Back to Journals » OncoTargets and Therapy » Volume 9
BRAF inhibitors and radiotherapy for melanoma brain metastases: potential advantages and disadvantages of combination therapy
Authors Chowdhary M, Patel KR, Danish HH, Lawson DH, Khan MK
Received 10 August 2016
Accepted for publication 20 October 2016
Published 12 December 2016 Volume 2016:9 Pages 7149—7159
DOI https://doi.org/10.2147/OTT.S119428
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Faris Farassati
Mudit Chowdhary,1,2 Kirtesh R Patel,1 Hasan H Danish,1 David H Lawson,3 Mohammad K Khan1
1Department of Radiation Oncology, Winship Cancer Institute, Emory University, Atlanta, GA, 2Department of Radiation Oncology, Rush University Medical Center, Chicago, IL, 3Department of Hematology and Medical Oncology, Winship Cancer Institute, Emory University, Atlanta, GA, USA
Abstract: Melanoma is an aggressive malignancy that frequently spreads to the brain, resulting in rapid deterioration in both quality and quantity of life. Historically, treatment options for melanoma brain metastases (MBM) have predominantly consisted of surgery and radiotherapy. While these options can help provide local control, the majority of patients still develop intracranial progression. Indeed, novel therapeutic options, including molecularly targeted agents and immunotherapy, have improved outcomes and are now changing the role of radiotherapy. Up to 50% of melanomas contain an activating BRAF mutation, resulting in hyperactive cellular proliferation and survival. Drugs that target BRAF have been introduced for the treatment of metastatic melanoma and offer hope in improving disease outcomes; however, many of these trials either excluded or had a limited amount of patients with MBM. Recent studies have revealed that melanoma cell lines become more radiosensitive following BRAF inhibition, thus providing a potential synergistic mechanism when combining BRAF inhibitor (BRAFi) and radiotherapy. However, neurotoxicity concerns also exist with this combination. This article reviews the efficacy and limitations of BRAFi therapy for MBM, describes current evidence for combining BRAFis with radiation, discusses the rationale and evidence for combination modalities, and highlights emerging clinical trials specifically investigating this combination in MBM.
Keywords: brain metastases, melanoma, radiation, BRAF inhibitors, vemurafenib, dabrafenib
Introduction
Brain metastases (BM) are the most feared and devastating neurologic complications of metastatic cancer.1 In 2013, 10%–30% of all adult cancer patients in the US developed intracranial metastases, which represents 170,000 newly diagnosed secondary brain malignancies.2 Melanoma is the third most frequent cause of BM, trailing only lung and breast cancers. Although the biological predilection for melanoma to spread to the brain is unknown, ~7% of melanoma patients present with brain involvement at the time of diagnosis,3 with incidence reaching up to 73% in autopsy series.4,5 Furthermore, these lesions are found to contribute to death in up to 95% of cases.4 Thus, the significance of melanoma brain metastases (MBM) cannot be overemphasized.
The prognosis of patients with MBM is dismal, with a median overall survival (OS) of <3 months without treatment.6 In 2008, Sperduto et al7 developed a point-scoring system to predict outcomes of patients with intracranial metastases. They applied this graded partitioning analysis (GPA) to a multi-institution database with 4,259 BM patients to develop disease-specific prognostic criteria.8 For MBM patients, only Karnofsky performance status (KPS) and the number of intracranial metastases predicted survival. The median survival for all MBM patients was 6.7 months; patients with a KPS <70 and more than three metastases had a median OS of 3.4 months, whereas patients with a KPS of 90–100 and a single metastasis had a median OS of 13.2 months.
Treatment options for BM consist of surgery, radiation, and more recently immune or targeted therapy; however, the quality of the efficacy data for MBM is variable. Very few studies assessing surgery and/or radiotherapy for BM have been confined to melanoma patients. Moreover, clinical trials of systemic therapies have traditionally excluded patients with MBM. Advancements in radiation therapy and the advent of newer, more effective systemic agents have offered renewed hope of improving survival in patients with MBM. Here, we review the current understanding and discuss the evolving multimodal management of MBM.
