Back to Journals » Breast Cancer: Targets and Therapy » Volume 12
Breast Cancer and Tamoxifen: A Nigerian Perspective to Effective Personalised Therapy
Authors Adehin A, Kennedy MA , Soyinka JO , Alatise OI, Olasehinde O , Bolaji OO
Received 8 June 2020
Accepted for publication 8 September 2020
Published 7 October 2020 Volume 2020:12 Pages 123—130
DOI https://doi.org/10.2147/BCTT.S266314
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Pranela Rameshwar
Ayorinde Adehin,1,2 Martin Alexander Kennedy,3 Julius Olugbenga Soyinka,1 Olusegun Isaac Alatise,4 Olalekan Olasehinde,4 Oluseye Oladotun Bolaji1
1Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Obafemi Awolowo University, Ile-Ife, Nigeria; 2Institute of Biomedical and Pharmaceutical Sciences, Guangdong University of Technology, Guangzhou, People’s Republic of China; 3Department of Pathology and Biomedical Science; Carney Centre of Pharmacogenomics, University of Otago, Christchurch, New Zealand; 4Department of Surgery, Faculty of Clinical Sciences, College of Health Sciences, Obafemi Awolowo University, Ile-Ife, Nigeria
Correspondence: Ayorinde Adehin Department of Pharmaceutical Chemistry, Faculty of Pharmacy
Obafemi Awolowo University, Ile-Ife, Nigeria
Tel +234 8022013470
Email [email protected]
Abstract: Estrogen-receptor positivity in tumour, often requiring long-term tamoxifen therapy, is thought to characterise between 43% and 65% of breast cancer cases in Nigeria. The patient population is further marked by late-stage diagnosis which significantly heightens the tendency for tumour relapse in the course of tamoxifen therapy. Despite tamoxifen being considered a reliable chemopreventive in high-risk individuals and an effective adjuvant therapy for hormone-sensitive tumours, mortality has remained high among breast cancer patients in the West African region where Nigeria belongs. The Nigerian breast cancer population, like other similar patient-populations in the West African region, provides a mix of intrinsic genome-diversity and perhaps unique tumour biology and evolution. These peculiarities suggest the need for a rational approach to tumour management and a personalised delivery of therapy in Nigeria’s dominant estrogen-receptor-positive patient population. Herein, critical indices of tamoxifen-therapy success are discussed in the context of the Nigerian breast cancer population with emphasis on salient aspects of tamoxifen-biotransformation, host- and tumour-genomics, and epigenetics.
Keywords: breast cancer, estrogen receptor, tamoxifen, Nigeria, epigenetics, genomics
Estrogen-Receptor-Positive Breast Cancer in Nigeria
The most recent statistics by the Global Cancer Observatory showed that incidence (age-standardized) of breast cancer in the West African region, home to about 5% of the world population, for 2018 stood at 37.2 per 100,000 females with an attributed mortality rate of 17.8 per 100,000 females. Nigeria, whose population accounts for over 50% of persons living in the West African region, recorded some 26,310 new cases of breast cancer in females during the same period,1 and a previous report had noted an age-standardized incidence of between 52 and 64.6 per 100,000 females in the population.2 Although breast cancer in males is quite rare in the population, a few cases have been reported. It is, however, noteworthy that formal data-gathering structures at healthcare facilities in the region are generally weak, and the actual cases of breast cancer could be much higher than what is currently known.
The estrogen receptor, often profiled in breast tumours alongside progesterone receptor and human epidermal growth factor 2 gene expression, is considered a primary driver of oncogenesis in hormone-positive tumours.3 Published data for Nigerian patients suggests that estrogen-receptor-positive (ER+) breast cancer is quite common. These cases often present late,4,5 and hormonal agents like tamoxifen are often deployed as adjuncts to surgical resection. The most recent report on hormone-receptor status of breast tumour in Nigeria noted a prevalence of about 43% (94/220) among patients who presented at six healthcare delivery centres between 2014 and 2016.6 Other older reports have noted frequencies of about 58% (n = 48)7 and 65% (n = 192)8 among Nigerian patients.
These data, however sparse, imply an important role of hormone therapy in the management of breast cancer patients in Nigeria, and perhaps the West African region. As such, tamoxifen therapy has largely remained a convenient choice when indicated due to the ease of access and a relatively cheaper cost.
