Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 12
Brodalumab for the treatment of moderate-to-severe psoriasis: case series and literature review
Authors Pinter A, Bonnekoh B, Hadshiew IM, Zimmer S
Received 11 April 2019
Accepted for publication 31 May 2019
Published 10 July 2019 Volume 2019:12 Pages 509—517
DOI https://doi.org/10.2147/CCID.S211938
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jeffrey Weinberg
Andreas Pinter,1 Bernd Bonnekoh,2 Ina Marion Hadshiew,3 Sebastian Zimmer4
1Department of Dermatology, Venereology, and Allergology, University Hospital Frankfurt am Main, Frankfurt am Main, Germany; 2Clinic for Dermatology, Otto-von-Guericke-University Hospital, Magdeburg, Germany; 3Derma Köln am Heilig-Geist-Krankenhaus, Köln, Germany; 4MediCorium, Oberursel, Germany
Abstract: Brodalumab, a recombinant fully human monoclonal immunoglobulin IgG2 antibody with high affinity to human interleukin (IL)-17RA, is approved for the treatment of moderate-to-severe plaque psoriasis. In controlled clinical trials, brodalumab 210 mg administered by subcutaneous injection at weeks 0, 1, and 2, then 210 mg every 2 weeks, produced a rapid onset and sustained clinical response. Consistently, >80% of patients achieved PASI-75 and efficacy was maintained for >2 years. The benefits are apparent soon after the start of therapy and are maintained in the long term. Such results, from the reviewed literature, support the findings from 4 ’real world’ cases in mainstream clinical practice which are reported here. Psoriatic plaques, including on the scalp, nails, soles and palms, were largely resolved, and quality of life improved markedly. Therapeutic success was achieved in patients naïve to biologics (2 cases) and in those responding inadequately to other biologics (2 cases). The high affinity of brodalumab to human IL-17RA blocks the biological activities of the pro-inflammatory cytokines IL-17A, IL-17C, IL-17E, IL-17F, and IL-17A/F heterodimer, resulting in inhibition of the inflammation and clinical symptoms associated with psoriasis. This mechanism of blocking multiple IL-17 family cytokines differs from that of other available biologics which selectively target some parts of the Th-17 axis and may account for the effectiveness of brodalumab in patients poorly responsive to other biologics, a feature which has also been shown where subgroup analysis has been undertaken in clinical trials. The drug is well tolerated during the normal 12-week induction phase and with prolonged treatment (52 to 120 weeks), as it was in the current case series.
Keywords: brodalumab, psoriasis, psoriatic arthritis, interleukin 17 axis, IL-17
Introduction
Psoriasis is a common condition, with a significant influence on physical and psychological well-being, and consequential economic impacts through days off work and health care costs. Lesions can result in itching, burning, aching and bleeding,1 as well as being unsightly and affecting quality of life and general well-being. The reported prevalence of psoriasis varies widely between countries but is generally between 1.5 and 5% in developed countries.2–4
The etiology of psoriasis is complex. For example, psoriasis can be triggered by trauma, obesity, infection, stress and medications, and it can affect any area of the skin, including the scalp, nails, palms and soles.1 There are central roles played by both the innate and adaptive immune systems.2,5 Alteration of the interleukin (IL)-23/type 17 helper T cells (Th17), IL-17A/F axis appears to be a key etiologic factor.6 IL-23, released by dendritic cells in response to an internal or external stimulus, such as an antigenic challenge, is involved in the activation and maintenance of Th17 cells.7 In turn, these cells release a swathe of cytokines, including IL-17A, IL-17F, IL-21, IL-22, and tumor necrosis factor (TNF)-α. The IL-17 family of cytokines bind to the IL-17 group of receptors with different affinities and consequences. Activation of the receptors, among other things, results in chemokine and antimicrobial peptide production which enhances the inflammatory cascade.2,5 There are five IL-17 cell surface receptors: RA to RE. IL-17RA forms heterodimers with each of IL-17RC, IL-17RB and IL-17RE.6,8 The complexity of the etiology explains the diversity of symptoms and variable influence of environmental factors in psoriatic disease.2
