Back to Journals » Clinical Ophthalmology » Volume 15
Brolucizumab: Evaluation of Compassionate Use of a Complex Anti-VEGF Therapy
Authors Murray JE, Gold AS, Latiff A, Murray TG
Received 7 October 2021
Accepted for publication 6 December 2021
Published 18 December 2021 Volume 2021:15 Pages 4731—4738
DOI https://doi.org/10.2147/OPTH.S339393
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Scott Fraser
Julianna E Murray, Aaron S Gold, Azeema Latiff, Timothy G Murray
Private Practice, Miami Ocular Oncology & Retina, Miami, FL, USA
Correspondence: Timothy G Murray
Miami Ocular Oncology & Retina, 6705 Red Road Suite 412, Miami, FL, 33143, USA
Email [email protected]
Objective: To report a consecutive series of compassionate, off-label use of intravitreal brolucizumab as a rescue therapy for complex, non-responsive macular edema. This report delineates primary diagnosis, indications for treatment, adverse events, and visual and anatomic outcomes after intravitreal brolucizumab.
Methods: A retrospective review of a consecutive clinical case series of 110 eyes treated with intravitreal brolucizumab between January 1st and March 1st. 2020. All patients were included if they received intravitreal brolucizumab in an off-label delivery and had ongoing macular edema in the setting of prior, multiple intravitreal anti-VEGF and/or intravitreal triamcinolone acetonide. All patients had spectral domain OCT documented before, at the time of, and in serial follow-up after intravitreal brolucizumab.
Results: Ninety-eight of 98 patients had marked decrease in macular edema. Indications for treatment were assigned to the primary etiologic diagnosis leading to the macular edema secondary to radiation retinopathy, complex epiretinal membrane, or complex diabetic retinopathy. In this series, sdOCT central point thickness decreased by an average of 71.5 microns, subretinal fluid resolved, and visual acuity was improved in 40% (greater than two Snellen lines) and stable in 60% (within two Snellen lines). No patient experienced a severe adverse event to specifically include vitritis and/or vasculitis.
Conclusion: In this series, brolucizumab intravitreal injection was associated with significant improvement in macular edema in each diagnostic category. No serious complications to treatment were found in this series. Brolucizumab, though associated with known intraocular inflammation and vasculitis, demonstrated marked benefit in these complex eyes previously unresponsive to aggressive intravitreal pharmacotherapy.
Keywords: brolucizumab, off-label usage, retinal vasculitis, vitreous inflammation, intraocular inflammation, macular edema
Introduction
Brolucizumab, an anti-vascular endothelial growth factor (anti-VEGF) for intravitreal injections (IVI), is the newest drug of its kind to be commercially available in the United States.1–3 This drug, a humanized single-cell chain antibody fragment, constrains VEGF-A attachment to VEGFR1 and VEGFR2, two VEGF receptors. After three monthly loading IVI of both brolucizumab 6mg/0.05mL dosed at 8 or 12 weeks and aflibercept 2mg/0.05mL dosed at 8 weeks, two separate Phase III clinical trials [HAWK (NCT02307682) and HARRIER (NCT02434328)] showed that brolucizumab was non-inferior to the comparative agent in terms of visual acuity for treatment-naïve neovascular age-related macular degeneration (AMD).4–7 Brolucizumab has a potential for increased durability and is the first drug in its class to be authorized for, after three loading doses, a dosing interval range of 8 to 12 weeks.6 This agent was granted approval on October 7, 2019, in the United States (US) by the Food and Drug Administration; following this, it gained approval for use in the European Union (EU) on February 17, 2020, by the European Commission.8
Using HAWK and HARRIER along with additional supportive safety data, provided by a Phase I (NCT01304693) and Phase II study (OSPREY; NCT01796964), brolucizumab underwent a systemic and ocular safety assessment.5 Brolucizumab was administered in various doses and regimens to treat about 1270 treatment-naïve eyes with neovascular AMD in these two studies combined. While the ocular safety analysis resembled that of other approved anti-VEGF drugs for the most part, some unexpected complications arose. These studies reported that 32 of the 730 eyes (4.4%) from the brolucizumab 6mg dose group had intraocular inflammation (IOI) adverse events (AEs); investigators recorded these AEs as per clinical trial procedures.5 Furthermore, six of the 32 eyes that experienced IOI post injection also showed signs of concurrent retinal artery occlusion (ROA) with a mean loss of 22.8 ETDRS letters (range: 18 letters gained to 62 letters lost).5 This complication has not yet been reported with other commercially available anti-VEGF agents. Subsequent to this, both the FDA and European Commission requested further close monitoring of intraocular inflammation as well as further investigations of these events.