Tamoxifen and Estrogen-Receptor-Positive Breast Cancer
Tamoxifen, a selective estrogen-receptor modulator, is an effective hormone therapy indicated for the management and chemoprevention of ER+ breast cancer.9,10 Maximum clinical benefit from tamoxifen therapy, often achieved after a minimum 5-year administration, occurs through its cytostatic effect on breast cancer cell proliferation.11,12 The therapeutic benefit derivable from tamoxifen is, however, known to be mediated by some key factors listed in Table 1. A 20 mg daily dose is considered best for efficacy as no superior clinical benefits have been attributed to other varied doses.13 The risk of tumour recurrence or contralateral cancer over a 20-year study period has been noted to be below 27% in patients administered long-term tamoxifen.14 Adverse reactions such as hot flashes, thromboembolic events, and the increased risk of endometrial cancer are not uncommon in patients on tamoxifen.15–17 Further, an increased risk of osteoporosis occasioned by a decrease in bone mineral density has been associated with the use of tamoxifen in pre-menopausal women. Tamoxifen therapy in postmenopausal women was, however, noted to result in an increase in bone mineral density which, perhaps, contributes to reduced risk of osteoporotic fracture rates.18–20
 |
Table 1 Some Keys Factors Mediating the Effectiveness of Tamoxifen in the Management of Hormone-Receptor Positive Breast Cancer |
Adjuvant Tamoxifen Therapy in Nigeria
Detailed clinical data, with wide geographical spread, for users of tamoxifen in Nigeria, is largely unavailable at this time. To this end, retrospective data on breast cancer patients placed on daily tamoxifen between 2008 and 2019 at the Obafemi Awolowo University Teaching Hospital, a major tertiary healthcare centre in Southwest Nigeria, was reviewed and summarised (Table 2). This record showed that tamoxifen was mostly recommended as an adjunct in a female-dominated patient-population with a comparable number of pre- and post-menopausal women. About 71% of these patients had stage 3 tumour at the time of diagnosis, and most (~68%) were between the ages of 40 and 65 years.
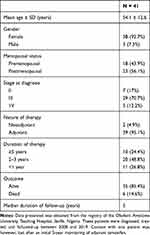 |
Table 2 Summary Data of Breast Cancer Patients on Tamoxifen Between 2008 and 2019 in a Tertiary Healthcare Institution in Nigeria |
Information on the tolerance, adverse events and effectiveness of adjuvant tamoxifen in this population was, however, not captured. To better assess the indices of usefulness of adjuvant tamoxifen in Nigeria, host genetic factors influencing tamoxifen metabolism, breast cancer predisposition, and epigenetics are discussed in the context of what is known about the Nigerian population.
Metabolism of Tamoxifen
Hepatic metabolism of tamoxifen yields the much more potent 4-hydroxytamoxifen and endoxifen amongst other metabolites.31 These bioactive hydroxylated-metabolites are dominantly generated by the activity of the polymorphic CYP2D6 enzyme,21 the deficiency of which is acquired as an autosomal recessive “poor metaboliser” trait22 and which has now been widely examined for its role in the effectiveness of adjuvant tamoxifen therapy. Although the impact of defective CYP2D6 protein on response to adjuvant tamoxifen in Nigerians is yet to be established, prevalence of key polymorphisms in the CYP2D6 gene across Nigerian ethnicities has been reported. CYP2D6*4, *10, *17, and *29 exist in the dominant Nigerian ethnicities at frequencies between 2% and 22%, and about 21% of the population is projected to comprise intermediate and poor metabolisers of CYP2D6 substrates.32
The Clinical Pharmacogenomics Implementation Consortium (CPIC) currently recommends a highly cautious dosing of tamoxifen in carriers of CYP2D6*4 and *10 haplotypes, which may not be uncommon in Nigeria, on the strength of clinical data available to date.33 Translational benefits of this and other recommendations of CPIC for the dosing of tamoxifen in Nigerian patients would, however, remain limited until richer population-specific data on CYP2D6 haplotypes are available for clinical evaluation.
BRCA1/BRCA2 Mutation, Adjuvant and Prophylactic Tamoxifen
Germline mutation in either BRCA1 or BRCA2 gene is known to increase the risk of breast cancer by 27% to 87% in persons over 70 years of age. Mutations in these breast cancer susceptibility genes are also reported to increase the risk of contralateral breast cancer by 26% to 40% in carriers.34 A 17-year study of some 1136 Nigerian breast cancer patients noted a prevalence of 7% and 4.1% of functional BRCA1 and BRCA2 mutations, respectively.35 Data presented by this study in the Nigerian breast cancer population also showed that about 78% and 83% of functional BRCA1 and BRCA2 mutation carriers, respectively, were persons below 60 years of age. This is consistent with a weak correlation between old-age and susceptibility to breast cancer in Nigerian carriers of functional BRCA mutations.