Levels of IL-17, and particularly IL-17A, −17C and −17E (also known as IL-25), are elevated in the blood and psoriatic lesions of patients with psoriasis, and the pro-inflammatory effects of IL-17A contribute to disruption of the skin barrier function.5,9–11 Thus, the Th17 axis plays a pivotal role in psoriatic disease.2,3,6,7
Brodalumab is a recombinant fully human immunoglobulin G2 monoclonal antibody which received FDA approval in February 2017 and EMA approval in September 2017. In the EU, brodalumab is indicated for the treatment of moderate-to-severe plaque psoriasis in adult patients who are candidates for systemic therapy. The antibody binds with high affinity to human IL-17RA and blocks the biological activities of the pro-inflammatory cytokines IL-17A, IL-17F, IL-17A/F heterodimer, IL-17C, and IL-17E, resulting in inhibition of the inflammation and clinical symptoms associated with psoriasis. IL-17RA is a protein expressed on the cell surface and is a required component of receptor complexes utilized by multiple IL-17 family cytokines. IL-17 family cytokine concentrations have been reported to be increased in psoriasis. IL-17A, IL-17F and IL-17A/F heterodimer have pleiotropic activities, including the induction of pro-inflammatory mediators such as IL-6, GROα, and G-CSF from epithelial cells, endothelial cells and fibroblasts that promote tissue inflammation. Blocking IL-17RA inhibits IL-17 cytokine-induced responses resulting in normalization of inflammation in the skin.12 This mechanism is different from that of other available biologics targeting the Th-17 axis (eg, secukinumab and ixekizumab each bind selectively to IL-17A).5,8
Meta analyses of controlled clinical trials of biologics for the treatment of moderate-to-severe psoriasis show that they are more effective than small molecules and conventional treatments, at least during the induction phase. However, there has been criticism that these trials are not necessarily representative of patients seen in daily clinical practice.13 The reporting of case studies from real-world clinical practice, such as those presented here, can help to address this criticism.
Case studies
All patients provided written informed consent to receive brodalumab and for their details and images to be published as a case study, subject to non-identification. Ethics committee or institutional review board approval was not necessary for the individual cases reported in this series because each case reflects a retrospective description of clinical findings. Except for one patient (Case 4) who had experienced vitiligo since childhood, no other concomitant disease or treatment was recorded.
Case 1
This patient was a 24-year-old female with a history of psoriasis since 2004. Her leading indication was plaque psoriasis. Nail involvement included discoloration of the nail on the right big toe. Scalp psoriasis was present, with a static Physicians Global Assessment (sPGA) score of 4 (sPGA measures psoriasis severity at a single point in time on a 6-point scale, ranging from 0 [no psoriasis] to 5 [very severe psoriasis]). There was no psoriatic arthritis.
Previous treatments had included cyclosporine for 1 year in 2015, fumaric acid esters (Fumaderm®) in 2016 and methotrexate for a few months (both stopped for abdominal pain), UV-therapy, calcipotriol and betamethasone (Daivobet®) and 1% hydrocortisone cream.
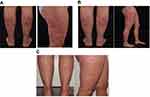
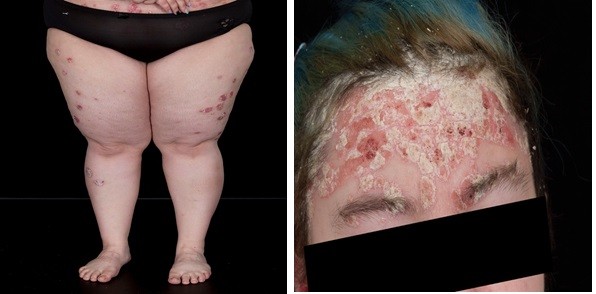
The patient presented with a Psoriasis Area Severity Index (PASI) score of 11.3. There were lesions on the trunk, arms, legs and especially the head (Figure 1A). The Body Surface Area (BSA) coverage was 14% and the Dermatology Life Quality Index (DLQI) score was 21.
 |
Figure 1 Case 1: psoriatic lesions on the legs and forehead (A) before and (B) after 14 months of brodalumab treatment. |
Treatment was initiated in September 2017 with brodalumab 210 mg at Week 0, Week 1, Week 2 and then every 2 weeks. 1% hydrocortisone cream was used to relieve itching (genital area).
