Back to Journals » Infection and Drug Resistance » Volume 15
Bronchial Artery-Pulmonary Artery Shunt by Apiotrichum mycotoxinivorans Infection in a Recurrent Hemoptysis Case
Authors Pang Y , Hu D , Dang Y, Huang S, Qin L, Li M
Received 6 May 2022
Accepted for publication 9 August 2022
Published 18 August 2022 Volume 2022:15 Pages 4611—4615
DOI https://doi.org/10.2147/IDR.S373615
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Yu Pang,1 Diefei Hu,1 Yiwu Dang,2 Siming Huang,3 Lanhui Qin,4 Meng Li5
1Department of Infectious Disease, First Affiliated Hospital of Guangxi Medical University, Nanning, Guangxi, 530021, People’s Republic of China; 2Department of Pathology, First Affiliated Hospital of Guangxi Medical University, Nanning, Guangxi, 530021, People’s Republic of China; 3Department of Respiratory Medicine, First Affiliated Hospital of Guangxi Medical University, Nanning, Guangxi, 530021, People’s Republic of China; 4Department of Radiology, First Affiliated Hospital of Guangxi Medical University, Nanning, Guangxi, 530021, People’s Republic of China; 5Department of Clinical Laboratory, First Affiliated Hospital of Guangxi Medical University, Nanning, Guangxi, 530021, People’s Republic of China
Correspondence: Meng Li, Department of Clinical Laboratory, First Affiliated Hospital of Guangxi Medical University, Nanning, Guangxi, 530021, People’s Republic of China, Tel/Fax +8613367809642, Email [email protected]
Background: Apiotrichum mycotoxinivorans is a rare mycotoxinivorans, and its pathogenicity is unknown. Bronchial artery shunt is a pathophysiological state following congenital or acquired chronic infection. We report a rare case of bronchial artery shunt by A. mycotoxinivorans infection in a recurrent hemoptysis patient.
Case Presentation: A 45-year-old female presented with recurrent cough and hemoptysis for 4 years. Before admission, she had been treated in several hospitals for pulmonary tuberculosis and bronchiectasis and received standardized anti-tuberculosis treatment for 1 year, but it was ineffective. After admission, CTPA and bronchial arterial angiography showed left bronchial artery–left pulmonary artery shunt and right bronchial artery–right pulmonary artery shunt. Fiber-optic bronchoscopy was performed, which revealed a large amount of purulent secretions, bronchoalveolar lavage fluid fungi (1-3)-β-d glucan: 728.06, and GM test: 3.239. Fungal hyphae and spores were observed by gram staining of BALF smear. Acid-fast bacilli were not found in BALF smear and brush smear. Two consecutive BALF fungal cultures grew A. mycotoxinivorans, the identity of which was confirmed by internal-transcribed-spacer (ITS) sequencing. Intravenous amphotericin B liposome (30mg; 0.5mg/kg, QD) was given for 2 weeks, embolization was performed, and itraconazole (voriconazole allergy) was taken orally for 9 months after operation. Hemoptysis and pulmonary lesions gradually improved after treatment.
Conclusion: We report the first case of bronchial artery–pulmonary artery shunt in a patient diagnosed with A. mycotoxinivorans infection. Phagocytosis of fungi by leukocytes was observed, and the pathogenicity of the fungus was confirmed in order to heighten the awareness of these infections.
Keywords: Apiotrichum mycotoxinivorans, bronchial artery–pulmonary artery shunt, hemoptysis, infection
Introduction
Apiotrichum mycotoxinivorans (formerly Trichosporon mycotoxinivorans)1 is a rare pathogenic Trichosporon that can inhabit soil, river, and farm animals. It is a yeast isolated from the hindgut of lower termite Mastotermes darwiniensis (Mastotermitidae) in 20042 and represents a new member of the genus Trichosporon. Its pathogenicity was unknown until 2009; Hickey et al3 reported the first human case of A. mycotoxinivorans infection, who died of histologically documented Trichosporon species pneumonia with cystic fibrosis (CF). It was suggested that A. mycotoxinivorans was a newly discovered pathogen, which seems to have a certain predisposition to CF patients. A. mycotoxinivorans has since been isolated from the sputum and blood samples of cystic fibrosis patients. Searches online has found that only 8 clinical cases of this fungal infection had been reported in PubMed until now, including CF patients (6/8) and non-CF patients (2/8). There was a case with CF died of disseminated infection for A. mycotoxinivorans after liver and lung transplantation.4 There was another case of disseminated infection for A. mycotoxinivorans after dialysis in chronic renal failure.5 In addition, there was a pediatric patient of A. mycotoxinivorans isolated in congenital ventricular septal defect complicated with pneumonia, which was found after surgery.6 There was another case of A. mycotoxinivorans isolated in a 70-year-old female patient with cirrhosis accompanied by chronic bronchial infection (CBI), which was detected with Cryptococcus.7 In these two cases, only fungi were isolated without sufficient evidence of pathogenicity.