While it appears that co-carriers of functional BRCA mutations and ER+ tumours are quite rare in Nigeria (~ 0.004%), the effectiveness of therapeutic options available is important for survival. It is now known that BRCA germline mutations define a subgroup of breast cancer patients whose treatment approach is better optimised by prior characterisation as carriers or non-carriers.35,36 For example, tamoxifen therapy is more effective in non-carriers of BRCA mutations as it has been associated with reduced risk of contralateral breast cancer. Further, the NSABP1-P1-trial which drew inference from a small number of patients noted that chemopreventive tamoxifen therapy was effective in BRCA2-mutation carriers but not in BRCA1-mutation carriers.24
Tamoxifen Resistance, Host Genetics and Epigenetics
Intrinsic or acquired resistance to tamoxifen therapy in ER+ tumour is known to occur in breast cancer patients.37 Particularly of interest in Nigerian patients is the development of acquired resistance to long-term tamoxifen therapy through a loss of estrogen-receptor function or expression. This is indeed so because of the documented increased susceptibility of ER+ patients who present late, prevalent in Nigeria (>70%),35,38 to relapse in the course of endocrine therapy.39 While the detailed mechanism underlying tamoxifen resistance is still unclear, the repression of estrogen-receptor-α (ERα, the biotarget of tamoxifen) gene due to epigenetic modification in its promoter region and those of accessory cellular machinery has been reported.28,40–42 This epigenetic phenotype appears to involve the aberrant methylation of CpG islands (CGIs), deacetylation at N-terminal lysine residue in histones, increased methylation at histone-H3-lysine-4 (H3K4) sites, and altered expression of relevant mRNA levels.28,43,44
While the complex web of interactions and changes in the activities of coactivators, corepressors, and transcriptional factors involved in the expression and normal physiological functions of ERα is being explored,39 the intrinsic genomic diversity characteristic of Nigerian populations poses an intriguing perspective. Examples are the single nucleotide polymorphisms (SNPs) in DNMT1, the maintenance methylase implicated in the aberrant methylation of CGIs. Although these SNPs have not been directly associated with epimutations in ER and tamoxifen response, positive correlation with the risk of breast cancer has been reported.45,46 DNMT1 rs22286121 and rs222861 SNPs, for example, have minor allele frequencies of 0.14–0.16 and 0.51–0.54, respectively, in Nigerian ethnicities.47 The significance of these and other DNMT1 SNPs for epigenetic changes in ERα and the success of adjuvant tamoxifen therapy in Nigerian patients are worthy of further research considering the fairly high prevalence.
Inhibitors of histone deacetylases such as Vorinostat, Entinostat, and Panobinostat have been deployed with some success in malignancies,48,49 and the application of some of these agents as therapeutics for the reversal of ERα repression in breast tumour is the subject of clinical trials. Expression levels of histone deacetylase (HDAC) 1, 2, and 3 have all been correlated with ER-status and disease progression of breast tumours,25,26 and the regulatory role of HDAC3 in the stability of ER mRNA has been reported.27 These genes, encoding HDAC isoforms, have been characterised for functional polymorphisms (eg the recurrence of hepatocellular carcinoma)50 that further invites a closer look at the role of variations in translated HDAC proteins in the repression of ERα.
A recent report detailed the crucial role of lysine N-methyltransferase 2C (KMT2C/MLL3) and - 2F (KMT2F/SET1A) in the regulatory methylation of ERα promoter/enhancer region.28 This report further confirmed that a knockout of the MLL3 and SET1A genes resulted in a decrease in ERα protein and mRNA level in breast cancer tissues and cell lines. While the epigenetic role of these proteins suggests that they might be useful targets in the development of drugs aimed at reversing decreased hormone-sensitivity in breast tumour, the impact of several mutations and polymorphisms47 already identified in these genes remain to be elucidated.
Breast Tumour Genome, Methylome and Tamoxifen Therapy
Cancer cells, generally, are in a constant state of evolution and as such, the myriad of mutations characteristic of tumours, and the significance of such mutations for therapy, continue to be a major field of study to date. In addition to the repertoire of somatic and germline mutations, epigenetic changes which bring about gene-silencing or overexpression of cellular components are also effected in the cancer cell genome over time.51 An interplay of these factors is thought to partly drive the complex machinery underpinning intrinsic or acquired resistance to tamoxifen and other breast cancer therapies.