By weeks 12–16, the psoriatic lesions had disappeared, and the patient was very satisfied with the therapy. By August 2018, after almost 1 year of treatment, the PASI score, DLQI score (Figure 2A) and BSA coverage were all zero, and these outcomes were maintained at the last follow-up (14 months after starting brodalumab; Figure 1B).
 |
Figure 2 Psoriasis Area Severity Index (PASI) and Dermatology Life Quality Index (DLQI) scores before and after treatment with brodalumab. (A) Case 1, (B) Case 2, (C) Case 3, (D) Case 4. |
Case 2
This 49-year-old female patient had experienced plaque psoriasis since the age of 20 years (no family history) and an additional diagnosis of disabling psoriatic arthritis since the age of 28 years. At the time of the current investigation, the patient presented with mild scalp psoriasis and psoriatic arthritis (mainly arms and legs; Figure 3A), and a PASI score of 11.9. The BSA was 17%, DLQI score was 19, and she had dactylitis on the left hand. These symptoms presented whilst undergoing treatment for plaque psoriasis (the leading indication) with ixekizumab 80 mg every 2 weeks for 12 weeks, then monthly, between April and December 2017 (this therapy was discontinued due to lack of efficacy and a flare-up of the psoriatic arthritis with dactylitis on the left hand).
The patient had a history of poor response to treatment. In 1995, fumaric acid was started and discontinued for secondary loss of efficacy. Between 2004 and 2006, she received methotrexate orally and then subcutaneously, up to 25 mg/week, until it was discontinued due to primary loss of efficacy. Subcutaneous adalimumab injections were administered for an extended period of time, from 2007 to January 2016. They were discontinued due to secondary loss of efficacy, despite the addition of methotrexate 7.5 mg subcutaneously and an increase in adalimumab dosage by switching to weekly injections. She participated in a clinical trial with secukinumab from May to October 2016. Any clinical benefit was lost after the induction phase of secukinumab 300 mg weekly for 4 weeks, and there was no improvement during the follow-up period when secukinumab was increased to 450 mg and subsequently to 600 mg monthly. Topical calcipotriol + betamethasone as a foam spray (Enstilar®) was added, but secukinumab was discontinued in March 2017, and ixekizumab treatment was started in April 2017.
Treatment with brodalumab 210 mg/week for 3 weeks and then every other week, began in December 2017, with no concurrent therapies. Psoriasis was markedly improved after 6 weeks (Figure 3B). By September 2018, the PASI score was reduced by over 90% to 0.8 (Figures 2B and 3C), BSA coverage was only 1%, and the DLQI score was zero (Figure 2B).
Case 3
This male patient, aged 61 years, had experienced plaque psoriasis for 13 years and concomitant psoriatic arthritis for another 10 years. On examination, the patient presented with mild plaques on the scalp, elbows, lower back and lower legs and severe nail involvement on both hands (Figure 4A) and feet. He had been diagnosed with psoriatic arthritis on his elbows (Figure 4A) and hips.
 |
Figure 4 Case 3: psoriasis on the left elbow and nails (A) before and (B) after 16 weeks on brodalumab, and on the nails (C) after 32 weeks on brodalumab. |
Previous systemic treatments included etanercept 50 mg weekly in combination with subcutaneous methotrexate 10 mg weekly and folic acid 5 mg weekly; prior topical therapy included betamethasone ointments, calcipotriol ointments and a foam containing clobetasol propionate for the scalp. During a 17-month treatment period, he achieved only a partial clinical response; therefore, in July 2016, this therapy was discontinued. The patient’s condition deteriorated continuously after January 2018, but the patient did not want to have multiple weekly injections. At this point, BSA coverage was 5%, the PASI score was 5, and the DLQI score was 20.