Chronic infection or long-term asymptomatic colonization was more common in CF patients with normal immune function. Shah et al8 reported a long-term follow-up of 4 children with CF, and none of their patients had fulminant disease associated with initial isolation of A. mycotoxinivorans and 2 of them could be improved without antifungal therapy. Goldenberger et al9 reported a case of CF patient with A. mycotoxinivorans, who was followed up for 9 years and presented mild pulmonary symptoms, which could be improved after antifungal treatment. Martinez Muniz et al10 reported a case of 37-year-old CBI patient with A. mycotoxinivorans, who showed no signs of clinical, radiological or functional deterioration during a 5-year follow-up.
These cases suggest that A. mycotoxinivorans infection is rare, whose host status and clinical manifestation varied in these patients. Few cases progress rapidly and can be severe or even fatal. In some cases, it could also manifest as nonpathogenic colonization or chronic airway infection. The exact host and mechanism are still unknown. Interestingly, bronchial artery–pulmonary artery shunt is also not common. We report the first case of bronchial artery–pulmonary artery shunt in a patient (non-CF patients) diagnosed with A. mycotoxinivorans infection. More importantly, the pathogenicity of the fungus was confirmed, and it is very rare and critical to our understanding and increasing our experience of the disease.
Case Presentation
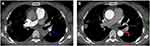
A 45-year-old female patient, a city greening worker and was in good health before onset, was admitted to a local hospital in October 2017 due to cough and hemoptysis. At that time, she was diagnosed with pneumonia and bronchiectasis. She was given cephalosporin anti-infective therapy, but it did not work. Then, between January and March 2018, she visited other hospitals several times and was diagnosed with tuberculosis due to imaging findings (no Mycobacterium tuberculosis was found in sputum). Therefore, from April 2018 to April 2019, the patient received conventional (2HRZE/9HE) anti-TB therapy for about 1 year, but it was ineffective; cough and hemoptysis continued to occur repeatedly. Therefore, in November 2019, she came to our hospital for treatment. After admission, tuberculosis bacteria culture in sputum in November 2019 showed no M. tuberculosis complex group. Blood tests showed that the WBC was 5.42 × 109/L, the percentage of neutrophil was 0.77, the erythrocyte sedimentation rate was 58mm, PCT was 0.078ng/mL, and the C-reactive protein was 62mg/L; sputum smear was negative for acid-fast bacilli; and HIV antibodies were negative and antinuclear antibody was positive (1:1000). CTPA showed the following: Filling defect was observed in the main pulmonary artery, bronchial artery diameter was widened, and irregular patchy and nodular density was observed in both lungs (Figure 1A and B). Fiber-optic bronchoscopy was performed, which revealed a large amount of purulent secretions, bronchoalveolar lavage fluid (BALF) fungi (1-3)-β-d glucan: 728.06, and GM test: 3.239. Pleomorphic fungi were observed by gram staining of BALF smear (Figure 2A and B). Acid-fast bacilli were not found in BALF smear and brush smear. Cryptococcus latex agglutination test was negative. No bacteria were cultured in BALF. No bacteria, fungi, viruses, parasites, M. tuberculosis, mycoplasma, chlamydia, or rickettsia were found by mNGS (BALF).
