Back to Journals » Neuropsychiatric Disease and Treatment » Volume 13
Brain metabolite values in children with breath-holding spells
Authors Calik M, Sen Dokumaci D, Sarikaya S, Demir M, Isik I, Kazanasmaz H, Kaya C, Kandemir H
Received 1 March 2017
Accepted for publication 16 May 2017
Published 26 June 2017 Volume 2017:13 Pages 1655—1660
DOI https://doi.org/10.2147/NDT.S135842
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Roger Pinder
Mustafa Calik,1 Dilek Sen Dokumaci,2 Suna Sarikaya,3 Mahmut Demir,4 Ilhan Isik,5 Halil Kazanasmaz,4 Cemil Kaya,4 Hasan Kandemir6
1Department of Pediatric Neurology, 2Department of Radiology, 3Department of Neurology, 4Department of Pediatrics, Harran University School of Medicine, 5Department of Pediatric Neurology, Eyyubiye Training and Research Hospital, 6Department of Child and Adolescent Psychiatry, Harran University School of Medicine, Sanliurfa, Turkey
Abstract: Breath-holding spells are benign, paroxysmal events with apnea and postural tone changes after a crying episode in infants. The objective of this study was to investigate the pathologies in brain metabolite values in the absence of seizure in children with breath-holding spells by using magnetic resonance spectroscopy (MRS). Brain MRS examination was performed on 18 children with breath-holding spells and 13 neurologically normal children who were included as the control group. There was no significant difference in terms of N-acetyl aspartate (NAA), choline (Cho), creatine (Cr), and myoinositol (mI) levels and also in terms of NAA/Cr, Cho/Cr, and mI/Cr ratios between the patients and the control group (all P>0.05). Our study suggested that there is no permanent neuronal damage in patients with breath-holding spells. This result confirms the previous studies, which reported no permanent neuronal damage in patients with breath-holding spells.
Keywords: brain metabolite, children, breath holding, magnetic resonance spectroscopy
Introduction
Breath-holding spells are benign, paroxysmal events with apnea and postural tone changes found in infants. They may resemble a seizure sometimes, although they are nonepileptic events. These spells have benign prognosis, in general, but occasionally some complications may be seen as a loss of consciousness, tonic–clonic movements, or seizures. In addition, it has been reported that severe breath-holding spells in children could cause cerebral hypoxemia.1–6
Nowadays, various biochemical7,8 and neuroimaging modalities including MRI9,10 and magnetic resonance spectroscopy (MRS)11,12 have often been used for the detection of neuronal damage in children with different neurological diseases. MRI is the modality of choice for evaluating the structural lesions in different neurological diseases. Its high specificity and sensitivity provide clues to the pathological basis of many developmental disorders including migrational disturbances and developmental tumors.13,14 On the other hand, MRS is clinically useful for identifying the biochemical state of the central nervous system, and it is a noninvasive method that metabolites such as N-acetyl aspartate (NAA), choline (Cho), myoinositol (mI), creatine (Cr), and lactate can be measured in different parts of the brain. These metabolites are considered markers of multiple facets of neural tissue such as Cr for high-energy phosphate metabolism, Cho for membrane phospholipid metabolism, NAA for neuronal and axonal integrity/density, and mI for glial cell density. MRS has been used for imaging of the brain with metabolic diseases, neurodegenerative disease, and epilepsy.15,16 In recent years, although there are some reports on the biochemical state of the central nervous system related to neuronal damage in patients with breath-holding spells, but these results are inconclusive.1–3
The objective of the present study was to examine the brain MRS findings in patients with breath-holding spells.
