion criteria. Medication was administered in a single-blind manner, as an inhaled capsule.
Measurements of IC, FEV1, FVC and 6MWT were performed before and after administration of the drug. Following a 7-day washout period, visit 1 (day 0) outcomes were assessed at 30, 60 and 240 mins after administration of a single dose of IND or TIO. At visit 2 (after 7 days of a second washout period), the same procedure was performed with the alternative bronchodilator (Figure 2).
 |
Figure 2 Flowchart of subject recruitment. |
The trough FEV1, FVC and IC were defined as the average of the two measurements taken at the end of the drug administration period, at approximately 23:10–23:45 hrs following drug administration.
Outcome variables
The primary outcome of this study was to determine the impact of a single dose of IND or TIO on IC, FEV1 and FVC from baseline to 30, 60 and 240 mins as well as 23:10 and 23:45 hrs (trough measurements).
The secondary outcome was to determine the impact of a single dose of IND or TIO on WD and exertional dyspnea during the 6MWT from baseline to 30, 60 and 240 mins as well as 23:10 and 23:45 hrs (trough measurements).
Study procedures
Pulmonary function testing
Pulmonary function was measured at the first visit according to previously described guidelines28 and using the Mexican standard reference equations,29 which are similar to the Third National Health and Nutrition Examination Survey values for Mexican-Americans.30
To obtain IC, patients quietly breathed into a spirometer, such that several reproducible readings of tidal volume were obtained. Patients were then asked to take a maximum inspiration and slowly and completely exhale.31
Six-minute walking test
All patients performed the 6MWT after pulmonary function testing. The 6-min walking distance (6MWD) was measured according to the guidelines of the American Thoracic Society,32 with standardized encouragement, in a 30-m corridor under the supervision of an experienced technician.
Sample size and statistical analysis
The sample size calculation was based on the premise that improvements in WD with TIO should be of similar magnitude to those seen with IND. A calculation was obtained from a previous study,33 taking into account a minimal clinical difference of 30 m with a standard deviation of 90 m. It was calculated that 22 patients in each treatment arm would be necessary for this study to achieve power equal to 0.80 at p<0.05 significance with a one-tailed statistical test. Taking into account a 20% attrition rate, we planned to recruit 44 patients for this study. The primary efficacy variables were the increase in IC, FEV1 and FVC from predose among the readings at 30, 60 and 240 mins as well as 24 hrs postdose. Statistical significance was tested by ANOVA. Possible crossover and period effects for all exploratory end points were first assessed. Treatment responses were compared using paired t-tests. A p<0.05 significance level was used for all analyses, with Bonferroni adjustments for multiple comparisons where appropriate. The results are reported as the mean± standard deviation.
Ethical considerations
The study protocol and informed consent were approved by the Ethics and Research Committee of the National Institute of Respiratory Diseases Ismael Cosío Villegas, of Mexico City, Mexico, with approval reference number C-2212.
The study was conducted in accordance with the statements of the Helsinki´s Declaration.
Results
Data was collected from August 2013 to December 2016. The study´s flow chart is presented in Figure 1. Fifty-five patients were screened, 13 were excluded, and 42 patients were randomized in a 1:1 ratio to TIO or IND at visit one. Subject characteristics are presented in Table 1. One hundred percent of participants were female, with a mean age of 75 years and a median biomass smoke exposure index of 310 hr-years.
 |
Table 1 Demographics and lung function characteristics on COPD-BE |
Patients had on average moderate airflow obstruction, 68% of patients were naïve for COPD treatment and the remaining subjects had the following treatment: 6 patients were on TIO treatment and 7 on IND. Data on FEV1, IC and FVC both for IND and for TIO after a single study dose of medication is shown in Table 2. Significant increases over all time periods were observed in IC (p<0.0001), FEV1(p<0.0001) and FVC (p<0.0001) after IND was administered. When TIO was administered, an increase over all time periods was observed only for FEV1 (p<0.0001) and FVC (p<0.0001), whereas for IC an increase was observed only at 30 mins and 24 hrs after TIO administration (Figure 3). No significant differences were observed among TIO and IND.