 |
Figure 2 (A and B) Pleomorphic fungi were observed by gram staining of BALF smear. |
CTPA and bronchial arterial angiography showed left bronchial artery–left pulmonary artery shunt (Figure 1A and B) and right bronchial artery–right pulmonary artery shunt. Fungal hyphae and spores were observed by gram staining of BALF smear. Two consecutive BALF fungal cultures grew A. mycotoxinivorans (Figure 2A and B), the identity of which was confirmed by internal-transcribed-spacer (ITS) sequencing. The phagocytosis of A. mycotoxinivorans by leukocytes was observed under the microscope (Figure 3). Intravenous amphotericin B liposome (30mg; 0·5mg/kg, QD) was given for 2 weeks, embolization was performed, and itraconazole (voriconazole allergy) was taken orally for 9 months after operation. Hemoptysis and pulmonary lesions gradually improved after treatment. Fiber-optic bronchoscopy was performed once again. Acid-fast bacilli were found on BALF smears two times, and Mycobacterium intracellulare was detected by mNGS. Oral rifabutin (0.3g qd), ethambutol (15–25mg/kg·d), and clarithromycin (0.5, bid) were given for 6 months at the same time. The patient had clinical remission of pulmonary infection. After infection control, oral methylprednisolone (30mg qd) and hydroxychloroquine (0.2g bid Po) were given for 2 months for SLE. Re-examination of the lesion showed absorption, and no hemoptysis occurred in the patient after 6 months of follow-up.
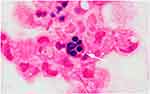 |
Figure 3 The white arrow shows that A. mycotoxinivorans were engulfed by leukocytes. |
Discussion and Conclusions
A. mycotoxinivorans infection is rare, and pathogenicity is the most hotly discussed topic among the limited reports on A. mycotoxinivorans. We know that virulence factors are closely related to pathogenicity because they allow fungi to establish and develop in host tissue species. Studies11 have confirmed that Apiotrichum has the highest temperature tolerance among yeast-like basidiomycetes and has lipase and biofilm production.5 These virulence factors also increase the possibility of long-term infection of Apiotrichum to some extent. In our case, leukocyte phagocytosis of the fungus was observed in gram-stained BALF pathological specimen, which is generally considered an evidence of infection rather than colonization, thus indicating the potential pathogenicity of A. mycotoxinivorans.
A review of relevant literature found that invasive infection is related to immunosuppression or invasive operation, risk factors for infection or colonization in CF patients are unknown, and invasive infection in non-CF patients is related to mucosal destruction or systemic immune dysfunction caused by tumor or HIV.12 The patient in our case had no neoplastic disease before onset and no history of use of immunosuppressant or hormone drugs. However, SLE was found in subsequent examination, which indirectly suggested the existence of potential immune disorder in our patient. The exact host and mechanism are still unclear. Mucosal damage may be a predisposing factor. So far, no relevant literature has reported the possible infection route of A. mycotoxinivorans. Our patient was a greening worker who had also grown vegetables for many years, which was suspected to be related to environmental inhalation. The exact pathophysiological mechanism of A. mycotoxinivorans is not clear.
Bronchial artery–pulmonary artery fistula is a rare congenital disorder that occurs mostly in children. Adult bronchial artery–pulmonary artery fistula could be associated with persistent lung inflammation, such as common actinomycete infection, tuberculosis, and bacterial pneumonia,13 leading to the reopening of potential traffic branches. In our case, A. mycotoxinivorans infection could have been an important factor in the formation of bronchial artery–pulmonary shunt in our patient. It provides a new diagnostic perspective and further evidence for the possibility of long-term infection of A. mycotoxinivorans. Mycobacterium was found after ITCZ treatment and surgical treatment. Therefore, Mycobacterium could have been a secondary infection. Due to its common symptoms of cough and hemoptysis, it can easily be misdiagnosed as tuberculosis or bronchiectasis. Up to now, the routine diagnosis of bronchial artery fistula still depends on further imaging interventions. In the case of shunt findings, catheter embolization should be performed.
In terms of treatment for A. mycotoxinivorans, Hickey et al3 believed that amphotericin B and lipid formulations as well as the echinocandins had limited efficacy against Trichosporon species and were even resistant to echinocandins. Triazole drugs are considered the first choice. Among the limited selection of antifungal triazole agents, voriconazole may be preferred for its intravenous and oral preparations and bioavailability. Do et al11 reported a case of A. mycotoxinivorans infection and found that the MIC of FLC was the highest, followed by AMB, and ITCZ was the lowest. In our case, after itraconazole was administered, the pulmonary infection foci did not increase, and triazole was proved to be effective. However, due to the small sample size, whether it is worth promoting remains to be supported by more clinical and microbiological research data.