Materials and methods
Study subjects
This prospective study was conducted at Radiology and Pediatric Neurology Departments of Harran University School of Medicine, Sanliurfa, Turkey. The study group included 18 patients (5 girls and 13 boys) who were consecutively diagnosed with breath-holding spells in our Department of Pediatric Neurology and 13 neurologically normal children (4 girls and 9 boys). Neurologically normal children who were admitted to the Department of Pediatric Neurology with mild head trauma were included as the control group. The children in control group were not admitted to the hospital. The mean ages of children were 19.1±9.3 months in the patient group and 19.4±9.4 months in the control group. All the patients were diagnosed with breath-holding spells according to clinical and biochemical findings, electroencephalography, electrocardiography, and echocardiography results. The patients included in this study were those with at least 2-month survival, multiple episodes of attack, and breath-holding seizures without any medical treatment. The brain MRS examinations were carried out in the normal period between the 2 spells. The breath-holding spells were classified as mild, moderate, and severe (Table 1). Patients were excluded from the study with spells associated with metabolic problems, electrolyte disturbances, and trauma to avoid including children with seizures that can mimic breath-holding spells.17,18 In addition, a total of 12 patients were excluded from the study due to hypocalcemia-related tremor (2), early childhood benign myoclonus (4), hyperekplexia (2), benign infantile epilepsy (3), and benign paroxysmal vertigo (1).
  | Table 1 Demographic findings of the patients and controls and types of spells |
Prior to subject recruitment, the study protocol was reviewed and approved by Harran University Ethics Committee, in accordance with the ethical principles for human investigations, as outlined by the Second Declaration of Helsinki, and written informed consents were obtained from the parents of all the patients.
Analysis of brain MRS
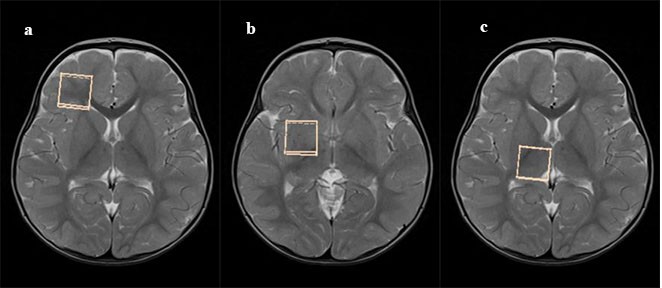
Brain MRS imaging was carried out by using a 1.5-T MRI unit (Magnetom Symphony A Tim System; Siemens, Erlangen, Germany) with a quadrature transmit/receive head coil. Each patient was sedated before the examination. The transfer and monitoring of the patients were done by pediatricians who accompanied all the patients during the entire procedure. All imaging procedures were completed by an experienced radiologist. Single voxel method of MRS was performed on all the patients and control group. Three baseline images were obtained orthogonally for each patient with automated magnetic field shimming. In both the patients and control group, 3 distinct neuroanatomic structures (ie, frontal white matter, putamen, and thalamus) were selected for the analysis in this study (Figure 1A–C). The raw spectroscopic data were collected following optimal water signal suppression. Metabolite signals included the metabolites: NAA (2.04 ppm), Cr (3.05 and 3.95 ppm), Cho (3.24 ppm), mI (3.58 ppm), and lactate (1.33 ppm; Figure 2). The total scanning time was ~20 minutes per patient. The collected data were transferred to a computer workstation (Leonardo; Siemens Medical Solutions, Forchheim, Germany), and peak integrals (areas under the peaks) for NAA, Cho, Cr, and mI were calculated by using the operator-independent, manufacturer-supplied, software package (Siemens). All metabolite peaks were evaluated for full width at half maximum peak height, and all widths were <0.1 ppm for all the metabolites. The ratios NAA/Cr, Cho/Cr, and mI/Cr were also calculated and noted.
Statistical analysis
We calculated the necessary sample size according to the frontal white matter NAA/Cr ratios of first 8 patients (Group 1) and of control group (Group 2), which were 2.21±0.70 in Group 1 and 2.85±0.40 in Group 2. From this initial sample variance and assuming a 2-tailed α value of 0.05 (sensitivity =95%) and a β value of 0.20 (study power =80%), we determined that at least 13 patients were required in each group (effect size =1.15; δ =2.93), (G*Power3 power analysis program; Düsseldorf University, Düsseldorf, Germany).19
SPSS for Windows Version 18.0 (SPSS Inc., Chicago, IL, USA) was used to analyze the obtained data. Conformity to normal distribution of the quantitative data was examined by using Kolmogorov–Smirnov test. All the data were expressed as mean ± SD. The χ2 test and Mann–Whitney U-test were used to compare the categorical variables and continuous variables between both the groups, respectively. P<0.05 was considered as statistically significant.