 |
Table 2 Measurements over time of FEV1, IC, FVC with IND and TIO |
Figure 4 shows the comparison of IC, FVC and FEV1 at 24 hrs postdose (trough) to predose values. There were significant differences for all variables at 24 hrs (p<0.0001). However, there were no differences between the treatments. Trough FEV1 increased by an average of 57 mL with IND and 90 mL with TIO (p=NS); trough FVC increased by an average of 73 mL with IND and 153 mL with TIO (p=NS); trough IC increased by an average of 116 mL with IND and 156 mL with TIO (p=NS), confirming a reduction in air trapping.
Distance walked and dyspnea after IND or TIO are shown in Table 3. Significant changes in walking distance (WD) over 24 hrs were observed after administering both bronchodilators. However, no difference was observed between them at any interval and the increase in WD did not reach a minimal clinically important difference (MCID). Dyspnea during the walking test did not show any significant decrease over time at any interval in comparison with baseline when either bronchodilator was used.
 |
Table 3 Measurements over time of walking distance and dyspnea (end 6MWT) with IND and TIO |
Table 4 shows the proportion of subjects who reached MCID in distance walked, dyspnea at the end of the 6MWT, IC and FEV1 during the different intervals after administration of IND or TIO. For distance walked and dyspnea score, a small number of patients reached a MCID with both bronchodilators. However, more than 40% of subjects showed a MCID in IC at all intervals after using both bronchodilators. Interesting to note is that for FEV1 a lower proportion of subjects reached a MCID when IND was administered in comparison to TIO. Nevertheless, these differences were not statistically significant.
 |
Table 4 Subjects achieving minimal clinically important difference (MCID) at different time periods with IND and TIO [n=number of subjects (proportion)] |
Safety
The most common drug-related adverse event was cough in 13.6% and 4.5% of patients after administration of IND and TIO, respectively. “Cough” resolved after a few minutes to inhale the drug in the majority of the patients (60%).
Discussion
Our results showed that both IND and TIO had a significant bronchodilator effect in patients with BE-COPD. This effect started in the first 30 mins following a single inhalation and remained 24 hrs later with both bronchodilators; however, this finding did not show a clinically relevant effect on the improvement of dyspnea and distance walked.
Inspiratory capacity has been used as a surrogate to demonstrate dynamic hyperinflation in patients with COPD.32 Likewise, improvement in IC has been associated with a reduction in exercise-induced dyspnea.17,21,34 Our data showed that both IND and TIO increased IC. Interestingly, these women with BE-COPD had moderate airflow limitation (FEV1% predicted of 55%) yet had hyperinflation, as indicated by increased IC following administration of the bronchodilators. Although changes in IC were not as high as reported elsewhere,18 this may be explained by the short stature of these women and the lower predicted values in spirometry and IC.
Both IND and TIO were the very first 24-hr long-acting bronchodilators in their class, as has been demonstrated in many clinical trials with smokers as patients. This study shows that this effect is also observed in BE-COPD, as can be observed in the significant trough effect on FEV1, FVC and IC. As in TE-COPD, our results showed that patients with BE-COPD might receive additional benefit from regular treatment with long-acting bronchodilators earlier in the course of their disease and that both airflow obstruction and lung hyperinflation should therefore be targeted. In this sense, either a LAMA or LABA is equally effective in their acute and long-acting bronchodilator effects.