Brodalumab treatment was initiated according to schedule, with a dose of 210 mg once a week for 3 weeks followed by injections at 2-week intervals. The first two doses were administered at the clinic with subsequent doses being self-administered (after training). Betamethasone ointments were used once daily, and betamethasone liquid was applied to the scalp once a week.
There was rapid regression of plaques during the first 2 weeks. The patient was happy, and he had no more joint pains. After 16 weeks, his skin was free of plaques and elbow swelling was markedly reduced (Figure 4B); also, his previously severely-affected nails started to grow normally from the proximal end (Figure 4B), and the PASI score was 1. At 24 weeks, there were no more plaques discernible, the scalp was disease-free, and nails were growing slowly with minimal residual dystrophy at the distal end. By 32 weeks, the patient’s nails were completely normal again (Figure 4C), and he was very satisfied with the treatment outcome. At that time, the PASI score and BSA coverage were zero, and the DLQI score was 1 (Figure 2C). The patient had no more itching and no joint pain either in his hands, elbows or hips. Previously, the patient’s nails had significantly impacted his everyday life, both at work and socially. After brodalumab treatment, the patient claimed to have a new life, not stigmatized by the look of his nails, with all functionality back and no tenderness upon touching things. He continues with brodalumab injections but no longer needs any topical treatment.
Case 4
This 33-year-old male patient experienced severe plaque psoriasis involving the whole body including the scalp, palms, soles and nails. Psoriasis first occurred at the age of 25 years, and there was no evidence of psoriatic arthritis. The patient had also been affected by vitiligo since childhood.
Previously, the patient had been treated with different topical medications, including corticosteroids and vitamin D derivatives, as well as with acitretin, methotrexate, fumarates, and apremilast. Limited efficacy was achieved, and when the patient began treatment with brodalumab, in January 2018, he still experienced severe psoriasis, with a PASI score 25, a DLQI of 26 and complained of a severe itch.
Brodalumab was administered at the recommended dosage of 210 mg/week for three weeks and then every second week, and the patient continued using a mild topical corticosteroid. Within 5 weeks, there was significant improvement (Figure 5A and B) and the PASI score had been reduced to 7.4. By week 20, the patient’s skin condition had improved even further, and he no longer required any topical treatment (Figure 5C).
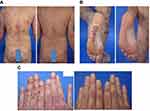 |
Figure 5 Case 4: psoriatic lesions on (A) the back and (B) the soles of the feet before and after 5 weeks on brodalumab, and (C) on the hands before and after 20 weeks on brodalumab. |
The last visit was in mid-November 2018 (Week 44) when the patient’s PASI score was 1.9 and the DLQI score was zero (Figure 2D).
Discussion
In early dose-finding studies with brodalumab in moderate-to-severe plaque psoriasis for a 12-week induction phase, optimal responses occurred at a dose of 210 mg by subcutaneous injection at weeks 0, 1, and 2, then every 2 weeks.14 All patients in the current series of cases received brodalumab at this recommended dosage.
All four patients improved rapidly (within 2 to 6 weeks) with significant improvement or complete clearance of psoriatic lesions. This response was further improved or remained stable at follow-up visits over 9 to 14 months. In the two cases with psoriatic arthritis, joint pain also disappeared. Notably, brodalumab was effective for severe and extensive psoriatic arthritis resistant to other treatments, and in difficult-to-manage lesions involving scalp, nails, palms of the hands and soles of the feet. Patients commented on their high level of satisfaction with brodalumab treatment, and DLQI scores were reduced from 19–26 to 0 in three cases, and to 1 in the other case.