In separate studies, clinical cases of retinal vasculitis after brolucizumab 6mg IVI were gathered from retinal specialists across the US.9–14 One real-world, non-consecutive series reported 15 eyes with retinal vasculitis and IOI after brolucizumab IVI. After the first brolucizumab injection, it took as long as 8 weeks (range: 2 to 8 weeks) after for retinal vasculitis, which in this series was typically occlusive and could involve the retinal arteries, veins, and potentially capillaries with a range of severity, to be diagnosed.9–14 These real-world reports reflect the results of the phase III HAWK and HARRIER trial mentioned previously. It was concluded that brolucizumab was generally well received, and as a whole, the AE rates paralleled those with aflibercept within each trial. One discrepancy between the two is that, in HAWK, iritis and uveitis occurred in 2.2% of patients in the brolucizumab 6mg group versus 0% with aflibercept, and in HARRIER, both groups had corresponding rates of <1%. Genetic predisposition may be a factor in this discrepancy. This study concluded that postmarketing safety data collection and risk assessment is vital to evaluate a drug’s risk profile and to come to further resolutions for risk management.
Currently, Brolucizumab is approved only for neovascular AMD.15–22 Recognition of the potential utility in a variety of retinal diseases, including orphan diseases, unresponsive to existing treatments provided the basis of this report. This study evaluated the “off-label” utilization of brolucizumab for “rescue therapy” in eyes showing progression on existing treatment regimens. The use of brolucizumab, in this context, was based on early recognition by the investigators that anatomical response appeared significantly enhanced over existing treatment regimens. Additionally, the evolving understanding of the unique adverse event profile necessitated extensive patient-physician discussion focused on potential benefit to risk for each individual patient.
Methods
This retrospective consecutive case series focused on patients receiving intravitreal brolucizumab injections as rescue therapy for eyes with macular edema. This study was approved by the Institutional Review Board of Larkin Community Hospital, conformed to the Declaration of Helsinki, and adhered to the rules of the Health Insurance Portability and Accountability Act. Inclusion criteria included all patients treated with intravitreal brolucizumab from January to March of 2020 within the outpatient clinic of Miami Ocular Oncology and Retina (MOOR). All patients had been previously treated with intravitreal bevacizumab and/or intravitreal triamcinolone acetonide. Mean number of injections prior to brolucizumab therapy was 15 (9–45) injections. All patients were injected by a single provider (TGM). Exclusion criteria included age younger than 18 years, inability to give informed consent, preexisting nontumoral or treatment-related macular disease, or media opacity precluding view of the tumor and macula. Injections were identified through review of our nursing logbooks and correlated with the patient’s individual Electronic Health Record. Electronic review captured patient demographics, clinical indications of treatment, and sdOCT imaging (Heidelberg Spectralis® OCT – HR scans), with all patients requiring a minimum follow-up of sixty days. Dilated funduscopic examination, sdOCT, BCVA assessment, and macular and tumor imaging were all components of each clinical examination. After treatment options were discussed with patients, each patient received a brolucizumab 6mg IVI following a standardized injection protocol, which includes the use of a sterile speculum, povidone-iodine administration, and topical proparacaine application accompanied with a lidocaine gel application using a cotton swab.