For WD during the 6MWT, previous papers have shown that TIO, either alone or in combination with other bronchodilators, improved 6MWD in COPD-TE.35–37 However, the modest improvement in distance observed with TIO at 24 hrs (19 m) and the lack of improvement observed for IND were not surprising because our study was designed to evaluate a single dose within 24 hrs, which was likely insufficient to show significant changes and is in contrast to the more than 4-week period reported in other studies.35–37 The 6MWT has been used as a tool to evaluate the submaximal exercise.36 However, its utility as a marker of bronchodilation is controversial. Celly et al38 evaluated the 6MWT for interventions and its responsiveness to pharmacologic agents in more than 14,000 COPD patients from observational and clinical trials and concluded that the 6MWT is not a responsive outcome for inhaled bronchodilators. In this sense, the benefits of bronchodilators on the 6MWT might be explained through mechanisms other than bronchodilation.38
It has been assumed that IND has a 24-hr duration of bronchodilator effect and a fast onset of action in COPD. In a double-blind, core-period study with 635 subjects with COPD, Rennard et al14 showed in an open-label crossover extension period with a subgroup of subjects that IND provided comparable or superior efficacy to TIO from 0 to 4 hrs on Day 1. In terms of bronchodilation, similar results were observed in the present sample of women with COPD.
This is the first clinical trial to evaluate the acute effects of LABAs in COPD-BE. No clinical information regarding clinical trials, in particular, with the new generation of bronchodilators, has been documented in COPD-BE. Notwithstanding that COPD in women in rural areas is a public worldwide health problem,39 all clinical trials have focused on COPD associated with tobacco smoking, thus giving the appearance that pharmaceutical companies are not interested in testing their products on this population. Therefore, physicians dealing with COPD-BE empirically treat these women and prescribe different drugs by following the same criteria of international COPD guidelines.8
It was important to test whether LABAs are equally as effective for COPD-BE as they are for COPD-TE. Our results suggest that when LABAs or different inhalers are prescribed to women with COPD-BE with moderate disease, the same therapeutic approach that is used for COPD-TE may be used.26,27 More studies are needed to evaluate the effects of LABAs over longer periods of time and their association with mortality, exacerbations, quality of life and lung function.
One of the problems with performing a clinical trial is that few centers are interested in testing drugs for COPD-BE. Another setback is the cost for medical centers to perform clinical trials without sponsorship from pharmaceutical companies. The importance of this clinical trial is that it gathered evidence to show the utility of drugs used in COPD by using a hard and reliable methodology for treating COPD-BE rather than relying strictly on empirically supported treatments.
Limitations
A potential limitation may be that this study only examined acute effects. By not examining the effects of the study drugs beyond a single day, we might have underestimated the potential long-term benefits of both drugs.
Despite the clinical intention to blind this study, the most important limitation was the absence of a control group, in this case, COPD-TE women. This control group would have helped to contrast both populations, in terms of their different or similar responses to the treatments. However, given that COPD associated with tobacco smoking has been widely studied, such a control group may not have been necessary.
Strengths
This is the first clinical trial using appropriate methodology to include BE-COPD women to test the impact of long-acting bronchodilators.
In summary, IND and TIO similarly improved IC, FEV1 and FVC during the first 24 hrs after their administration in women with moderate BE-COPD. Both bronchodilators had a higher proportion of subjects with a clinically significant response in IC than the rest of the outcomes.
Acknowledgements
This work was supported by a non-restricted grant allocated for the care of women with respiratory diseases associated with biomass, by the legislature of the Chamber of Deputies and by a restricted grant of Novartis.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Available from: http://www.who.int/en/news-room/fact-sheets/detail/household-air-pollution-and-health.
2. Gordon SB, Bruce NG, Grigg J, et al. Respiratory risks from household air pollution in low and middle-income countries. Lancet Respir Med. 2014;2(10):823–860. doi:10.1016/S2213-2600(14)70168-7
3. Balmes JR. When smoke gets in your lungs. Proc Am Thorac Soc. 2010;7(2):98–101. doi:10.1513/pats.200907-081RM
4. Armstrong JR, Campbell H. Indoor air pollution exposure and lower respiratory infections in young Gambian children. Int J Epidemiol. 1991;20(2):424–429. doi:10.1093/ije/20.2.424
5. Sood A, Assad NA, Barnes PJ, et al. ERS/ATS workshop report on respiratory health effects of household air pollution. Eur Respir J. 2018;51:1700698. doi:10.1183/13993003.00698-2017
6. Naeher LP, Brauer M, Lipsett M, et al. Woodsmoke health effects: a review. Inhal Toxicol. 2007;19(1):67–106. doi:10.1080/08958370600985875
7. Perez-Padilla R, Schilmann A, Riojas-Rodriguez H. Respiratory health effects of indoor air pollution. Int J Tuberc Lung Dis. 2010;14(9):1079–1086.