Radiation therapy for BM
Historically, cytotoxic drugs have played a limited role in the management of MBM, partly due to inadequate penetration across the blood–brain barrier (BBB) and the overall poor prognosis of MBM patients.9 As a result, surgical resection for solitary metastases or very large symptomatic lesions and/or radiotherapy have been the standard of care. While surgical resection is effective for symptomatic control, it commonly results in high rates of local failure.10 Consequently, adjuvant whole brain radiation therapy (WBRT) has been utilized to maximize intracranial control.
Recently, quality of life concerns due to late neurocognitive toxicities from WBRT11 have resulted in a paradigm shift toward more conformal radiation treatments.12 Stereotactic radiosurgery (SRS) is a technique that delivers higher radiation doses to a target while limiting radiation exposure to the surrounding normal tissue. Despite lower rates of distant intracranial control with SRS, a prospective study13 noted similar survival rates between WBRT and SRS alone. Furthermore, the addition of WBRT to SRS did not improve OS,10 leading to SRS being recommended as an initial treatment in patients with one to four intracranial metastases. Most recently, a prospective study14 determined SRS to be non-inferior in patients with five to 10 BM to that in patients with two to four BM. While these randomized studies illustrate the efficacy of SRS, MBM were not highly represented. Furthermore, there are several criticisms regarding study design and interpretations of the mentioned trials.15,16 To address these gaps, a prospective Phase 3 clinical trial is currently ongoing that compares local control (LC) and neurocognitive preservation with SRS versus WBRT for patients with more than three MBM (ClinicalTrials.gov NCT01644591). Several retrospective studies, however, have demonstrated good LC (>80%) and median survival (5.3–7 months) in patients with one to four MBM.17–19
However, SRS is not without its limitations. Prospective SRS trials have reported grade 3 toxicity rates of 10%–15%.20 Radiation necrosis (RN) is the most feared long-term radiation-induced complication. Vascular endothelial cell damage, secondary to radiation, results in white matter tissue demyelination, surrounding tissue edema, and eventually normal tissue necrosis.21 The most significant predictive factors related to the development of RN include cumulative dose, treated tumor volume, and number of fractions.22 For larger BM (>3 cm), our institution, among others, has demonstrated improved grade 3 toxicity rates (0%–5%) with hypofractionated (two to five fractions) SRS without sacrificing LC.22,23 Nevertheless, further prospective trials comparing fractionation schemes may help elucidate the optimal dose and fractionation for MBM.
Role of BRAF mutation in melanoma
The mitogen-activated protein kinase (MAPK) pathway (Figure 1) plays an important role in melanoma pathogenesis. This pathway is physiologically activated once extracellular signals bind to their respective membrane receptor, typically a receptor tyrosine kinase (RTK). In turn, the receptor transmits activation signals via the RAS guanosine triphosphatase (GTP) located on the inner surface of the cell membrane. The GTP-bound RAS activates effector proteins, RAF kinases (BRAF). Activated BRAF is a critical component of the MAPK pathway. BRAF functions by phosphorylating and activating MEK1/2, which in turn phosphorylates and activates ERK1/2, leading to cellular proliferation (cyclin D, RBL2), survival (Bim, MCl-1), and differentiation.24
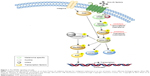  | Figure 1 The MAPK signaling cascade. |
Up to 50% of melanomas harbor an activating BRAF mutation. The most common mutations are V600E (80%) and V600K (14%), which result from a single point mutation at codon 600 that replaces valine (V) with glutamate (E) and lysine (K), respectively. Consequently, cells with this mutation display constitutive kinase activity and ensuing unregulated cellular growth.