Each radiation maculopathy sdOCT was graded using the modification of the Shields grading scale: grade 1, extrafoveal, non-cystoid edema; grade 2, extrafoveal cystoid edema; grade 3, foveal non-cystoid edema; grade 4 foveal cystoid edema mild to moderate; grade 5, foveal cystoid edema severe; and grade 6, foveal cystoid edema severe with sub-retinal fluid.
Statistical analysis utilized Mann–Whitney U-test for evaluation. Statistical analyses were performed with SAS, Version 9.4 (SAS Institute, North Carolina, USA). Sample size was determined by power analysis. P-values reported as significant if less than 0.05. Analysis focused on pre/post best corrected visual acuity and pre/post sdOCT central point thickness (CPT). Evaluations were performed for the total study, and then subset analysis was performed for each of three major treatment indications.
Results
A total of 110 eyes from 98 patients (52 men, 46 women) were injected with brolucizumab, including 51 Caucasian patients (52%), 42 Hispanic patients (42.9%), 3 African American patients (3.1%), and 2 Asian patients (2%). The mean patient age was 71.4 (median – 73, range: 34–95) (Table 1). No adverse effects were reported at the post-injection follow-ups.
 |
Table 1 Study Population |
Primary treatment indications for macular edema included radiation retinopathy (43 cases, 42.6% of all cases), macular edema secondary to a primary epiretinal membrane (17, 16.8%), and diabetic macular edema secondary to diabetic retinopathy (13, 12.9%) (Table 2). The mean age of patients varied by treatment indications from 69.0 (range: 47–87) for radiation retinopathy, 72.6 (range: 49–92) for diabetic retinopathy, and 75.4 (range: 57–95) for primary epiretinal membrane (Table 3). Post-treatment evaluation was conducted at 6 weeks (range 4–8).
 |
Table 2 Treatment Indication Distribution |
 |
Table 3 Demographics: Major Treatment Indications |
Pre- and post-treatment visual acuity were statistically analyzed in LogMar and presented with Snellen acuity correlation (Table 4). Pretreatment evaluation of the entire study cohort revealed a mean visual acuity of 0.886 (20/153.8) (median – 0.6505 [20/89.4], range: 0.097–2.9 [20/25 – LP]). Post-treatment visual acuity achieved a mean visual acuity of 0.701 (20/100.5) (median – 0.477 [20/60], range: 0–2.9 [20/20 – LP]) (p < 0.05). Subset analysis by primary treatment indication noted a baseline mean visual acuity for primary epiretinal membrane of 0.598 (20/79.2) (median – 0.477 [20/60], range: 0.097–1.097 [20/23-20/250]), diabetic retinopathy of 0.879 (20/151.4) (median – 0.602 [20/80], range: 0.176–2.301 [20/30-1/200]), and radiation retinopathy of 1.153 (20/284.2) (median – 1 [20/200], range: 0.097–2.9 [20/25 – LP]). Post-treatment subset analysis noted a mean visual acuity for primary epiretinal membrane of 0.511 (20/64.9) (median – 0.398 [20/50], range: 0.097–1 [20/25-20/20]) (p < 0.05), diabetic retinopathy of 0.724 (20/105.9) (median – 0.544 [20/70] (p < 0.1), range: 0.097–1.824 [20/25-3/200]), and radiation retinopathy of 0.900 (20/158.8) (median – 0.875 [20/150], range: 0.097–2.9 [20/25 – LP]) (p < 0.02) (Table 5).