Results
There was no statistically significant difference between the patient group and the control group in terms of age or gender (P>0.05). Breath-holding spells were pallid in 3 (16.6%) cases, cyanotic in 9 (50.0%) cases, and mixed in 6 (33.2%) cases. The severity of the spells was categorized as mild (10 cases; 55.5%), moderate (5 cases; 27.7%), and severe (3 cases; 16.6%). Table 1 shows the demographic findings of patients and control group and types and durations of the spells. The time elapsed after the first diagnosis of breath-holding spells was ranging from 5 to 28 months.
In brain MRS, there were no significant differences between the patients and control group in terms of NAA/Cr, Cho/Cr, and mI/Cr ratios in the 3 regions (all P>0.05). Table 2 shows the results of brain MRS in the patients with breath-holding spells and the control group.
Discussion
Breath-holding spells are usually benign attacks found in infants. The etiology of breath-holding spells is not fully understood.20 As the child grows, they often disappear spontaneously without any treatment. In various studies, it had been reported that seizures may develop 0.5%–4.8%, and neurodevelopmental abnormalities may occur up to 3.6% of these patients in follow-up.1,2
Within the last 10–15 years, many important advances have been made in imaging modalities. Currently, MRI is the preferred imaging method for identifying the underlying pathologies in patients with epilepsy and various neurological disorders.9,10
Despite structural abnormalities in the brain, MRI occasionally could be normal.21 In the case of the more subtle types of cortical dysplasia associated with tumors and mesial temporal sclerosis which can be overlooked, advanced imaging techniques including diffusion tensor imaging and MRS can provide additional findings in these patients.22 Therefore, these techniques are becoming increasingly available to clinicians for the evaluation of neurological diseases in children. Various investigators used MRS to determine metabolite changes in brain during normal maturation and white matter myelination, which may provide additional information to assess the developmental delay; neurodegenerative, inflammatory, metabolic, neuropsychiatric disorders; hypoxic ischemic brain injury; and epilepsy.23
Numerous studies, both clinically24,25 and on animals,26 have reported decreases in NAA levels that are associated with either neuronal dysfunction or neuronal damage in epilepsy.27,28 Studies on children with newly diagnosed temporal lobe epilepsy have also shown a significantly decreased NAA/Cr level in the temporal lobe compared with normal controls.29 Similarly, Holopainen et al30 have evaluated metabolite abnormalities by using MRS of children with febrile convulsions and epilepsy, and they found significantly decreased NAA/(Cho + Cr) and NAA/Cr ratios in both febrile convulsion and epilepsy groups when compared with the control group.
In a study by Koepp et al,31 it was reported that brain NAA levels were reduced in the thalamus of juvenile myoclonic epilepsy patients. In addition, Varho et al32 found that the mean NAA level was significantly decreased in the hippocampus of children with infrequent seizures and nonsymptomatic localization-related epilepsy.
To the best of our knowledge, this is the first MRS study in the literature conducted on these patients. In our study, patients with breath-holding spells showed no significant differences in metabolite values compared with the control subjects.
The results of the current study are not consistent with most previous MRS studies showing neuronal damage of the brain in children with epilepsy. In contrast to some studies on epilepsy patients, no findings of neuronal cell loss, increased membrane phospholipid turnover, or cellular proliferation were observed in the present study.32,33 There were different results among the studies because most of our cases were not exposed to severe hypoxia. In our recently published study, we found increased serum S-100B protein levels reflecting neuronal damage in patients with breath-holding spells.34 However, we now consider that those previous results may be due to reversible neuronal metabolic dysfunction rather than permanent neuronal damage in breath-holding spells.