Early results from metastatic melanoma models confirmed that gain of function BRAF signaling was strongly associated with in vivo tumorigenicity while conditional BRAF suppression slowed systemic tumor growth,25 thus making BRAF a rational target for therapeutic inhibition. Early preclinical results demonstrated that the targeted BRAF inhibitor (BRAFi), PLX4032, led to potent antitumor activity of PLX4032 against melanomas harboring the mutant BRAF V600E gene.26
BRAFi therapy in melanoma patients
PLX4032, later renamed as vemurafenib (VMF), was the first BRAFi to be approved by the US Food and Drug Administration (FDA) for the treatment of BRAF-mutated metastatic melanoma. A Phase 2 trial27 of VMF in previously treated BRAF-mutated metastatic melanoma demonstrated an overall response rate of 53% (95% CI, 44%–62%), median progression-free survival (PFS) of 6.8 months (95% CI, 5.6–8.1 months), and a median OS of 15.9 months (95% CI, 11.6–18.3 months). Furthermore, in a subsequent randomized Phase 3 trial of 675 patients with untreated metastatic melanoma,28 VMF significantly improved objective response rates (48% vs 5%) and 6-month OS (84% [95% CI, 78%–89%] vs 64% [95% CI, 56%–73%]) in comparison to the prior standard of care chemotherapy, dacarbazine.
Similar promising results led the FDA to approve another BRAFi, dabrafenib (DAB), for the treatment of metastatic melanoma.29–31 The Phase 2 trial30 of DAB reported a 59% confirmed response (95% CI, 48.2%–70.3%). Median PFS and OS were 6.3 months and 13.1 months, respectively. A subsequent Phase 3 trial of DAB demonstrated an objective response rate of 50% (95% CI, 42.4%–57.1%) with DAB versus 6% (95% CI, 1.8%–15.5%) with dacarbazine. The median PFS was 5.1 months versus 2.7 months, also in favor of DAB. Furthermore, the OS hazard ratio [HR] was 0.61 (95% CI, 0.25–1.48) in favor of DAB.
Despite the potency of BRAFi monotherapy, acquired drug resistance frequently develops through reactivation of the MAPK pathway.32–34 Inhibiting the downstream target of BRAF, MEK kinase, in congruence with BRAF inhibition, has helped overcome limitations of single-agent BRAFi and actually enhanced antitumor activity in metastatic melanoma.35–37 Recently, the results from two Phase 3 trials38,39 support the choice of combined BRAF + MEK inhibitor (MEKi) as the standard treatment in BRAF-mutated metastatic melanoma. In the COMBI-v study,38 DAB and the MEKi trametinib (TRA) demonstrated superior 12-month OS (72% vs 65%; HR: 0.69, P=0.005) and median PFS (11.4 months vs 7.3 months; HR: 0.56, P<0.001) versus VMF monotherapy. Adverse events were decreased with combination therapy, 91% versus 98%. In the COMBI-d study,39 OS was 25.1 months (95% CI, 19.2–unreached) with DAB and TRA versus 18.7 months (95% CI, 15.2–23.7 months) with DAB alone (HR: 0.71, P=0.0004). The 1-year OS and 2-year OS were also superior with BRAF + MEKi (74% and 51% vs 68% and 42%, respectively). Again, adverse events were decreased in the combination group, 87% versus 90%.
BRAFi therapy in MBM
The BBB is thought to be composed of various cells,40 which normally work together to limit intracranial penetration of non-lipophilic agents. As a result, patients with active BM were either excluded or minimally represented in earlier trials. Thus, questions remained whether these drugs would provide a similar benefit intracranially.
The first evidence to support the use of BRAFi in this setting was demonstrated by Rochet et al.41 An MBM patient was started on VMF 960 mg orally twice daily, who despite having been treated with SRS had developed rapid disease progression and neurological deterioration. One month after treatment initiation, the patient displayed a dramatic symptomatic improvement, and within 6 months, all visualized sites of melanoma in the brain were substantially reduced. A pilot study42 of 24 patients with unresectable, previously treated symptomatic MBM helped to validate Rochet et al’s results. The median PFS was 3.9 months (95% CI 3.0–5.5 months) and median OS was 5.3 months (95% CI 3.9–6.6 months) in the study. Forty-two percent (95% CI, 22.1%–63.4%) of patients developed a partial response both intra- and extracranially. Furthermore, >30% intracranial tumor regression was noted in 37% of patients with measurable intracranial disease. A retrospective review43 of 22 metastatic melanoma patients harboring the BRAF mutation with asymptomatic MBM treated with VMF similarly demonstrated a 50% intracranial response and a median survival of 10.6 months. Most recently, another retrospective study44 of 27 patients with BRAF-mutated MBM displayed similar intracranial (50%) and even greater extracranial response (71%) with VMF. The median intracranial PFS was 4.6 months (95% CI, 2.7–7.9 months), and the 1-year OS was 30.4%. Notably, patients with worse performance status and concomitant mutations in the PI3K-AKT pathway had poor outcomes despite VMF therapy.