The clinical efficacy and safety of brodalumab have been demonstrated in the AMAGINE controlled clinical trials.15,16 AMAGINE-2 and −3 were replicate phase 3, international, multicenter, randomized, double-blind, head-to-head trials of brodalumab versus ustekinumab and placebo in patients with moderate-to-severe psoriasis (sPGA ≥3, PASI score ≥12 and >10% BSA).16 Over 1800 patients were randomized in each trial and treated with either brodalumab 140 mg or 210 mg subcutaneously, weekly for 3 weeks then at 2-week intervals, ustekinumab 45 or 90 mg (according to body weight) subcutaneously according to label, or placebo. The 12-week treatment period (induction) was followed by a 40-week open-label period during which patients who had been on placebo and those inadequately responding to ustekinumab, received brodalumab 210 mg every 2 weeks.16 After the induction phase, brodalumab was statistically (P<0.001) superior to placebo with respect to PASI-75 and sPGA. It was also superior to ustekinumab (P<0.001) with respect to PASI-100 (44% vs 22% in one study and 37% vs 19% in the other). PASI-75 was achieved in 86% and 85% of patients receiving brodalumab (210 mg dose) in the two separate studies. According to pooled long-term data from AMAGINE-2 and −3, >60% of patients receiving any dose of brodalumab for >2 years (108 weeks) achieved PASI 100.17 A further pooled analysis of AMAGINE-2 and −3 data showed that, in patients who received brodalumab 210 mg every 2 weeks for 52 weeks, PASI 100 was achieved in 63.4% and 62.9% of patients at 52 and 108 weeks, respectively.18 In patients who received ustekinumab for 52 weeks, PASI 100 was achieved in 42.3% of patients, but after switching to brodalumab 210 mg every 2 weeks at week 52, PASI 100 increased to 62.5% at week 108.18
The AMAGINE-1 study followed a similar design to the other AMAGINE studies during a 12-week induction phase but without the ustekinumab treatment group. 661 patients with moderate-to-severe plaque psoriasis were included. Brodalumab (210 mg dose) resulted in a PASI-75 in 83% of patients and an sPGA score of 0 or 1 (classified as ‘success’) in 76% of patients.15
Among the collective AMAGINE trial populations, the psoriasis symptom inventory (PSI) total score and itch score improvements were significantly greater with brodalumab than with placebo (P<0.001) from week 2 to week 12. Compared with ustekinumab, the brodalumab 210 mg dose led to a faster response and a superior improvement of itching at 52 weeks.19 Among the cases reported in this paper, the first patient who had extensive plaque psoriasis, including his scalp, achieved complete resolution of plaques within 12–16 weeks which was maintained through the latest follow-up (14 months after starting brodalumab). Case 4, with severe and extensive psoriasis, had substantial improvement within 5 weeks.
The use of brodalumab in patients with psoriatic arthritis is less well investigated and not in the current European label. In a randomized, double-blind, placebo-controlled trial in 159 patients with psoriatic arthritis, significantly more patients (P=0.05) achieved 50% improvement according to American College of Rheumatology response criteria (ACR50) with brodalumab 140 or 280 mg (standard treatment protocol) compared with placebo, at 12 weeks. During a 40-week open-label phase, when all patients received the 280 mg dose, there were significant improvements in those who had previously received placebo or the 140 mg dosage regimen and sustained benefits in those continuing with brodalumab 280 mg.20
These continuous improvements with brodalumab, as demonstrated in the current case reports over 9–14 months, confirm the positive results of earlier trials. In an open-label study, 144 patients with moderate-to-severe psoriasis completed 120 weeks of treatment with brodalumab 140 or 210 mg every 2 weeks. Response rates achieved at week 12 were maintained at week 120.21 In the AMAGINE-2 and −3 studies, the response rate to brodalumab was stable from week 16 to week 52.16
In a subgroup analysis of data from a controlled trial in patients with moderate-to-severe plaque psoriasis in which brodalumab was administered for 12 weeks, patients’ history or prior treatment with biologics had no influence on brodalumab efficacy.22 Similarly, the response rate among patients with psoriatic arthritis treated for 12+40 weeks was not influenced by previous treatment with other biologics.20 In the AMAGINE-2 and −3 studies, most patients who did not respond to ustekinumab had significantly improved symptoms with brodalumab treatment (PASI-75 and sPGA reduced to 0 or 1).16 Two of the four cases reported here (Cases 2 and 3) responded well to brodalumab after having inadequate responses to other biologic treatments: etanercept (anti-TNF-alpha) in one case, and adalimumab (anti-TNF-alpha), ixekizumab and secukinumab (IL-17 antagonists) in the other. The latter case demonstrates distinctly the different mechanism of action for brodalumab compared with other drugs targeting the Th-17 axis. Brodalumab binds selectively to the IL-17RA receptor rather than just binding to IL-17A.8 Brodalumab was equally effective in the two biologics-naïve patients in this case series.