 |
Table 4 LOGMAR Visual Acuity for Total Population (Snellen VA Equivalent) |
 |
Table 5 LOGMAR Visual Acuity by Major Treatment Indication (Snellen VA Equivalent) |
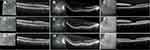
OCT analysis, evaluating CPT, noted a pretreatment baseline mean of 412.2 microns (median – 398, range: 234–701). Post-treatment evaluation at 6 weeks noted a mean of 340.7 (median – 324.5, range: 209–599) (p < 0.03) (Figure 1) (Table 6). Subset analysis by primary treatment indication revealed a pretreatment baseline OCT CPT for radiation retinopathy of 453.7 (median = 412, range = 265–695), diabetic retinopathy of 418.3 (median = 454, range = 279–541), and primary epiretinal membrane of 371.8 (median – 365, range: 234–544). Post-treatment subset analysis revealed OCT CPT for radiation retinopathy of 362.6 (median – 334, range: 209–599) (p < 0.02), diabetic retinopathy of 353.4 (median – 349, range: 231–498) (p < 0.04), and primary epiretinal membrane of 309.9 (median – 296, range: 226–401) (P < 0.05) (Table 7).
 |
Table 6 OCT CST of Total Population |
 |
Table 7 OCT CST of Major Treatment Indications (Microns) |
Discussion
Brolucizumab is the most recently FDA-approved biological agent for neovascular AMD, approved on October 9, 2019.20,22,23 Its advantages were both the duration of action and enhanced ability to decrease retinal and subretinal fluid. The two agents studied in the pivotal stage 3 clinical trials, brolucizumab and aflibercept, had intraocular inflammation rates that were statistically comparable.24,25 In addition, the HAWK and HARRIER approval trials determined that brolucizumab’s visual acuity outcomes were non-inferior to the aflibercept. At the onset of this study, FDA approval was focused on brolucizumab usage in neovascular AMD. This study presents the results for the “off-label” compassionate usage of brolucizumab in patients failing anti-VEGF and/or triamcinolone acetonide intravitreal injection with persistent vision compromising macular edema. Macular edema occurs from many clinical conditions, but in this study, the three main treatment indications for use of brolucizumab as a rescue/salvage therapy were radiation maculopathy, primary epiretinal membrane, and diabetic maculopathy.26 Kulikov et al showed a statistically significant improvement in CPT when utilizing anti-VEGF therapy to treat macular edema secondary to ERM involving the macular center.26 In our study, all patients had failed prior anti-VEGF therapy and/or combination anti-VEGF and intravitreal steroid treatment, utilizing aggressive, repetitive, short-interval re-injection and were offered the compassionate usage of brolucizumab as a secondary salvage therapy. Of note, in this study, brolucizumab was provided without charge to the patient in the setting of informed consent.
Salvage, rescue and/or compassionate use therapy requires unique understandings of risk and benefit and typically applies to non-standardized treatments. These treatments often employ an FDA-approved drug or device that is used “outside” of the approval. Off-label drug usage is, in fact, common in the field of medicine and has played a major role in ophthalmological and oncological patient care. For many patients with unique/rare diseases, atypical presentations of more common diseases, or poor response to treatment, no option exists for the use of an FDA approved drug or device. In 1998, Beck and Azari reported that 85% of all oncology prescriptions were off-label, while up to 60% of comprehensive medical prescriptions fell outside of the FDA approved labelling.15,27–35 They noted that this usage fell under the “Prerogative of the individual physician to apply their best professional judgment in the individual treatment of each unique patient”. This highlighted the unique application of personalized, patient-specific care. Further, the CDER (Center for Drug Evaluation and Research) addressed this issue directly when it noted that,
Neither the FDA nor the Federal Government regulate the practice of medicine. Any approved product may be used by a licensed practitioner for uses other than those stated in the product label.
Fortunately for our patients, this enables the clinician to use broad information sources to deliver informed, individualized care, even in the absence of approved therapeutics. In Richardson v. Miller, Tenn. Ct. App. 2000, it was noted that
Because the pace of medical discovery runs ahead of the FDA’s regulatory machinery, the off-label use of some drugs is frequently considered to be “state-of-the-art” treatment. In some instances, an off-label use of a particular drug or device may even define the standard of care.