DAB monotherapy has also been shown to reduce intracranial tumor progression in Phase 1–2 trials involving MBM patients.29,45 Nine out of 10 patients in a Phase 1 dose-escalation trial29 achieved an objective response, with four having a complete response. The Phase 2 BREAK-MB trial45 recruited 172 patients with MBM, of which 139 (81%) had histologically confirmed BRAF V600E mutation. The patients were split into two cohorts, those with no prior local treatment (n=74) and those with disease progression despite local treatment (n=65). Overall, intracranial response was achieved in 39.2% and 30.8% of patients, respectively, following DAB treatment. Additionally, impressive overall disease control (79.7% vs 83.1%) and OS (33.1 weeks vs 31.4 weeks) were seen. These results were confirmed by another study46 of 23 MBM patients treated with DAB. Intracranial and extracranial disease response rates were 78% and 90%, respectively. Median PFS and median OS were 16.3 weeks and 36.6 weeks, respectively.
Radiosensitization effect in melanoma cell lines following BRAF inhibition
Historically, adjuvant radiation for melanoma has been a controversial choice47 as early radiation studies of melanoma demonstrated wide survival curves,48 leading to the belief that melanoma is radioresistant. However, this principle has not been fully supported by clinical data. Chang et al49 examined 56 cutaneous melanoma patients treated with adjuvant radiation and demonstrated equal 5-year locoregional control of 87% with hypofractionation and conventional fractionation. More recently, the ANZMTG 01.02/TROG 02.0150 randomized controlled trial of melanoma patients at high risk of regional relapse displayed superior results for patients treated with adjuvant radiation and lymphadenectomy when compared to lymphadenectomy alone. At a median follow-up of 73 months, 21% of patients experienced relapses in the adjuvant radiotherapy group compared with 36% in the observation group (HR: 0.52 [95% CI, 0.31–0.88], P=0.023).50 An observational, population-based investigation of the National Cancer Database confirmed these results outside the controlled setting of a randomized trial.51 While these studies show that melanoma is not uniformly radioresistant, the use of radiation as a first-line treatment in primary melanoma remains uncommon. Thus, there is a great need to identify those melanoma patients who are most likely to respond to radiotherapy and to discover novel targets that can enhance radiosensitivity.
Sambade et al52 examined the relative sensitivities of multiple irradiated (0–8 Gy) melanoma cell lines in order to determine mechanisms that promote radiosensitivity versus resistance. The various melanoma cell lines displayed a very large range of surviving fraction values, ranging from highly radioresistant to highly radiosensitive. Interestingly, many of the highly radioresistant cell lines were BRAF mutated. The authors thus sought to examine whether inhibition of BRAF with PLX4032 could selectively sensitize BRAF-mutated melanoma cells. Four highly or moderately radioresistant BRAF-mutated cell lines in addition to wild-type cells were pretreated with PLX4032 prior to irradiation and compared to cells incubated with only control. All the BRAF-mutated cell lines demonstrated a statistically significant radiosensitization effect by PLX4032, while no such effect was noticed in the non-mutated BRAF cell lines. Further analysis determined that PLX4032 plus radiation led to an increase in G1 cell cycle arrest in the BRAF-mutated cell lines. By decreasing the amount of cells progressing to the highly resistant S phase, a greater fraction of cells could be destroyed.
Subsequently, Hecht et al53 assessed individual radiosensitivities in 35 blood samples of melanoma patients with or without BRAF inhibition. Each blood sample was divided into two aliquots; one sample was irradiated with 2 Gy and the other was not. Chromosomal aberrations were then analyzed via three-color fluorescence in situ hybridization (FISH). Again, patients who were or had taken BRAFi demonstrated increased radiosensitivity. Interestingly, this increased effect was significantly associated with VMF but not DAB.