In conclusion, A. mycotoxinivorans infection is rare, and infection or colonization of A. mycotoxinivorans is controversial. The histopathophysiological changes caused by the disease are unknown. We report a rare case of A. mycotoxinivorans in a patient with bronchial artery–pulmonary artery fistula. Phagocytosis of fungi by leukocytes was observed. This is the first case in China and is also not reported worldwide before. Clinical reports on A. mycotoxinivorans are rare, and due to the difficulties in clinical isolation and identification of pathogens, it is particularly difficult to diagnose A. mycotoxinivorans, which is easy to be missed or misdiagnosed. We provide new data on the clinical manifestations of this emerging microbe under current knowledge, providing evidence for further clinical research to better understand A. mycotoxinivorans and bronchial artery–pulmonary fistula.
Ethics Approval and Informed Consent
Written informed consent to have the case details and any accompanying images published has been provided by the patient. No ethical committee approval was required for this study as the data were analyzed in a retrospective manner.
Acknowledgments
All authors thank the patient and her families for their support.
Author Contributions
All authors made substantial contributions to the conception and design, acquisition of data, or analysis and interpretation of data; participated in drafting the article or revising it substantially; agreed to submit to the current journal; gave final approval of the version to be published; and agreed to take responsibility for all aspects of the work.
Funding
There is no funding to report.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Liu XZ, Wang QM, Goker M, et al. Towards an integrated phylogenetic classification of the Tremellomycetes. Stud Mycol. 2015;81:85–147.
2. Molnar O, Schatzmayr G, Fuchs E, Prillinger H. Trichosporon mycotoxinivorans sp. nov., a new yeast species useful in biological detoxification of various mycotoxins. Syst Appl Microbiol. 2004;27(6):661–671.
3. Hickey PW, Sutton DA, Fothergill AW, et al. Trichosporon mycotoxinivorans, a novel respiratory pathogen in patients with cystic fibrosis. J Clin Microbiol. 2009;47(10):3091–3097.
4. Hirschi S, Letscher-Bru V, Pottecher J, et al. Disseminated Trichosporon mycotoxinivorans, Aspergillus fumigatus, and Scedosporium apiospermum coinfection after lung and liver transplantation in a cystic fibrosis patient. J Clin Microbiol. 2012;50(12):4168–4170.
5. Almeida JN, Francisco EC, Barberino M, et al. Emergence of Trichosporon mycotoxinivorans (Apiotrichum mycotoxinivorans) invasive infections in Latin America. Mem Inst Oswaldo Cruz. 2017;112(10):719–722.
6. Peng L, Jiang YQ, Jiang GM, et al. Molecular identification and biological characteristic analysis of an Apiotrichum mycotoxinivorans (formerly Trichosporon mycotoxinivorans) strain isolated from sputum specimens of a pediatric patient with pneumonia. J Mycol Med. 2019;29(2):120–126.
7. Sadamatsu H, Takahashi K, Tashiro H, et al. A rare case of Trichosporon mycotoxinivorans and Cryptococcus neoformans co-infection in lung. J Infect Chemother. 2020;26(8):838–842.
8. Shah AV, McColley SA, Weil D, Zheng X. Trichosporon mycotoxinivorans infection in patients with cystic fibrosis. J Clin Microbiol. 2014;52(6):2242–2244.
9. Goldenberger D, Hinic V, Prince SS, et al. A case report of a cystic fibrosis patient with repeated isolation of Trichosporon mycotoxinivorans identified by a novel short-extraction method. BMC Infect Dis. 2016;16(1):601.
10. Martinez Muniz Fde B, Martinez Redondo M, Prados Sanchez C, Garcia Rodriguez J. Chronic lung infection caused by Trichosporon mycotoxinivorans and tricosporin mucoides in an immunocompetent cystic fibrosis patient. Arch Bronconeumol. 2016;52(7):400.
11. Do Espirito Santo EPT, Monteiro RC, da Costa ARF, Marques-da-Silva SH. Molecular identification, genotyping, phenotyping, and antifungal susceptibilities of medically important Trichosporon, Apiotrichum, and Cutaneotrichosporon species. Mycopathologia. 2020;185(2):307–317.
12. Kröner C, Kappler M, Grimmelt A-C, Laniado G, Würstl B, Griese M. The basidiomycetous yeast Trichosporon may cause severe lung exacerbation in cystic fibrosis patients – clinical analysis of Trichosporon positive patients in a Munich cohort. BMC Pulm Med. 2013;13(1):1–10.
13. Yon JR, Ravenel JG. Congenital bronchial artery-pulmonary artery fistula in an adult. J Comput Assist Tomogr. 2010;34(3):418–420.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.