Back to Journals » Infection and Drug Resistance » Volume 15
Brain Abscess Caused by Nocardia brevicatena in an Immunocompetent Patient: A Case Report
Authors Li X, Zhuang S, He L, Wang S, Zhao M, Lyu X
Received 5 November 2022
Accepted for publication 14 December 2022
Published 28 December 2022 Volume 2022:15 Pages 7693—7697
DOI https://doi.org/10.2147/IDR.S396085
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Xiaoxu Li,1,* Shifang Zhuang,2,* Lin He,2 Shanmei Wang,3 Ming Zhao,1 Xiaodong Lyu4
1Department of Neurosurgery, the Affiliated Cancer Hospital of Zhengzhou University, Henan Cancer Hospital, Zhengzhou, People’s Republic of China; 2Genskey Medical Technology Co., Ltd, Beijing, People’s Republic of China; 3Department of Clinical Laboratory, Henan Provincial People’s Hospital, People’s Hospital of Zhengzhou University, Zhengzhou, People’s Republic of China; 4Central Laboratory, the Affiliated Cancer Hospital of Zhengzhou University, Henan Cancer Hospital, Zhengzhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xiaodong Lyu, Central Laboratory, the Affiliated Cancer Hospital of Zhengzhou University, Henan Cancer Hospital, 127 Dongming Road, Zhengzhou, 450000, People’s Republic of China, Tel/Fax +8613523417973, Email [email protected]
Abstract: Nocardia brain abscess is relatively rare and generally occurs in immunodeficient patients. Here, we present the first case of brain abscess due to Nocardia brevicatena in an immunocompetent patient, with unknown origin. In this case, a 49-year-old man was admitted to our hospital with limb twitching and complained of a history of intermittent headache. He was diagnosed with brain abscess through brain imaging and cured after craniotomy for abscess excision and targeted antibiotic treatment. Surgical specimens were sent for further detection. The causative organism was identified by weak acid-fast staining, culture and metagenomic next-generation sequencing (mNGS). We hope this case could provide a reference for incoming patients as well as their clinical management.
Keywords: brain abscess, Nocardia brevicatena, non-immunocompromised, mNGS, surgical treatment
Introduction
Nocardia are Gram-positive, aerobic, branching filamentous bacteria belonging to Actinomycetales. They are universally present in soil, decaying vegetation and water. Around 54 Nocardia species are known to cause infections in human beings.1 Nocardia are relatively uncommon pathogens, which cause infections mostly in immunocompromised patients.2
Nocardia brain abscess constitutes approximately 1–2% of all brain abscesses. The mortality rate of a Nocardia brain abscess is three times higher than that of other bacterial brain abscesses.3 Pathogens found in brain abscesses enter the brain tissue through hematogenous spread, closer spread (such as sinusitis) or traumatic infection after head trauma or surgery.4
Nocardia brevicatena was first described in 1961, which was isolated from two human respiratory specimens.5 Nevertheless, there have been no case reports of human infection with N. brevicatena since then. Here, we present the first case of brain abscess due to N. brevicatena in an immunocompetent patient, to our best knowledge.
Case Presentation
A 49-year-old man was admitted to our hospital with limb twitching and complained of a 7-day history of intermittent headache. The patient was a truck driver without immunosuppression. He had no significant past medical history and did not report any foci of infection, sinusitis, or head trauma. Physical examination showed conscious state, temperature of 36.6°C, sensitivity to light reflex, a soft neck, and normal limb muscle strength.
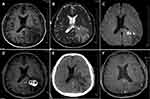
On admission, laboratory investigations showed white blood cell of 9.95×109/L (with 64.3% neutrophil, 4.7% monocytes, and 29.4% lymphocytes), procalcitonin of < 0.01 ng/mL (normal 0–0.05 ng/mL), C-reactive protein of 7.97 mg/L (normal 0–6 mg/L). Brain magnetic resonance imaging (MRI) revealed irregular lesions and surrounding edema in the left temporo-occipital lobe (Figure 1A and B). Enhanced diffusion-weighted imaging (DWI) signals were observed in the center of the lesions (Figure 1C). The signal intensity of the ring wall around the lesions was markedly increased after enhancement (Figure 1D). Magnetic resonance spectrum (MRS) indicated an elevated lactic acid peak, and low N-acetyl aspartate, creatine, and choline peaks. The patient was diagnosed with brain abscess based on the imaging results. He was given empiric treatment with intravenous metronidazole (1 g, q12h) and ceftriaxone (2 g, q12h). Thoracoabdominal computed tomography (CT) scan did not show any mass or solid organ lesion. Transthoracic and transesophageal echocardiograms excluded endocarditis. Blood and cerebrospinal fluid cultures proved negative. Cerebrospinal fluid analysis showed white blood cell of 0.01×109/L, glucose of 3.65 mmol/L (normal 2.5–4.5 mmol/L), and total protein of 1.262 g/L (normal 0.15–0.45 g/L).