BRAFis with radiation therapy for MBM
The radiosensitization effect demonstrated by the prior studies gives hope that BRAFi might enhance the antitumor effect of radiotherapy. This is especially valuable for MBM patients as they are often treated with radiation alone. Although cerebral tumor LC can be achieved with radiotherapy, OS remains poor. Thus, the synergistic effects of concomitant ionizing radiation and BRAF inhibition could improve the prognosis in these patients.
Narayana et al54 first reported on 12 patients treated with radiation and VMF; 50% were treated with SRS alone and 25% each with partial brain radiation therapy and/or WBRT. VMF was administered prior to radiation (58%) or concurrently (42%). The median survival for this study population was 13.7 months. Six-month LC, distal intracranial failure, and OS were 75%, 57%, and 92%, respectively. Although combination therapy demonstrated an impressive response, seven patients were also previously treated with ipilimumab, which may have impacted the overall response.
Two studies subsequently published their own institutional results in order to account for the radiotherapy heterogeneity in the prior analysis. Gaudy-Marqueste et al55 first reported their results of 30 patients who received Gamma Knife Radiosurgery (GKRS) and BRAFi. The majority of patients (86.6%) received VMF, while the others received DAB. Twenty-four (80%) patients received concurrent BRAF inhibition; four (16.7%) of these patients underwent a transient drug interruption (2.5 times the half-lives of VMF or DAB, before and after GKRS). The additional 20 (83.3%) patients had BRAFi following GKRS. Patients receiving no BRAFi interruption in the concomitant cohort were younger when compared to the other cohorts, but were otherwise similar at baseline. The median time to new BM and OS was 12.9 weeks (95% CI, 11.6–14.07 weeks) and 24.8 weeks (95% CI, 10.1–39.6 weeks), respectively. The 6-month survival estimate was 78.8%. Furthermore, 13.3% of lesions had a >20% decrease in size. Ahmed et al56 then reported on 24 patients treated with linear accelerator (LINAC)-based SRS and VMF. The 6- and 12-month LC and distal intracranial failure were 92% and 75%, and 45% and 23%, respectively. There was a trend toward improved survival with higher diagnosis-specific GPA (DS-GPA); 6- and 12-month OS was 61% and 38%, respectively, for classes 1–1.5 and 83% at both time points for class 2 (P=0.07). The median OS was 11.9 months from the date of intracranial metastases; however, this cohort was heavily pretreated with multiple systemic agents, including 70.8% of patients treated with immunotherapy or other targeted agents prior to starting VMF, which could lead to potential bias. Nevertheless, without controls, no conclusion could be drawn about the efficacy of combination treatment.
Most recently, three studies directly compared outcomes between patients treated with SRS alone and SRS with BRAFi. Ly et al57 published results on 52 patients treated with SRS for MBM. Twenty-one (40.4%) patients were BRAF wild type, while 31 (59.6%) were positive for the BRAF mutation; 17 (54.8%) of the 31 BRAF-mutated patients received BRAFi either before or concurrently with SRS (53% – DAB). The authors demonstrated a significantly improved 1-year LC rate for BRAF-mutated patients treated with BRAFi + SRS, 85%, when compared to BRAF-mutated patients treated with SRS alone, 51.5%, and wild-type patients, 67.1% (P=0.0077). BRAFi use did not affect survival: at 1-year, the OS rate was 50.2% and 42.9% for patients with BRAFi + SRS treatment and SRS alone, respectively (P=0.82). Patel et al58 reported on 87 MBM patients treated with SRS, of which 15 (17.2%) also received BRAFi. Fourteen of 15 (93.3%) patients were treated with VMF. Three (20%) patients received BRAFi before SRS (within 5 drug half-lives), 1 (13.3%) received BRAFi concurrently, and 10 (66.7%) patients received BRAFi following SRS. At 1 year, the combined therapy cohort had a trend toward improved OS (64.3% vs 40.4%, P=0.205), LC (96.7% vs 90.4%, P=0.423), and distal intracranial control (36.1% vs 34.9%, P=0.450), though the results were not significant. Finally, Xu et al59 reported their institutional results on 65 patients with MBM treated with GKRS. The patients were stratified into 3 groups: Group A – BRAF-mutated without BRAFi (n=13), Group B – BRAF-mutated with BRAFi (n=17), and Group C – wild-type BRAF (n=35). Twelve of 17 (70.6%) patients who received BRAFi were treated with VMF (2 – concurrent; 10 – post-GKRS), the remainder with DAB (1 – concurrent; 4 – post-GKRS). Six and 12-month OS after SRS was 31% and 31%, 71% and 52%, and 46% and 28%, amongst the three groups respectively (P=0.0018). At 1-year, LC in Groups A, B, and C was 82.4%, 92%, and 69.2%, respectively (P=0.022).