In a meta-analysis of controlled clinical trials involving the treatment of moderate-to-severe psoriasis, comparing drug classes, biologics were considered the best choice for achieving PASI-90. Anti-IL-17, anti-IL-12/23, anti-IL-23 and anti-TNF-alpha antibodies were significantly more effective than small molecules and conventional systemic therapies.13
The National Institute for Health and Care Excellence in the UK commissioned an independent review of the clinical and cost effectiveness of brodalumab for the treatment of moderate-to-severe plaque psoriasis, concluding that the drug appears to be as effective as other anti-IL-17 agents and is cost effective (based on the discount agreed in the patient access scheme). In particular, brodalumab is recommended for patients with severe plaque psoriasis who have not responded to systemic non-biologic therapies.23 The self-administration of brodalumab by subcutaneous injection every 2 weeks provides for great convenience and enhances patient adherence to therapy. This was particularly true for patient 3 who expressed that previous treatment regimens with multiple weekly injections were intolerable.
No adverse reactions were reported during brodalumab therapy by the four patients in our study. The FDA approval of brodalumab was issued with a warning relating to suicidal ideation and behavior (SIB). A higher incidence of SIB was noted in clinical trials with brodalumab;8 however, although there is a known association of increased suicide among patients with psoriasis compared to those without,5,24 patients with depression or anxiety were not excluded in the AMAGINE trials (in contrast to other trials), and no causal link between brodalumab and increased risk of SIB has been established.25–27 The most common side effects reported with brodalumab in controlled clinical trials have been nasopharyngitis, upper respiratory tract infections, headache and arthralgia.6,16,21,28 The rate of serious infection episodes in the AMAGINE-2 and −3 trials, respectively, were 1.0 and 1.3 per 100 patient years with brodalumab.16 In phase 3 trials, fewer than 2% of patients receiving brodalumab 140 or 210 mg for 12 weeks experienced neutropenia or candida, and none required treatment discontinuation.6
In a meta-analysis of randomized, controlled clinical trials, the incidence of infections and serious adverse events was not significantly different for patients treated with Th17 pathway inhibitors or placebo.3 The safety profile of brodalumab for moderate-to-severe psoriasis is similar to that of other IL-17 antagonists.27
Although only four clinical cases are reported here, these cases represent real-world clinical conditions and, as such, they provide a valuable adjunct to results obtained in the carefully selected patients included in controlled clinical trials.
Conclusion
Brodalumab rapidly improves psoriatic lesions and relieves associated symptoms in patients with moderate-to-severe psoriasis, including those with psoriatic arthritis. These benefits are also evident in patients who have responded poorly to other biologics and, moreover, they are maintained in the long term.
Acknowledgments
Medical writing support, under the direction of the authors, was provided by Jon Monk and David P. Figgitt PhD, ISMPP CMPP™, Content Ed Net, with funding from LEO Pharma GmbH, Neu-Isenburg, Germany. Funding for the case series described in this review article was provided by LEO Pharma GmbH, Neu-Isenburg, Germany. LEO Pharma-sponsored clinical trials have been conducted at the University Hospital Mainz and MediCorium, Germany.