Somatic mutations in the ERα gene, ESR1 (eg the Y537S and D538G mutants),29 known to precipitate hormone-therapy resistance and which are presently unstudied in Nigerian patients, present yet another perspective to tamoxifen resistance in the population. These mutations in ESR1 appear to also evolve with the disease and have been documented in metastatic lesions.52 Although existing data does suggest that mutations in ESR1 are quite rare in primary tumours,53–55 comparable studies establishing similar rarity in Nigerian patients are unavailable at this time. This present knowledge-gap also extends to the possibility of race playing a crucial role in the propensity for evolutionary changes in genes critical for hormone-therapy resistance such as ESR1. While there is no robust evidence, to date, showing that race influences the molecular evolution of breast tumours, the tendency for such has been suggested going by the greater genome instability observed in persons of African ancestry.56,57 The significance of these observations for ESR1 mutations and tamoxifen resistance in Nigerian patients remain to be seen in future studies.
The methylome has become an important topic in tumour studies for its crucial link with processes of tumour proliferation, repression, and by extension, choice and success of therapy. An example of this is seen in the methylation-induced silencing of SALL2, a gene encoding a zinc finger C2H2 transcription factor. The downregulation of SALL2 is reported to repress ESR1 transcription, thus creating tamoxifen-resistant tumours.30 Further, breast-tissue-specific hypermethylation of CpG islands strongly linked with estrogen-receptor positivity and with consequences for gene transcription has been reported.58 Amidst the rich genomic-diversity of African populations,59 which remains poorly explored, evaluating the possibility of a crosstalk between methylome evolution and a heightened genome instability56,57 in Nigerian patients may prove pivotal in the delivery of effective tamoxifen therapy for the population.
The Future of Effective Tamoxifen Therapy and Recommendations for Nigeria
Gaps in Genome Research
Curiously, germline mutations in a larger proportion of driver genes profiled for breast cancer are mostly associated with DNA repair processes underpinning genome integrity.60 What is presently known about the Nigerian breast cancer population, however, appears to question the benefits of this lopsided focus on genes associated with DNA repair processes in determining tumour risks and disease clinicopathology for the population. A sizeable study35 (n=1136) observed that the present conservative list of mutations in breast cancer driver genes, with the exception of BRCA genes (127/1136; ~0.1%), is largely rare (29/1136 for PALB2, TP53, ATM, BARD1, BRIP1, CHEK1, CHEK2, GEN1, NBN, RAD51C, RAD51D, XRCC2; ~0.03%) in Nigerian patients.
This would suggest that drivers of breast cancer biology and determinants of the effectiveness of therapy, tamoxifen inclusive, may lie in other parts of the host and cancer genome unique to the Nigerian population. With the assumption that ER+ breast tumours account for over 40% of diagnoses in Nigeria,7,8 radical approaches geared towards defining the functional significance of more germline mutations in factors mediating epigenetic gene suppression/expression (such as DNMT1, HDAC1-3, MLL3, SALL2, and SET1A) are more needed than ever. Further, the Nigerian ER+ breast cancer population might benefit from extensive studies of methylome in primary and metastatic tumours to identify unique signatures relevant for therapy, and as well profile the molecular drivers of evolutionary changes that might be uncovered.
Gaps in the Therapy Approach
For a number of long-term therapeutic interventions, strict patient compliance with defined therapeutic regimen that ensures adequate drug exposure is crucial for success. While this may not have been established for tamoxifen therapy,61 low intra-tumoral levels of tamoxifen have been observed in patients with acquired resistance.62 The possibility, hence, exists that prolonged exposure to suboptimal therapeutic levels of tamoxifen, due to poor regimen adherence, might become a critical factor in the development of acquired resistance to endocrine therapy. Sparse data gathered for 114 Nigerian patients on long-term tamoxifen identified non-adherence to prescribed regimen in about 25% of these tamoxifen users. Intolerable adverse events, particularly in the first six months of therapy commencement, were identified as the major driver of non-adherence.63
Alternative approaches aimed at minimising tamoxifen intolerance have been studied in the last two decades. For example, trials of low-dose tamoxifen (1 to10 mg per day) in limited number of patients and populations have noted uncompromised therapeutic benefits in addition to reduced risks and frequency of adverse events.64–66 Further, topical tamoxifen which provides the benefit of lesser systemic side effects has been successfully trialled in the management of cyclical mastalgia.67 Raloxifene, also a selective estrogen-receptor modulator like tamoxifen, has been shown to be equally effective in reducing the incidence and mortality from hormone-positive breast cancer.68,69 These alternatives are, however, untested in Nigerians.
To address these gaps, research efforts in a cohort of Nigerian patients, devoted to improving quality-of-life and regimen-adherence, are needed at this time. Since Nigerian breast cancer patients are largely unrepresented in the several previous studies of varied tamoxifen doses and formulation, and raloxifene trials, studies of the effectiveness of these alternative approaches in the population are required for policy direction in the management of hormone-positive breast tumour.