In no field has this been more obvious than in retina specialty care within the field of ophthalmology. For ROP, the use of intravitreal bevacizumab as first a rescue therapy, and then as “standard of care” has redefined primary ROP treatment and prevented blindness in thousands of children worldwide.
Important ethical implications always accompany the use of off-label therapies.36 While the Food and Drug Association (FDA) restricts commercial promotion of off label drugs, it does not restrict a physician’s ability to prescribe them (Hackett on-label and off-label) Many large institutions provide guidance for the process of off label prescribing that includes informing the patient on all risks, documenting the indication for off label use, and receiving and recording informed consent (J Simon off label med. u). Many physicians believe that off-label treatments should not be promoted to consumers and that the FDA approval process protects the public from harmful or ineffective drugs (pdf physicians perspective approval). While the latter is true, off-label therapies often provide the best, and potentially only, option, especially in certain rescue therapeutic treatments. Instead of limiting promotion or use of off label therapies, a further emphasis must be placed on assessing appropriate use of off label treatments since off-label indications include different safety profiles than that of the indication originally approved by the FDA (J Simon off label med. Hackett on-label and off-label). Additionally, while increased adverse drug events may be associated with the off-label use of prescription drugs lacking strong scientific backing, the same is not the case for those with sufficient scientific evidence (in defense of response). If an off-label use of a prescription drug has strong scientific evidence, it may have the same, more, or less risk of complications as on label therapy.
During this study, an enhanced awareness of potentially novel complications was noted in the postmarketing surveillance of brolucizumab. The ASRS ReST committee received reports of potentially severe visual loss associated with brolucizumab usage. The ReST committee characterized these events as retinal vasculitis and retinal vascular occlusion in the setting of intraocular inflammation. The evolving focus of the retinal specialty community, focused on these events, significantly curtailed the use of brolucizumab. Of interest, ongoing clinical trials were continued in the setting of a significantly revised informed consent. With this enhanced information, patients in our review were only offered brolucizumab, in an off-label application, after extensive informed consent. This informed consent included a clear discussion of the increased risk of retinal vasculitis and vascular occlusion. Within our practice, we felt ethically compelled to limit access to patients who had failed all prior therapies and were at risk for blinding disease progression. This focus on benefit-to-risk assessment coupled with informed consent enabled both the treating retina specialist and the patient to understand the decision to treat with brolucizumab.
This recognition of risk to benefit is integral in virtually all treatment paradigms irrespective of on-label or off-label use of a drug or device. In diseases where no treatment, or ineffective treatment, may lead to unacceptable outcomes such as death (oncology) or blindness (ophthalmology) the risk to benefit may shift FDA approved but off-label therapies into acceptable treatment strategies for an individual patient. In fact, this is exactly what drives much of the treatment decisions in rare diseases. For the patient series reported here, every treated patient had shown progressive macular edema in spite of repetitive, short interval (q 2 to 3 week), combined anti-VEGF and triamcinolone acetonide treatment. For these patients, historical visual and anatomic outcomes have been poor typically leading to severe visual limitation and even globe loss. Each treated patient, though acknowledging the uncertainty of treatment, was desirous of a treatment option that could lead to improvement or stabilization of both vision and anatomy.