In the present study, the metabolites selected for analysis were in 3 distinct anatomic regions, especially the putamen and thalamus, as they are known to be more sensitive to hypoxia than other regions in the brain. Measurements were taken of NAA, Cho, Cr, and mI, but only the results of the ratios based on these measures were reported because the study sample size was small and metabolite ratios can be used when there is a reduction in sample size.31
The aim of the present study was not to address the etiology of brain metabolite and neurological disorders associated with breath-holding spells. It was attempted to reveal the presence of neuronal damage associated with hypoxia, which may occur secondary to breath-holding spells. Moreover, there were no potential side effects of sedation on patients with respect to measuring the metabolites such as NAA, Cr, Cho, and mI values.
The limitations of the current study can be said to be that the sample size was low. At the time of the breath-holding spells, the brain metabolites could not be investigated on brain MRS for technical reasons. Previous studies have also reported that these metabolites in epileptic patients were examined after the seizure.30–33
Conclusion
In conclusion, according to these findings, no permanent neuronal damage was found in the patients with breath-holding spells, indicating that it is a benign event indeed. However, further studies with larger populations of children with severe spells are needed to confirm the present findings.
Acknowledgment
The authors received no financial support for the research, authorship, and/or publication of this article.
Author contributions
All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
DiMario FJ Jr. Breath-holding spells in childhood. Am J Dis Child. 1992;146(1):125–131. | ||
DiMario FJ Jr. Prospective study of children with cyanotic and pallid breath-holding spells. Pediatrics. 2001;107(2):265–269. | ||
Calik M, Abuhandan M, Aycicek A, Taskin A, Selek S, Iscan A. Increased oxidant status in children with breath-holding spells. Childs Nerv Syst. 2013;29(6):1015–1019. | ||
Işikay S, Hizli Ş. Frequency of coeliac disease in children with breath-holding spells. J Paediatr Child Health. 2014;50(11):916–919. | ||
Carman KB, Ekici A, Yimenicioglu S, Arslantas D, Yakut A. Breath holding spells: point prevalence and associated factors among Turkish children. Pediatr Int. 2013;55(3):328–331. | ||
Eliacik K, Bolat N, Kanik A, et al. Parental attitude, depression, anxiety in mothers, family functioning and breath-holding spells: a case control study. J Paediatr Child Health. 2016;52(5):561–565. | ||
Gazzolo D, Abella R, Marinoni E, et al. New markers of neonatal neurology. J Matern Fetal Neonatal Med. 2009;22(Suppl 3):57–61. | ||
Calik M, Abuhandan M, Kandemir H, et al. Interictal serum S-100B protein levels in intractable epilepsy: a case-control study. Neurosci Lett. 2014;558:58–61. | ||
Koepp MJ, Woermann FG. Imaging structure and function in refractory focal epilepsy. Lancet Neurol. 2005;4(1):42–53. | ||
Woermann FG, Vollmar C. Clinical MRI in children and adults with focal epilepsy: a critical review. Epilepsy Behav. 2009;15(1):40–49. | ||
Park YD, Allison JD, Weiss KL, Smith JR, Lee MR, King DW. Proton magnetic resonance spectroscopic observations of epilepsia partialis continua in children. J Child Neurol. 2000;15(11):729–733. | ||
Kabay SC, Gumustas OG, Karaman HO, Ozden H, Erdinc O. A proton magnetic resonance spectroscopic study in juvenile absence epilepsy in early stages. Eur J Paediatr Neurol. 2010;14(3):224–228. | ||