Conclusion
Without a doubt, very little is known about the spread, severity, and mortality of ER+ breast cancer and the effectiveness of tamoxifen in Nigeria, and the road to a personalised dosing of tamoxifen appears distant. These challenges are further exacerbated by the weak infrastructure available for research and data collection. Importantly at this time, a clearer picture can be gained from renewed efforts in the direction of data capture covering tumour relapse, survival, and drug-tolerance in Nigerian patients on tamoxifen therapy.
Notwithstanding, the sparse data available can still be put to use in helping to chart a course for the effective management of ER+ breast cancer in a population presently burdened with inadequate health insurance platforms. Newer approaches would likely go beyond administering monotherapy tamoxifen as chemopreventive or adjuvant to accommodating rational multitherapy approaches in instances of predicted heightened-tamoxifen-therapy-failure. The time for these coordinated efforts to start is now.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi:10.3322/caac.21492
2. Jedy-Agba E, Curado MP, Ogunbiyi O, et al. Cancer incidence in Nigeria: a report from population-based cancer registries. Cancer Epidemiol. 2012;36:e271–e278. doi:10.1016/j.canep.2012.04.007
3. Lim E, Metzger-Filho O, Winer EP. The natural history of hormone receptor-positive breast cancer. Oncology. 2012;26:688–694, 696.
4. Awofeso O, Roberts AA, Salako O, et al. Prevalence and Pattern of Late-Stage Presentation in Women with Breast and Cervical Cancers in Lagos University Teaching Hospital, Nigeria. Niger Med J. 2018;59:74–79. doi:10.4103/nmj.NMJ_112_17
5. Adesunkanmi AR, Lawal OO, Adelusola KA, et al. The severity, outcome and challenges of breast cancer in Nigeria. Breast. 2006;15:399–409. doi:10.1016/j.breast.2005.06.008
6. Jedy-Agba E, McCormack V, Olaomi O, et al. Determinants of stage at diagnosis of breast cancer in Nigerian women: sociodemographic, breast cancer awareness, health care access and clinical factors. Cancer Causes Control. 2017;28(7):685–697. doi:10.1007/s10552-017-0894-y
7. Nwafor CC, Keshinro SO. Pattern of hormone receptors and human epidermal growth factor receptor 2 status in sub-Saharan breast cancer cases: private practice experience. Niger J Clin Pract. 2015;18(4):553–558. doi:10.4103/1119-3077.156905
8. Adebamowo CA, Famooto A, Ogundiran TO, et al. Immunohistochemical and molecular subtypes of breast cancer in Nigeria. Breast Cancer Res Treat. 2008;110(1):183–188. doi:10.1007/s10549-007-9694-5
9. Eggemann H, Altmann U, Costa SD, et al. Survival benefit of tamoxifen and aromatase inhibitor in male and female breast cancer. J Cancer Res Clin Oncol. 2018;144(2):337–341. doi:10.1007/s00432-017-2539-7
10. Sauter ER. Breast Cancer Prevention: current Approaches and Future Directions. Eur J Breast Health. 2018;14:64–71. doi:10.5152/ejbh.2018.3978
11. Lee WL, Cheng MH, Chao HT, et al. The role of selective estrogen receptor modulators on breast cancer: from tamoxifen to raloxifene. Taiwan J Obstet Gynecol. 2008;47(1):24–31. doi:10.1016/S1028-4559(08)60051-0
12. Davies C, Pan H, Godwin J, et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet. 2013;381(9869):805–816. doi:10.1016/S0140-6736(12)61963-1
13. Group EBCTC. Tamoxifen for early breast cancer: an overview of the randomised trials. Lancet. 1998;351(9114):1451–1467. doi:10.1016/S0140-6736(97)11423-4
14. Pan H, Gray R, Braybrooke J, et al. 20-Year Risks of Breast-Cancer Recurrence after Stopping Endocrine Therapy at 5 Years. N Engl J Med. 2017;377:1836–1846. doi:10.1056/NEJMoa1701830
15. Hernandez RK, Sorensen HT, Pedersen L, et al. Tamoxifen treatment and risk of deep venous thrombosis and pulmonary embolism: a Danish population-based cohort study. Cancer. 2009;115(19):4442–4449. doi:10.1002/cncr.24508