After 3 weeks of initial treatment with metronidazole and ceftriaxone, the patient’s symptoms persisted. A CT scan showed enlarged lesions and surrounding edema. Then we performed craniotomy to excise the lesions (Figure 1E). The consistency of the excised lesions resembled a mixture of necrotic tissue with hematoma. Surgical specimens were sent for pathology, culture, and metagenomic next-generation sequencing (Genskey, Beijing, China). Histopathology analysis indicated extensive necrosis and abscess formation. Weak acid-fast staining showed acid-fast, branching and uneven staining of filamentous bacilli (Figure 2A), suggestive of Nocardia. mNGS analysis reported N. brevicatena with 28,770 unique reads distributing across regions of the genome (Figure 3). It is not a random mapping and can be identified as N. brevicatena. Metagenome data is now available at NCBI under the Sequence Read Archive (SRA) database with accession no. PRJNA908993. Specimen culturing on blood agar plates at 37°C under aerobic conditions yielded pale yellow, rough, and dry colonies (Figure 2B). The isolate also presented filamentous branched bacilli and positive weak acid-fast staining. These findings demonstrated a brain abscess caused by N. brevicatena.
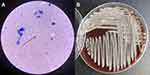 |
Figure 2 The results of staining and culture. (A) Weak acid-fast staining of the abscess wall (×1000). (B) An image of pure bacteria after 10 days of culture. |
 |
Figure 3 The distribution of mapping read number on the genome of N. brevicatena. |
After surgery, the patient had no fever and his headache relieved. A repeat CT confirmed total excision of the space-occupying lesions (Figure 1E). On the 3th day after surgery, he switched to intravenous vancomycin (1 g, q12h) for 10 days and oral trimethoprim/sulfamethoxazole (80 mg/400 mg, q12h) for 6 months. He was discharged home 14 days after surgery. The patient had a return visit and denied clinical symptoms at 4 months after discharge. No residual lesion was observed through gadofluorine-enhanced magnetic resonance imaging (Figure 1F).
Discussion and Conclusion
The incidence of nocardiosis seems to be increasing each year in the population, especially in those with deficient cell-mediated immunity, such as persons with organ transplantation, malignancy, diabetes mellitus, autoimmune diseases, AIDS infection, and prolonged corticosteroid therapy.2,6 Brain is the most commonly involved extrapulmonary nocardiosis site, and abscess formation is an important feature.7 In this report, we present a case of primary brain abscess due to N. brevicatena in an immunocompetent patient, without a known origin.
Brain imaging is considered as the cornerstone for the diagnosis of brain abscess. The time course of brain abscess consists of three stages: meningoencephalitis stage, suppuration stage, and capsule stage.8 The abscess commonly develops a vascularized wall made of astrocytes and glia in the capsule stage, which can be identified by CT scan or MRI as a ring enhancement.9 However, brain abscess is similar to gliomas, cystic foci, and necrotic foci on imaging modalities in the capsule stage. MRS is a good method for distinguishing them. The center of brain abscess lacks the metabolites of normal brain tissue; the peaks of N-acetyl aspartate, creatine, and choline are low but the peak of lactic acid is elevated.10 In addition, typical DWI of brain abscess shows a hyperintense signal in the abscess cavity, which is also a valuable method to distinguish between brain abscess, glioma, and metastases.11 In this case, we report a multiloculated brain abscess corresponding to the capsule stage.
The microbiology laboratory plays an important role in the diagnosis of nocardiosis, because the clinical presentation mimics other common infections.12 The possibility of nocardiosis should always be alerted in abscess samples. Special staining techniques like weak acid-fast staining may be required for diagnosis, when nocardiosis is suspected.13 Nocardia can grow on routine laboratory media such as blood agar, and most isolates grow significantly within 3–5 days. In the current case, the colony morphology of the isolate was similar to that of N. brevicatena described before.5 The patient with brain abscess was diagnosed with N. brevicatena infection by weak acid-fast staining, culture, and mNGS. As an emerging technique, mNGS represents an unbiased and rapid diagnostic tool, which is useful for the diagnosis of infectious diseases, especially for rare or difficult cases.14 When compared with culture, the mNGS method can improve the detection rate of Nocardia, identify different Nocardia species, and greatly reduce the turnaround time.15
The therapies for brain abscess include chemotherapy and surgery. Nocardia have a tendency to relapse due to their slow replication and intracellular presence. Therefore, prolonged antimicrobial therapy for a few to several months is required. The most widely used antibiotic is trimethoprim/sulfamethoxazole due to good tolerance and better cerebrospinal fluid penetration; alternative drugs are meropenem, ceftriaxone, linezolid, ampicillin, vancomycin, or amikacin.16 Surgical treatment is required in most cases of cerebral nocardiosis after multiloculated and thick walled lesions form. However, surgical treatment can be avoided based on accurate diagnosis of Nocardia and prompt antibiotic treatment in some cases.17 Overall prognosis is favorable on administration of appropriate surgical therapy and antibiotic cover.18,19
In summary, we present a case of brain abscess due to N. brevicatena in an immunocompetent patient. Although the majority of patients with cerebral nocardiosis are immunocompromised, cerebral nocardiosis could occur without any predisposing factors. It is very important for clinicians to be familiar with the characteristics of nocardial infections. mNGS detection can help clinicians provide direction for the diagnosis of infectious diseases. Timely surgical intervention and targeted antibiotic treatment are both significant for the patient with Nocardia brain abscess. Finally, we hope this case could provide a reference for incoming patients as well as their clinical management.