Patel et al60 recently published a preliminary report of six patients treated with SRS combined with BRAFi and MEKi. All patients received DAB and TRA within 3 months of SRS. With a median follow-up of 10.6 months, the authors reported a median OS of 20.0 months from the time of SRS treatment and 23.1 months from the date of combined BRAFi + MEKi administration. At 1 year, the OS and distant intracranial control were 100% and 80%, respectively. Local failure was seen in one lesion 21.7 months following SRS.
When looking at these studies together (Table 1), their findings suggest that indeed there may be a benefit with combining BRAFi and radiation; however, prospective clinical trials are required to confirm these results.
Toxicity of intracranial radiation in conjunction with BRAFis
For systemic agents that act as radiosensitizers, damage is amplified, sometimes synergistically, when combined with radiotherapy; however, there is also potential of worsening toxicity. Indeed, several single institution case reports of patients treated with VMF concurrently or soon after intra- and extracranial radiation resulted in severe dermatologic toxicities.61–64 A larger series by Hecht et al53 involving 70 patients treated with BRAFi and radiotherapy at various sites reported grade 2 and 3 radiodermatitis to be 27% and 9%, respectively. In addition, the authors found that BRAFi given concurrently with WBRT significantly increased the dermatitis rate compared with WBRT alone (44% vs 8%, P<0.001). Nevertheless, the results showed that this combination was feasible with an acceptable increase in toxicity. Further clinical trials (Table 2) investigating this combination will help to prospectively report on these clinical toxicities.
Case series of patients treated with BRAFi and SRS has also noted the development of symptomatic RN. Liebner et al65 reported on two patients who developed RN following VMF and radiotherapy, which was confirmed after salvage surgery. Another group also reported RN in a patient treated with concurrent SRS and VMF.66 However, from case reports alone, the rates of RN following SRS alone, BRAFi alone, or combination therapy are unknown. Gaudy-Marqueste et al55 and Patel et al60 reported 0% radiographic or symptomatic RN, while Narayana et al54 and Ahmed et al56 both reported very low rates of RN, 8.3% and 4.2%, respectively. Xu et al59 found no difference in the development of RN between BRAF-mutated patients treated with BRAFi and GKRS versus BRAF-mutated patients treated with GKRS alone (17.6% vs 8.6%, P=0.721). In contrast, Patel et al58 found a statistically significant difference in symptomatic RN between SRS and BRAFi and SRS alone cohorts (28.2% vs 11.1%, P<0.001). Unlike the other prior studies that used Kaplan–Meier statistics to determine rates of RN or adverse events, the authors utilized a cumulative incidence model with death as a competing risk for intracranial outcomes. Furthermore, the BRAFi cohort was a significant factor for symptomatic RN in univariate and multivariate analyses.
Another concerning side effect for MBM treated with radiation therapy and BRAFi may be intracranial hemorrhage (ICH). Although initial studies of BRAFi alone in the setting of BM42,45 reported minimal intracranial toxicity, including hemorrhage, Lee et al67 noted the development of ICH in a patient treated with Cyberknife radiosurgery in combination with DAB and the MEKi TRA. Although Xu et al59 did note an increased rate of ICH in BRAF-mutated patients treated with BRAFi and radiation versus BRAF-mutated patients treated with radiotherapy alone (29.4% vs 8.6%), the results were not significant (P=0.487). In contrast, Ly et al57 reported a significantly increased risk of hemorrhage in BRAF-mutated patients treated with BRAFi and radiation. The 1-year rates of freedom from intratumoral hemorrhage were 39.3% and 77.0% in patients treated with SRS and BRAFi and SRS alone, respectively (P=0.0003).