16. Fisher B, Costantino JP, Redmond CK, et al. Endometrial cancer in tamoxifen-treated breast cancer patients: findings from the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-14. J Natl Cancer Inst. 1994;86:527–537. doi:10.1093/jnci/86.7.527
17. Cuzick J, Sestak I, Bonanni B, et al. Selective oestrogen receptor modulators in prevention of breast cancer: an updated meta-analysis of individual participant data. Lancet. 2013;381:1827–1834. doi:10.1016/S0140-6736(13)60140-3
18. Cooke AL, Metge C, Lix L, et al. Tamoxifen use and osteoporotic fracture risk: a population-based analysis. J Clin Oncol. 2008;26(32):5227–5232. doi:10.1200/JCO.2007.15.7123
19. Tzeng HE, Muo CH, Chen HT, et al. Tamoxifen use reduces the risk of osteoporotic fractures in women with breast cancer in Asia: a nationwide population-based cohort study. BMC Musculoskelet Disord. 2015;16(1):123. doi:10.1186/s12891-015-0580-8
20. Powles TJ, Hickish T, Kanis JA, et al. Effect of tamoxifen on bone mineral density measured by dual-energy x-ray absorptiometry in healthy premenopausal and postmenopausal women. J Clin Oncol. 1996;14(1):78–84. doi:10.1200/JCO.1996.14.1.78
21. Desta Z, Ward BA, Soukhova NV, et al. Comprehensive evaluation of tamoxifen sequential biotransformation by the human cytochrome P450 system in vitro: prominent roles for CYP3A and CYP2D6. J Pharmacol Exp Ther. 2004;310(3):1062–1075. doi:10.1124/jpet.104.065607
22. Bertilsson L, Dahl ML, Dalen P, et al. Molecular genetics of CYP2D6: clinical relevance with focus on psychotropic drugs. Br J Clin Pharmacol. 2002;53(2):111–122. doi:10.1046/j.0306-5251.2001.01548.x
23. Goetz MP, Kamal A, Ames MM. Tamoxifen pharmacogenomics: the role of CYP2D6 as a predictor of drug response. Clin Pharmacol Ther. 2008;83(1):160–166. doi:10.1038/sj.clpt.6100367
24. King MC, Wieand S, Hale K, et al. Tamoxifen and breast cancer incidence among women with inherited mutations in BRCA1 and BRCA2: national Surgical Adjuvant Breast and Bowel Project (NSABP-P1) Breast Cancer Prevention Trial. JAMA. 2001;286(18):2251–2256. doi:10.1001/jama.286.18.2251
25. Krusche CA, Wulfing P, Kersting C, et al. Histone deacetylase-1 and −3 protein expression in human breast cancer: a tissue microarray analysis. Breast Cancer Res Treat. 2005;90(1):15–23. doi:10.1007/s10549-004-1668-2
26. Muller BM, Jana L, Kasajima A, et al. Differential expression of histone deacetylases HDAC1, 2 and 3 in human breast cancer–overexpression of HDAC2 and HDAC3 is associated with clinicopathological indicators of disease progression. BMC Cancer. 2013;13:215. doi:10.1186/1471-2407-13-215
27. Oie S, Matsuzaki K, Yokoyama W, et al. HDAC3 regulates stability of estrogen receptor alpha mRNA. Biochem Biophys Res Commun. 2013;432(2):236–241. doi:10.1016/j.bbrc.2013.02.007
28. Kim SS, Lee MH, Lee MO. Histone methyltransferases regulate the transcriptional expression of ERalpha and the proliferation of tamoxifen-resistant breast cancer cells. Breast Cancer Res Treat. 2020;180(1):45–54. doi:10.1007/s10549-019-05517-0
29. Fanning SW, Mayne CG, Dharmarajan V, et al. Estrogen receptor alpha somatic mutations Y537S and D538G confer breast cancer endocrine resistance by stabilizing the activating function-2 binding conformation. Elife. 2016;5. doi:10.7554/eLife.12792
30. Ye L, Lin C, Wang X, et al. Epigenetic silencing of SALL 2 confers tamoxifen resistance in breast cancer. EMBO Mol Med. 2019;11(12):e10638. doi:10.15252/emmm.201910638
31. Lim YC, Desta Z, Flockhart DA, et al. Endoxifen (4-hydroxy-N-desmethyl-tamoxifen) has anti-estrogenic effects in breast cancer cells with potency similar to 4-hydroxy-tamoxifen. Cancer Chemother Pharmacol. 2005;55:471–478. doi:10.1007/s00280-004-0926-7
32. Ebeshi BU, Bolaji OO, Masimirembwa CM. CytochromeP450 2D6 (CYP2D6) Genotype and Phenotype Determination in the Nigerian Populations. Asian J Pharm Hea Sci. 2011;1:47–54.