Interestingly, in this review of all 110 off-label brolucizumab treated eyes, no patient experienced visual loss with mean visual acuity improving from 20/153 to 20/89, while sdOCT improved from 412 microns to 340 microns. Statistically significant improvements were seen for both best visual acuity and sdOCT CPT for the entire study cohort and for each of the three major disease categories for treatment. The absence of severe adverse events in this population is important but does not detract from the concerns for both retinal vasculitis and occlusive vasculitis associated with brolucizumab. From a treatment perspective, all patients had received anti-VEGF therapy prior to brolucizumab, and most patients had also been treated with triamcinolone acetonide. Though prior treatment does not guarantee the avoidance of intraocular inflammation, it may reduce the risk for the first injection of brolucizumab. Further, patients were treated with primary underlying conditions that were excluded from the FDA approval. These excluded groups may also have a lower risk suggesting that either neovascular AMD or co-factors such as age may be associated with severe adverse events in the use of this drug. Recent conclusion of the KITE and KESTREL studies of brolucizumab for DME have documented retinal vasculitis and vascular occlusion suggesting that, at least for neovascular AMD and DME, these severe adverse events do occur.37
Conclusion
It must be recognized that the use of advanced biologics targeting enhanced efficacy may come at the cost of a greater complication profile. In the field of oncology, advanced biologics, including check-point inhibitors and immunomodulators, have been associated with never before seen retinal complications. In this setting, where patients are experiencing life-threatening malignancy, the use, even with these potential complications, of these advanced biologics has been pivotal to enhanced patient’s survival. These advanced biologics define the importance of risk to benefit and set the stage for our ophthalmological understanding of the use of agents with enhanced benefit but associated with greater risk. When the serious adverse events associated with brolucizumab were first recognized, many in the retina specialty community felt that this drug should never be used. For patients with unrelenting disease, intractable to existing therapies, novel treatments, even with greater risk, provide an opportunity to save both vision and anatomy. For these patients, brolucizumab along with other, developing advanced biologics may be the “rescue” that these patients need even in the face of “risk”.
Precis
Brolucizumab has been shown to significantly reduce macular edema but is also associated with a complex vasculitis. Utilization of this novel anti-VEGF shows significant promise in eyes with macular edema unresponsive to prior intravitreal therapy.
Disclosure
Timothy G. Murray is part of the FDA advisory board, a consultant for Alcon Surgical, and reports grants from Regeneron. Aaron S Gold reports grants from Regeneron. The authors report no other conflicts of interest in this work.
References
1. Tadayoni R, Sararols L, Weissgerber G, Verma R, Clemens A, Holz FG. Brolucizumab: a newly developed Anti-VEGF molecule for the treatment of neovascular age-related macular degeneration. Ophthalmologica. 2020;244:93–101. doi:10.1159/000513048
2. Patel NA, Berrocal AM, Murray TG, Villegas VM. Advanced Coats’ disease treated with intravitreal brolucizumab combined with laser photocoagulation. Am J Ophthalmol Case Rep. 2020;19:eCollection. doi:10.1016/j.ajoc.2020.100815
3. Rispoli M, Eandi CM, Di Antonio L, Kilian R, Montesel A, Savastano MC. Biomarkers in early response to brolucizumab on pigment epithelium detachment associated with exudative age-related macular degeneration. Biomedicines. 2021;9(6):668. doi:10.3390/biomedicines9060668
4. Yu JS, Carlton R, Agashivala N, Hassan T, Wykoff CC. Brolucizumab vs aflibercept and ranibizumab for neovascular age-related macular degeneration: a cost-effectiveness analysis. J Manag Care Spec Pharm. 2021;27(6):743–752. doi:10.18553/jmcp.2021.27.6.743
5. Singer M, Albini TA, Seres A, et al. Clinical characteristics and outcomes of eyes with intraocular inflammation after brolucizumab: post hoc analysis of HAWK and HARRIER. Ophthalmol Retina. 2021. doi:10.1016/j.oret.2021.05.003
6. Ogura Y, Jaffe GJ, Cheung CMG, et al. Efficacy and safety of brolucizumab versus aflibercept in eyes with polypoidal choroidal vasculopathy in Japanese participants of HAWK. Br J Ophthalmol. 2021. doi:10.1136/bjophthalmol-2021-319090
7. Sacconi R, Giuffrè C, Corbelli E, Borrelli E, Querques G, Bandello F. Emerging therapies in the management of macular edema: a review. F1000Research. 2019;8:1413. doi:10.12688/f1000research.19198.1
8. European Medicines Agency: EMA/MB/69923/2010 – annual report of the European Medicines Agency; 2009. Available from: https://www.ema.europa.eu/en/documents/annual-report/annual-report-european-medicines-agency-2009_en.pdf.