Disclosure
Andreas Pinter has received honoraria as a speaker and for participating in advisory boards for AbbVie, Almirall-Hermal, Amgen, Biogen Idec, Boehringer-Ingelheim, Celgene, GSK, Eli-Lilly, Galderma, Hexal, Janssen, LEO Pharma, MC2, Medac, Merck Serono, Mitsubishi, MSD, Novartis, Pascoe, Pfizer, Tigercat Pharma, Regeneron, Roche, Sandoz Biopharmaceuticals, Schering-Plough and UCB Pharma. Bernd Bonnekoh has received funding for clinical studies and honoraria for scientific presentations and counseling by the following pharma companies: AbbVie, Almirall, Biogen, Celgene, Janssen, LEO Pharma, Lilly, Medac, Merck Sharp & Dohme, Novartis, Pfizer and UCB. Ina Marion Hadshiew has received funding from LEO Pharma. Sebastian Zimmer has received honoraria as a speaker and for participating in advisory boards for LEO Pharma, and as a speaker and/or advisor for AbbVie, Almirall, BMS, Celgene, Janssen, Lilly, MSD and Novartis. The authors report no other conflicts of interest in this work.
References
1. Schadler ED, Ortel B, Mehlis SL. Biologics for the primary care physician: review and treatment of psoriasis. Dis Mon. 2019;65(3):51–90. doi: 10.1016/j.disamonth.2018.06.001
2. Marinoni B, Ceribelli A, Massarotti MS, et al. The Th17 axis in psoriatic disease: pathogenetic and therapeutic implications. Auto-Immun Highlights. 2014;5(1):9–19. doi:10.1007/s13317-013-0057-4
3. Naik GS, Ming WK, Magodoro IM, et al. Th17 inhibitors in active psoriatic arthritis: a systematic review and meta-analysis of randomized controlled clinical trials. Dermatology. 2017;233(5):366–377. doi:10.1159/000484520
4. World Health Organisation. Global report on psoriasis. 2016;1–48. WHO Press, Geneva. Available from: https://apps.who.int/iris/bitstream/handle/10665/204417/9789241565189_eng.pdf.psoriasis;jsessionid=3EFBDE720BAD52FD683811E05737FA18?sequence=1 (
5. Silfvast-Kaiser A, Paek SY, Menter A. Anti-IL17 therapies for psoriasis. Expert Opin Biol Ther. 2019;19(1):45–54. doi:10.1080/14712598.2019.1555235
6. Galluzzo M, D’adamio S, Bianchi L, et al. Brodalumab for the treatment of psoriasis. Expert Rev Clin Immunol. 2016;12(12):1255–1271. doi:10.1080/1744666X.2016.1246957
7. Mease PJ. Inhibition of interleukin-17, interleukin-23 and the TH17 cell pathway in the treatment of psoriatic arthritis and psoriasis. Curr Opin Rheumatol. 2015;27(2):127–133. doi:10.1097/BOR.0000000000000147
8. Roman M, Chiu MW. Spotlight on brodalumab in the treatment of moderate-to-severe plaque psoriasis: design, development, and potential place in therapy. Drug Des Devel Ther. 2017;11:2065–2075. doi:10.2147/DDDT.S113683
9. Nograles KE, Zaba LC, Guttman-Yassky E, et al. Th17 cytokines interleukin (IL)-17 and IL-22 modulate distinct inflammatory and keratinocyte-response pathways. Br J Dermatol. 2008;159(5):1092–1102.
10. Johansen C, Usher PA, Kjellerup RB, Lundsgaard D, Iversen L, Kragballe K. Characterization of the interleukin-17 isoforms and receptors in lesional psoriatic skin. Br J Dermatol. 2009;160(2):319–324. doi:10.1111/bjd.2009.160.issue-2
11. Jeon C, Sekhon S, Yan D, Afifi L, Nakamura M, Bhutani T. Monoclonal antibodies inhibiting IL-12, −23, and −17 for the treatment of psoriasis. Hum Vaccin Immunother. 2017;13(10):2247–2259. doi:10.1080/21645515.2017.1356498
12. Beringer A, Noack M, Miossec P. IL-17 in chronic inflammation: from discovery to targeting. Trends Mol Med. 2016;22(3):230–241. doi:10.1016/j.molmed.2016.01.001
13. Sbidian E, Chaimani A, Garcia-Doval I, et al. Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis. Cochrane Database Syst Rev. 2017;12:CD011535.