33. Consortium CPI Guideline for CYP2D6 and Tamoxifen Therapy https://cpicpgx.org/.
34. Kuchenbaecker KB, Hopper JL, Barnes DR, et al. Risks of Breast, Ovarian, and Contralateral Breast Cancer for BRCA1 and BRCA2 Mutation Carriers. JAMA. 2017;317(23):2402–2416. doi:10.1001/jama.2017.7112
35. Zheng Y, Walsh T, Gulsuner S, et al. Inherited Breast Cancer in Nigerian Women. J Clin Oncol. 2018;36:2820–2825. doi:10.1200/JCO.2018.78.3977
36. Reding KW, Bernstein JL, Langholz BM, et al. Adjuvant systemic therapy for breast cancer in BRCA1/BRCA2 mutation carriers in a population-based study of risk of contralateral breast cancer. Breast Cancer Res Treat. 2010;123:491–498. doi:10.1007/s10549-010-0769-3
37. Early Breast Cancer Trialists’ Collaborative G. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials.. Lancet. 2005;365(9472):1687–1717. doi:10.1016/S0140-6736(05)66544-0
38. Adejumo AA, Ajamu OJ, Akanbi OO, et al. Epidemiology and Challenges of Managing Breast Cancer in Keffi, North-Central Nigeria: A Preliminary Report. Niger Med J. 2019;60(4):193–197. doi:10.4103/nmj.NMJ_45_19
39. Legare S, Basik M. Minireview: the Link Between ERalpha Corepressors and Histone Deacetylases in Tamoxifen Resistance in Breast Cancer. Mol Endocrinol. 2016;30(9):965–976. doi:10.1210/me.2016-1072
40. Sharma D, Blum J, Yang X, et al. Release of methyl CpG binding proteins and histone deacetylase 1 from the Estrogen receptor alpha (ER) promoter upon reactivation in ER-negative human breast cancer cells. Mol Endocrinol. 2005;19(7):1740–1751. doi:10.1210/me.2004-0011
41. Zhou Q, Atadja P, Davidson NE. Histone deacetylase inhibitor LBH589 reactivates silenced estrogen receptor alpha (ER) gene expression without loss of DNA hypermethylation. Cancer Biol Ther. 2007;6:64–69. doi:10.4161/cbt.6.1.3549
42. Izadi P, Noruzinia M, Karimipoor M, et al. Promoter hypermethylation of estrogen receptor alpha gene is correlated to estrogen receptor negativity in Iranian patients with sporadic breast cancer. Cell J. 2012;14:102–109.
43. Sproul D, Meehan RR. Genomic insights into cancer-associated aberrant CpG island hypermethylation. Brief Funct Genomics. 2013;12(3):174–190. doi:10.1093/bfgp/els063
44. de Souza Rocha Simonini P, Breiling A, Gupta N, et al. Epigenetically deregulated microRNA-375 is involved in a positive feedback loop with estrogen receptor alpha in breast cancer cells. Cancer Res. 2010;70(22):9175–9184. doi:10.1158/0008-5472.CAN-10-1318
45. Kullmann K, Deryal M, Ong MF, et al. DNMT1 genetic polymorphisms affect breast cancer risk in the central European Caucasian population. Clin Epigenetics. 2013;5(1):7. doi:10.1186/1868-7083-5-7
46. Xiang G, Zhenkun F, Shuang C, et al. Association of DNMT1 gene polymorphisms in exons with sporadic infiltrating ductal breast carcinoma among Chinese Han women in the Heilongjiang Province. Clin Breast Cancer. 2010;10(5):373–377. doi:10.3816/CBC.2010.n.049
47. The International Genome Sample Resource. Human Genome Project. Available from: https://www.internationalgenome.org/. Accessed September 25, 2020.