Gaillard WD, Chiron C, Cross JH, et al. ILAE, Committee for Neuroimaging, Subcommittee for Pediatric. Guidelines for imaging infants and children with recent-onset epilepsy. Epilepsia. 2009;50(9):2147–2153. | ||
Fellah S, Callot V, Viout P, et al. Epileptogenic brain lesions in children: the added-value of combined diffusion imaging and proton MR spectroscopy to the presurgical differential diagnosis. Childs Nerv Syst. 2012;28(2):273–282. | ||
Soares DP, Law M. Magnetic resonance spectroscopy of the brain: review of metabolites and clinical applications. Clin Radiol. 2009;64(1):12–21. | ||
Oguz KK, Celebi A, Anlar B. MR imaging, diffusion-weighted imaging and MR spectroscopy findings in acute rapidly progressive subacute sclerosing panencephalitis. Brain Dev. 2007;29(5):306–311. | ||
Facini C, Spagnoli C, Pisani F. Epileptic and non-epileptic paroxysmal motor phenomena in newborns. J Matern Fetal Neonatal Med. 2016;29(22):3652–3659. | ||
Chen L, Knight EM, Tuxhorn I, Shahid A, Lüders HO. Paroxysmal non-epileptic events in infants and toddlers: a phenomenologic analysis. Psychiatry Clin Neurosci. 2015;69(6):351–359. | ||
Faul F, Erdfelder E, Buchner A, Lang AG. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav Res Methods. 2009;41(4):1149–1160. | ||
Vurucu S, Karaoglu A, Paksu SM, et al. Breath-holding spells may be associated with maturational delay in myelination of brain stem. J Clin Neurophysiol. 2014;31(1):99–101. | ||
Vézina LG. MRI-negative epilepsy: protocols to optimize lesion detection. Epilepsia. 2011;52(Suppl 4):25–27. | ||
Krsek P, Hajek M, Dezortova M, et al. (1) H MR spectroscopic imaging in patients with MRI-negative extratemporal epilepsy: correlation with ictal onset zone and histopathology. Eur Radiol. 2007;17(8):2126–2135. | ||
Kosucu P, Erdemli S, Sönmez M, Kul S, Aksoy A. MR spectroscopic evaluation of psychomotor delay of unknown cause in children. AJR Am J Roentgenol. 2010;194(4):1110–1115. | ||
Cendes F, Andermann F, Preul MC, Arnold DL. Lateralization of temporal lobe epilepsy based on regional metabolic abnormalities in proton magnetic resonance spectroscopic images. Ann Neurol. 1994;35(2):211–216. | ||
Tsuchida TN, Barkovich AJ, Bollen AW, Hart AP, Ferriero DM. Childhood status epilepticus and excitotoxic neuronal injury. Pediatr Neurol. 2007;36(4):253–257. | ||
Hiremath GK, Najm IM. Magnetic resonance spectroscopy in animal models of epilepsy. Epilepsia. 2007;48(4):47–55. | ||
Kuzniecky R, Palmer C, Hugg J, et al. Magnetic resonance spectroscopic imaging in temporal lobe epilepsy: neuronal dysfunction or cell loss? Arch Neurol. 2001;58(12):2048–2053. | ||
Pan JW, Williamson A, Cavus I, et al. Neurometabolism in human epilepsy. Epilepsia. 2008;49(Suppl 3):31–41. | ||
Miller SP, Li LM, Cendes F, et al. Neuronal dysfunction in children with newly diagnosed temporal lobe epilepsy. Pediatr Neurol. 2000;22(4):281–286. | ||
Holopainen IE, Valtonen ME, Komu ME, et al. Proton spectroscopy in children with epilepsy and febrile convulsions. Pediatr Neurol. 1998;19(2):93–99. | ||
Hoch SE, Kirov II, Tal A. When are metabolic ratios superior to absolute quantification? A statistical analysis. NMR Biomed. Epub 2017 Mar 8. | ||
Koepp MJ, Woermann F, Savic I, Wandschneider B. Juvenile myoclonic epilepsy-neuroimaging findings. Epilepsy Behav. 2013;28(Suppl 1):S40–S44. | ||
Varho T, Komu M, Sonninen P, Lähdetie J, Holopainen IE. Quantitative HMRS and MRI volumetry indicate neuronal damage in the hippocampus of children with focal epilepsy and infrequent seizures. Epilepsia. 2005;46(5):696–703. | ||
Calik M, Ciftci A, Sarikaya S, et al. Assessment of both serum S-100B protein and neuropeptide-Y levels in childhood breath-holding spells. Epilepsy Behav. 2015;47:34–38. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.