14. Papp KA, Leonardi C, Menter A, et al. Brodalumab, an anti-interleukin-17-receptor antibody for psoriasis. N Engl J Med. 2012;366(13):1181–1189. doi:10.1056/NEJMoa1114705
15. Papp KA, Reich K, Paul C, et al. A prospective phase III, randomized, double-blind, placebo-controlled study of brodalumab in patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2016;175(2):273–286. doi:10.1111/bjd.14493
16. Lebwohl M, Strober B, Menter A, et al. Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. N Engl J Med. 2015;373(14):1318–1328. doi:10.1056/NEJMoa1503824
17. Lebwohl M, Papp KA, Tyring S, et al. Long-term efficacy of brodalumab for the treatment of moderate-to-severe psoriasis in two pivotal phase 3 clinical trials [abstract 6616]. J Am Acad Dermatol. 2018;79(3Suppl 1):AB180. doi:10.1016/j.jaad.2018.03.023
18. Green L, Hsu S, Papp KA, et al. Long-term efficacy of brodalumab in ustekinumab-responsive patients with moderate-to-severe psoriasis [abstract 6832]. J Am Acad Dermatol. 2018;79(3Suppl 1):AB181. doi:10.1016/j.jaad.2018.03.023
19. Gottlieb AB, Gordon K, Hsu S, et al. Improvement in itch and other psoriasis symptoms with brodalumab in phase 3 randomized controlled trials. J Eur Acad Dermatol Venereol. 2018;32(8):1305–1313. doi:10.1111/jdv.14282
20. Mease PJ, Genovese MC, Greenwald MW, et al. Brodalumab, an anti-IL17RA monoclonal antibody, in psoriatic arthritis. N Engl J Med. 2014;370(24):2295–2306. doi:10.1056/NEJMoa1315231
21. Papp K, Leonardi C, Menter A, et al. Safety and efficacy of brodalumab for psoriasis after 120 weeks of treatment. J Am Acad Dermatol. 2014;71(6):1183–1190. doi:10.1016/j.jaad.2014.08.039
22. Papp K, Menter A, Strober B, et al. Efficacy and safety of brodalumab in subpopulations of patients with difficult-to-treat moderate-to-severe plaque psoriasis. J Am Acad Dermatol. 2015;72(3):436–439. doi:10.1016/j.jaad.2014.10.026
23. Wade R, Grosso A, South E, et al. Brodalumab for the treatment of moderate-to-severe plaque psoriasis: an evidence review group evaluation of a NICE single technology appraisal. Pharmacoeconomics. 2019;37(2):131–139. doi:10.1007/s40273-018-0698-2
24. Liang SE, Cohen JM, Ho RS. Psoriasis and suicidality: a review of the literature. Dermatol Ther. 2019;32(1):e12771. doi:10.1111/dth.12771
25. Chiricozzi A, Romanelli M, Saraceno R, et al. No meaningful association between suicidal behavior and the use of IL-17A-neutralizing or IL-17RA-blocking agents. Expert Opin Drug Saf. 2016;15(12):1653–1659. doi:10.1080/14740338.2016.1228872
26. Hashim PW, Chen T, Lebwohl MG, et al. What lies beneath the face value of a BOX WARNING: a deeper look at brodalumab. J Drugs Dermatol. 2018;17(8):s29–s34.
27. Rusta-Sallehy S, Gooderham M, Papp K. Brodalumab: a review of safety. Skin Therapy Lett. 2018;23(2):1–3.
28. Yamasaki K, Nakagawa H, Kubo Y, Japanese Brodalumab Study Group, et al. Efficacy and safety of brodalumab in patients with generalized pustular psoriasis and psoriatic erythroderma: results from a 52-week, open-label study. Br J Dermatol. 2017;176(3):741–751. doi:10.1111/bjd.15380
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.