48. Halsall JA, Turner BM. Histone deacetylase inhibitors for cancer therapy: an evolutionarily ancient resistance response may explain their limited success. Bioessays. 2016;38:1102–1110. doi:10.1002/bies.201600070
49. Connolly RM, Rudek MA, Piekarz R. Entinostat: a promising treatment option for patients with advanced breast cancer. Future Oncol. 2017;13(13):1137–1148. doi:10.2217/fon-2016-0526
50. Yang Z, Zhou L, Wu LM, et al. Combination of polymorphisms within the HDAC1 and HDAC3 gene predict tumor recurrence in hepatocellular carcinoma patients that have undergone transplant therapy. Clin Chem Lab Med. 2010;48(12):1785–1791. doi:10.1515/CCLM.2010.353
51. Stratton MR, Campbell PJ, Futreal PA. The cancer genome. Nature. 2009;458:719–724. doi:10.1038/nature07943
52. Fumagalli D, Wilson TR, Salgado R, et al. Somatic mutation, copy number and transcriptomic profiles of primary and matched metastatic estrogen receptor-positive breast cancers. Ann Oncol. 2016;27(10):1860–1866. doi:10.1093/annonc/mdw286
53. Wang P, Bahreini A, Gyanchandani R, et al. Sensitive Detection of Mono- and Polyclonal ESR1 Mutations in Primary Tumors, Metastatic Lesions, and Cell-Free DNA of Breast Cancer Patients. Clin Cancer Res. 2016;22(5):1130–1137. doi:10.1158/1078-0432.CCR-15-1534
54. Takeshita T, Yamamoto Y, Yamamoto-Ibusuki M, et al. Droplet digital polymerase chain reaction assay for screening of ESR1 mutations in 325 breast cancer specimens. Transl Res. 2015;166:540–553 e542. doi:10.1016/j.trsl.2015.09.003
55. Griffith OL, Spies NC, Anurag M, et al. The prognostic effects of somatic mutations in ER-positive breast cancer. Nat Commun. 2018;9(1):3476. doi:10.1038/s41467-018-05914-x
56. Pitt JJ, Zheng Y, Olopade OI. Genetic Ancestry May Influence the Evolutionary Trajectory of Cancers. Cancer Cell. 2018;34:529–530. doi:10.1016/j.ccell.2018.09.006
57. Yuan J, Hu Z, Mahal BA, et al. Integrated Analysis of Genetic Ancestry and Genomic Alterations across Cancers. Cancer Cell. 2018;34:549–560 e549. doi:10.1016/j.ccell.2018.08.019
58. Benevolenskaya EV, Islam AB, Ahsan H, et al. DNA methylation and hormone receptor status in breast cancer. Clin Epigenetics. 2016;8(1):17. doi:10.1186/s13148-016-0184-7
59. Genomes Project C, Auton A, Brooks LD, et al. A global reference for human genetic variation. Nature. 2015;526:68–74. doi:10.1038/nature15393
60. Rajendran BK, Deng CX. Characterization of potential driver mutations involved in human breast cancer by computational approaches. Oncotarget. 2017;8:50252–50272. doi:10.18632/oncotarget.17225
61. Weaver KE, Camacho F, Hwang W, et al. Adherence to adjuvant hormonal therapy and its relationship to breast cancer recurrence and survival among low-income women. Am J Clin Oncol. 2013;36(2):181–187. doi:10.1097/COC.0b013e3182436ec1
62. Johnston SR, Haynes BP, Smith IE, et al. Acquired tamoxifen resistance in human breast cancer and reduced intra-tumoral drug concentration. Lancet. 1993;342(8886–8887):1521–1522. doi:10.1016/S0140-6736(05)80088-1
63. Oguntola AS, Adeoti ML, Akanbi OO. Non-adherence to the Use of Tamoxifen in the First year by the Breast Cancer Patients in an African Population. East Central African J Surg. 2011;16:52–56.
64. Decensi A, Gandini S, Serrano D, et al. Randomized dose-ranging trial of tamoxifen at low doses in hormone replacement therapy users. J Clin Oncol. 2007;25(27):4201–4209. doi:10.1200/JCO.2006.09.4318
65. Guerrieri-Gonzaga A, Botteri E, Lazzeroni M, et al. Low-dose tamoxifen in the treatment of breast ductal intraepithelial neoplasia: results of a large observational study. Ann Oncol. 2010;21(5):949–954. doi:10.1093/annonc/mdp408
66. DeCensi A, Puntoni M, Guerrieri-Gonzaga A, et al. Randomized Placebo Controlled Trial of Low-Dose Tamoxifen to Prevent Local and Contralateral Recurrence in Breast Intraepithelial Neoplasia. J Clin Oncol. 2019;37(19):1629–1637. doi:10.1200/JCO.18.01779
67. Singh DD, Dharanipragada K, D S, et al. Oral versus topical tamoxifen in cyclical mastalgia-A randomized controlled trial. Breast J. 2020;26(4):743–747. doi:10.1111/tbj.13674
68. Vogel VG, Costantino JP, Wickerham DL, et al. Effects of Tamoxifen vs Raloxifene on the Risk of Developing Invasive Breast Cancer and Other Disease Outcomes The NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 Trial. JAMA. 2006;295(23):2727–2741. doi:10.1001/jama.295.23.joc60074
69. Pinsky PF, Miller EA, Heckman-Stoddard BM, et al. Breast Cancer Characteristics and Survival among Users versus Nonusers of Raloxifene. Cancer Prev Res. 2020;13(1):83–90. doi:10.1158/1940-6207.CAPR-19-0393
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