9. Nguyen QD, Das A, Do DV, et al. Brolucizumab: evolution through preclinical and clinical studies and the implications for the management of neovascular age-related macular degeneration. Ophthalmology. 2020;127(7):963–976. doi:10.1016/j.ophtha.2019.12.031
10. Baumal CR, Bodaghi B, Singer M, et al. Expert opinion on management of intraocular inflammation, retinal vasculitis, and vascular occlusion after brolucizumab treatment. Ophthalmol Retina. 2021;5(6):519–527. doi:10.1016/j.oret.2020.09.020
11. Cox JT, Eliott D, Sobrin L. Inflammatory complications of intravitreal anti-VEGF injections. J Clin Med. 2021;10(5):981. doi:10.3390/jcm10050981
12. Anderson WJ, da Cruz NFS, Lima LH, Emerson GG, Rodrigues EB, Melo GB. Mechanisms of sterile inflammation after intravitreal injection of antiangiogenic drugs: a narrative review. Int J Retina Vitreous. 2021;7(1):37. doi:10.1186/s40942-021-00307-7
13. Dugel PU, Singh RP, Koh A, et al. HAWK and HARRIER: ninety-six-week outcomes from the Phase 3 trials of brolucizumab for neovascular age-related macular degeneration. Ophthalmology. 2021;128(1):89–99. doi:10.1016/j.ophtha.2020.06.028
14. Witkin AJ, Hahn P, Murray TG, et al. Occlusive retinal vasculitis following intravitreal brolucizumab. J Vitreoretin Dis. 2020;4(4):269–279. doi:10.1177/2474126420930863
15. Bell JS, Richards GC. Off-label medicine use: ethics, practice and future directions. Aust J Gen Pract. 2021;50(5):329–331. doi:10.31128/AJGP-08-20-5591
16. Bilgic A, Kodjikian L, March de Ribot F, et al. Real-world experience with brolucizumab in wet age-related macular degeneration: the REBA Study. J Clin Med. 2021;10(13):2758. doi:10.3390/jcm10132758
17. Monés J, Srivastava SK, Jaffe GJ, et al. Risk of inflammation, retinal vasculitis, and retinal occlusion-related events with brolucizumab: post hoc review of HAWK and HARRIER. Ophthalmology. 2021;128(7):1050–1059. doi:10.1016/j.ophtha.2020.11.011
18. Dugel PU, Koh A, Ogura Y, et al. HAWK and HARRIER: phase 3, multicenter, randomized, double-masked trials of brolucizumab for neovascular age-related macular degeneration. Ophthalmology. 2020;127(1):72–84. doi:10.1016/j.ophtha.2019.04.017
19. Baumal CR, Spaide RF, Vajzovic L, et al. Retinal vasculitis and intraocular inflammation after intravitreal injection of brolucizumab. Ophthalmology. 2020;127(10):1345–1359. doi:10.1016/j.ophtha.2020.04.017
20. Agostini H, Mulyukov Z, Tsilimbaris M, et al. Comparison of the efficacy of brolucizumab with natural disease progression in wet AMD using clinical data from the Phase III HAWK and HARRIER trials and modelled placebo data. Curr Eye Res. 2020;45(10):1298–1301. doi:10.1080/02713683.2020.1731832
21. Iyer PG, Albini TA. Drug-related adverse effects of antivascular endothelial growth factor agents. Curr Opin Ophthalmol. 2021;32(3):191–197.