8. Available from: https://goldcopd.org/gold-2017-global-strategy-diagnosis-management-prevention-copd/Last.
9. Montes de Oca M, López Varela MV, Acuña A, et al. ALAT-2014 Chronic obstructive pulmonary disease (COPD) clinical practice guidelines: questions and answers. Arch Bronconeumol. 2015;51(8):403–416. doi:10.1016/j.arbres.2014.11.017
10. Vogelmeier C, Hederer B, Glaab T, et al. Tiotropium versus salmeterol for the prevention of exacerbations of COPD. N Engl J Med. 2011;364(12):1093–1103. [PubMed]. doi:10.1056/NEJMoa1008378
11. Wedzicha JA, Decramer M, Ficker JH, et al. Analysis of chronic obstructive pulmonary disease exacerbations with the dual bronchodilator QVA149 compared with glycopyrronium and tiotropium (SPARK): a randomised, double-blind, parallel-group study. Lancet Respir Med. 2013;1(3):199–209. doi:10.1016/S2213-2600(13)70052-3
12. Littner MR, Ilowite JS, Tashkin DP
13. Tashkin DP, Celli B, Senn S, et al; UPLIFT Study Investigators. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med. 2008;359(15):1543–1554. doi:10.1056/NEJMoa0805800
14. Rennard S, Bantje T, Centanni S, et al. A dose-ranging study of indacaterol in obstructive airways disease, with a tiotropium comparison. Respir Med. 2008;102(7):1033–1044. doi:10.1016/j.rmed.2008.02.001
15. Rossi A, Polese G. Indacaterol: a comprehensive review. Int J Chron Obstruct Pulmon Dis. 2013;8:353–363. doi:10.2147/COPD.S21625
16. O’Donnell DE, Flüge T, Gerken F, et al. Effects of tiotropium on lung hyperinflation, dyspnoea and exercise tolerance in COPD. Eur Respir J. 2004;23(6):832–840. doi:10.1183/09031936.04.00116004
17. Watz H, Krippner F, Kirsten A, Magnussen H, Vogelmeier C. Indacaterol improves lung hyperinflation and physical activity in patients with moderate chronic obstructive pulmonary disease–a randomized, multicenter, double-blind, placebo-controlled study. BMC Pulm Med. 2014;4(14):158. doi:10.1186/1471-2466-14-158
18. Cazzola M, Biscione GL, Pasqua F, et al. Use of 6-min and 12-min walking test for assessing the efficacy of formoterol in COPD. Respir Med. 2008;102:1425–1430. doi:10.1016/j.rmed.2008.04.017
19. Singh SJ, Puhan MA, Andrianopoulos V, et al. An official systematic review of the European Respiratory Society/American Thoracic Society: measurement properties of field walking tests in chronic respiratory disease. Eur Respir J. 2014;44:1447–1478. doi:10.1183/09031936.00003814
20. Puente-Maestu L, Palange P, Casaburi R, et al. Use of exercise testing in the evaluation of interventional efficacy: an official ERS statement. Eur Respir J. 2016;47:429–460. doi:10.1183/13993003.00745-2015
21. Spruit MA, Polkey MI, Celli B, et al; Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) study investigators. Predicting outcomes from 6 min walk distance in chronic obstructive pulmonary disease. J Am Med Dir Assoc. 2012;13(3):291–297. doi:10.1016/j.jamda.2011.06.009
22. O´Donnell DE. Hyperinflation dyspnea and exercise intolerance in chonic obstructive pulmonary disease. Proc Am Thorac Soc. 2006;3(2):180–184. doi:10.1513/pats.200508-093DO
23. Holland AE, Spruit MA, Troosters T, et al. An official European Respiratory Society/American Thoracic Society technical standard: field walking tests in chronic respiratory disease. Eur Respir J. 2014;44:1428–1446. doi:10.1183/09031936.00003814
24. Pérez-Padilla R. Population distribution residing at different altitudes: implications for hypoxemia. Arch Med Res. 2002;33(2):162–166.