Consent for Publication
Written informed consent has been obtained from the patient for the case details and images to be published. Details of the case can be published without institutional approval.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Conville PS, Brown-Elliott BA, Smith T, Zelazny AM. The Complexities of Nocardia Taxonomy and Identification. J Clin Microbiol. 2017;56(1):e01419–17. doi:10.1128/JCM.01419-17
2. Wadhwa T, Baveja U, Kumar N, Govil D, Sengupta S. Clinical manifestations of nocardiosis: study of risk factors and outcomes in a tertiary care hospital. J Lab Physicians. 2017;9(4):288–295. doi:10.4103/JLP.JLP_111_16
3. Kim S, Lee KL, Lee DM, et al. Nocardia brain abscess in an immunocompetent patient. Infect Chemother. 2014;46(1):45–49. doi:10.3947/ic.2014.46.1.45
4. Sonneville R, Ruimy R, Benzonana N, et al. An update on bacterial brain abscess in immunocompetent patients. Clin Microbiol Infect. 2017;23(9):614–620. doi:10.1016/j.cmi.2017.05.004
5. Lechevalier HA, Solotorovsky M, McDurmont CI. A new genus of the actinomycetales: micropolyspora gen. nov. J Gen Microbiol. 1961;26:11–18. doi:10.1099/00221287-26-1-11
6. Fatahi-Bafghi M. Nocardiosis from 1888 to 2017. Microb Pathog. 2018;114:369–384. doi:10.1016/j.micpath.2017.11.012
7. Beaman BL, Beaman L. Nocardia species: host-parasite relationships. Clin Microbiol Rev. 1994;7(2):213–264. doi:10.1128/CMR.7.2.213
8. Zhou W, Shao X, Jiang X. A clinical report of two cases of cryptogenic brain abscess and a relevant literature review. Front Neurosci. 2018;12:1054. doi:10.3389/fnins.2018.01054
9. Moorthy RK, Rajshekhar V. Management of brain abscess: an overview. Neurosurg Focus. 2008;24(6):E3. doi:10.3171/FOC/2008/24/6/E3
10. Hsu SH, Chou MC, Ko CW, et al. Proton MR spectroscopy in patients with pyogenic brain abscess: MR spectroscopic imaging versus single-voxel spectroscopy. Eur J Radiol. 2013;82(8):1299–1307. doi:10.1016/j.ejrad.2013.01.032
11. Brouwer MC, Coutinho JM, van de Beek D. Clinical characteristics and outcome of brain abscess: systematic review and meta-analysis. Neurology. 2014;82(9):806–813. doi:10.1212/WNL.0000000000000172
12. Kandi V. Human nocardia infections: a review of pulmonary nocardiosis. Cureus. 2015;7(8):e304. doi:10.7759/cureus.304
13. Mathur S, Sood R, Aron M, Iyer VK, Verma K. Cytologic diagnosis of pulmonary nocardiosis: a report of 3 cases. Acta Cytol. 2005;49(5):567–570. doi:10.1159/000326207
14. Chiu CY, Miller SA. Clinical metagenomics. Nat Rev Genet. 2019;20(6):341–355. doi:10.1038/s41576-019-0113-7
15. Weng SS, Zhang HY, Ai JW, et al. Rapid detection of nocardia by next-generation sequencing. Front Cell Infect Microbiol. 2020;10:13. doi:10.3389/fcimb.2020.00013
16. Duggal SD, Chugh TD. Nocardiosis: a Neglected Disease. Med Princ Pract. 2020;29(6):514–523. doi:10.1159/000508717
17. Yang J, Xie S, Li J, Xia H, Liu X. Brain abscess caused by nocardia farcinica and diagnosed by metagenomic next-generation sequencing: a case report. Front Med. 2022;9:803554. doi:10.3389/fmed.2022.803554
18. Valarezo J, Cohen JE, Valarezo L, et al. Nocardial cerebral abscess: report of three cases and review of the current neurosurgical management. Neurol Res. 2003;25(1):27–30. doi:10.1179/016164103101201076
19. Soto-Hernández JL, Moreno-Andrade T, Góngora-Rivera F, Ramírez-Crescencio MA. Nocardia abscess during treatment of brain toxoplasmosis in a patient with aids, utility of proton MR spectroscopy and diffusion-weighted imaging in diagnosis. Clin Neurol Neurosurg. 2006;108(5):493–498. doi:10.1016/j.clineuro.2005.01.010
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.