22. Enríquez AB, Baumal CR, Crane AM, et al. Early experience with brolucizumab treatment of neovascular age-related macular degeneration. JAMA Ophthalmol. 2021;139(4):441–448. doi:10.1001/jamaophthalmol.2020.7085
23. Bulirsch LM, Saßmannshausen M, Nadal J, Liegl R, Thiele S, Holz FG. Short-term real-world outcomes following intravitreal brolucizumab for neovascular AMD: SHIFT. Br J Ophthalmol. 2021. doi:10.1136/bjophthalmol-2020-318672
24. Haensli C, Pfister IB, Garweg JG. Switching to brolucizumab in neovascular age-related macular degeneration incompletely responsive to ranibizumab or aflibercept: real-life 6 month outcomes. J Clin Med. 2021;10(12):2666. doi:10.3390/jcm10122666
25. Dugel PU, Jaffe GJ, Sallstig P, et al. Brolucizumab versus aflibercept in participants with neovascular age-related macular degeneration: a randomized trial. Ophthalmology. 2017;124(9):1296–1304. doi:10.1016/j.ophtha.2017.03.057
26. Kulikov AN, Sosnovskii SV, Berezin R, et al. Vitreoretinal interface abnormalities in diabetic macular edema and effectiveness of anti-VEGF therapy: an optical coherence tomography study. Clin Ophthalmol. 2017;11:1995–2002. doi:10.2147/OPTH.S146019
27. Ashkenazy N, Henry CR, Abbey AM, McKeown CA, Berrocal AM, Murray TG. Successful treatment of juvenile xanthogranuloma using bevacizumab. J AAPOS. 2014;18(3):295–297. doi:10.1016/j.jaapos.2014.01.007
28. Sisk RA, Berrocal AM, Albini TA, Murray TG. Bevacizumab for the treatment of pediatric retinal and choroidal diseases. Ophthalmic Surg Lasers Imaging. 2010;41(6):582–592. doi:10.3928/15428877-20100830-03
29. Graversen VK, Hamichi SE, Gold A, Murray TG. History through the eyes of a pandemic. Curr Opin Ophthalmol. 2020;31(6):538–548. doi:10.1097/ICU.0000000000000711
30. Eguale T, Buckeridge DL, Verma A, et al. Association of off-label drug use and adverse drug events in an adult population. JAMA Intern Med. 2016;176(1):55–63. doi:10.1001/jamainternmed.2015.6058
31. Eguale T, Verma A, Tamblyn R. In defense of off-label prescribing-reply. JAMA Intern Med. 2016;176(6):861–862. doi:10.1001/jamainternmed.2016.1406
32. Gazarian M, Kelly M, McPhee JR, Graudins LV, Ward RL, Campbell TJ. Off-label use of medicines: consensus recommendations for evaluating appropriateness. Med J Aust. 2006;185(10):544–548. doi:10.5694/j.1326-5377.2006.tb00689.x
33. Murray TG, Berrocal AM. Ethics in the practice of retina. Retin Physician. 2019;54:67–78.
34. Radley DC, Finkelstein SN, Stafford RS. Off-label prescribing among office-based physicians. Arch Intern Med. 2006;166(9):1021–1026. doi:10.1001/archinte.166.9.1021
35. Gopal AD, Wallach JD, Shah SA, Regillo C, Ross JS. On-label and off-label clinical studies of FDA-approved ophthalmic therapeutics. Ophthalmology. 2021;128(2):332–334. doi:10.1016/j.ophtha.2020.07.028
36. Borysowski J, Ehni HJ, Górski A. Ethics codes and use of new and innovative drugs. Br J Clin Pharmacol. 2019;85(3):501–507. doi:10.1111/bcp.13833
37. Brown DM, Wolf S, Garweg J, et al. Brolucizumab for the treatment of visual impairment due to diabetic macular edema: 52-week results from the KITE and KESTREL studies. Invest Ophthalmol Vis Sci. 2021;62(8):1045.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.