25. Perez-Padilla R, Regalado J, Vedal S, et al. Exposure to biomass smoke and chronic airway disease in Mexican women: a case-control study. Am J Respir Crit Care Med. 1996;154:701–706. doi:10.1164/ajrccm.154.3.8810608
26. Ramírez-Venegas A, Sansores RH, Quintana-Carrillo RH, et al. FEV1 decline in patients with chronic obstructive pulmonary disease associated with biomass exposure. Am J Respir Crit Care Med. 2014;190(9):996–1002. doi:10.1164/rccm.201404-0720OC
27. Ramírez-Venegas A, Sansores RH, Pérez-Padilla R, et al. Survival of patients with chronic obstructive pulmonary disease due to biomass smoke and tobacco. Am J Respir Crit Care Med. 2006;173(4):393–397. doi:10.1164/rccm.200504-568OC
28. Miller MR, Hankinson J, Brusasco V, et al; ATS/ERS Task Force. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi:10.1183/09031936.05.00034805
29. Perez-Padilla R, Regalado J, Vazquez Garcıa JC. Reproducibilidad espirometrica y adecuacion a valores de referencia internacionales en trabajadores Mexicanos demandando incapacidad. Salud Publica Mex. 2001;43:113–121. doi:10.1590/S0036-36342001000200006
30. Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179–187. doi:10.1164/ajrccm.159.1.9712108
31. Tantucci C, Pinelli V, Cossi S, Guerini M, Donato F, Grassi V; SARA Study Group. Reference values and repeatability of inspiratory capacity for men and women aged 65-85. Respir Med. 2006;100(5):871–877. doi:10.1016/j.rmed.2005.08.017
32. ATS statement: guidelines for the six-minute walk test. ATS committee on proficiency standards for clinical pulmonary function laboratories. Am J Respir Crit Care Med. 2002;166(1):111–173. doi:10.1164/ajrccm.166.1.at1102
33. Zutler M, Singer JP, Omachi TA, et al. Relationship of obesity with respiratory symptoms and decreased functional capacity in adults without established COPD. Prim Care Respir J. 2012;21(2):194–201. doi:10.4104/pcrj.2012.00028
34. Marin JM, Carrizo SJ, Gascon M, Sanchez A, Gallego B, Celli BR. Inspiratory capacity, dynamic hyperinflation, breathlessness, and exercise performance during the 6 min-walk test in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;163:1395–1399. doi:10.1164/ajrccm.163.6.2003172
35. Bédard ME, Brouillard C, Pepin V, et al. Tiotropium improves walking endurance in COPD. Eur Respir J. 2012;39(2):265–271. doi:10.1183/09031936.00059511
36. Fernandes FL, Pavezi VA, Dias SA
37. Jayaram L, Wong C, McAuley S, Rea H, Zeng I, O’Dochartaigh C. Combined therapy with tiotropium and formoterol in chronic obstructive pulmonary disease: effect on the 6 min walk test. COPD. 2013;10(4):466–472. doi:10.3109/15412555.2013.771162
38. Celli B, Tetzlaff K, Criner G, et al; COPD Biomarker Qualification Consortium. The 6 min-walk distance test as a chronic obstructive pulmonary disease stratification tool. Insights from the COPD biomarker qualification consortium. Am J Respir Crit Care Med. 2016;194(12):1483–1493. doi:10.1164/rccm.201508-1653OC
39. Pérez-Padilla R, Ramirez-Venegas A, Sansores-Martinez R. Clinical characteristics of patients with biomass smoke-associated COPD and chronic bronchitis, 2004-2014. Chronic Obstr Pulm Dis. 2014;1(1):23–32. doi:10.15326/jcopdf.1.1.2013.0004
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.