Because of these inconclusive reports (Table 1), the new consensus guidelines from the Eastern Cooperative Oncology Group (ECOG) recommend holding BRAFi ≥1 day before and after SRS and ≥3 days before and after fractionated radiosurgery.68 In addition, ECOG recommends a radiation dose per fraction <4 Gy unless using a stereotactic approach or if the patient has very poor prognosis/performance status.
Upcoming clinical trials investigating BRAFi ± MEKi or radiotherapy for MBM
There are currently several Phase 2 clinical trials of BRAFi ± MEKi or radiotherapy for MBM (Table 2). Two trials, both evaluating VMF monotherapy in MBM, have been completed, with the final results currently pending. The first (ClinicalTrials.gov NCT01378975) is a multicenter study of 146 BRAF-mutated patients divided into a cohort of previously untreated participants (Cohort A) and another cohort of participants previously treated with SRS, WBRT, or surgery for MBM (Cohort B). The primary outcome is the best overall response rate in the brain in Cohort A. The second trial (ClinicalTrials.gov NCT01781026), at Yale University, is assessing intracranial response activity in two patients with BRAF V00E or K mutation receiving neoadjuvant VMF for untreated MBM with a goal of providing definitive local therapy at 8 weeks.
Three studies are assessing combined BRAFi ± MEKi therapy for MBM. The first (ClinicalTrials.gov NCT02039947) is an ongoing, but not currently recruiting, multicenter study of 120 patients that is investigating DAB and TRA in patients with histologically confirmed BRAF V600E, K, D, or R mutation and at least one measurable, previously untreated MBM. The primary outcome is intracranial response rate. The second trial (ClinicalTrials.gov NCT02537600), better known as the CONVERCE trial, is a multi-institution, European single-arm, Phase 2 study with a planned enrollment of 137 BRAF-mutated patients with documented BRAF-mutation; patients must have at least one measurable MBM between 5 and 40 mm in one dimension. The patients will be divided into three cohorts, Cohort A (neurologically asymptomatic patients without prior local therapy), Cohort B (neurologically asymptomatic patients with prior local therapy), and Cohort C (neurologically symptomatic patients with or without prior local therapy). All patients will be treated with combined VMF and cobimetinib with a primary outcome of intracranial response rate in Cohort A. The third ongoing trial (ClinicalTrials.gov NCT01978236) is a multicenter study of 30 BRAF V600E/K-mutated patients with resectable MBM being treated with either DAB alone or DAB and TRA. The primary purpose of this study is to determine the levels and distribution of DAB, its metabolites, and TRA (Cohort B only) in parenchymal MBM, extracranial metastases, and peripheral blood.
There is currently one Phase 2 trial investigating the combination of BRAFi and radiotherapy. This study (ClinicalTrials.gov NCT01721603), at The University of California, San Francisco (UCSF), will primarily assess intracranial response in 39 BRAF V600E-mutated patients with four or less MBM and no lesion of >3 cm treated with GKRS following 28 days of DAB therapy.
Conclusion
Over the past 10 years, the arrival of novel therapeutics, including BRAFi and immunotherapy, has changed the overall landscape for malignant melanoma. Despite these advancements, 20%–50% of patients still develop intracranial metastases for which the mainstay of therapy remains radiation, with surgery reserved for symptomatic or refractory cases. BRAFis for MBM have demonstrated promising results both intra- and extracranially. Retrospective studies54–59 suggest that the combination of BRAFi with radiation may have a synergistic effect leading to improved outcomes. These clinical studies, however, are limited by their retrospective design and likely selection bias. Currently, there is one ongoing prospective trial combining BRAFi and SRS for MBM (ClinicalTrials.gov NCT01721603). This Phase 2 trial at UCSF will assess intracranial response in patients with MBM treated with GKRS following 28 days of DAB therapy. Further clinical investigations such as this, as well as studies combining BRAFi and MEKi ± radiation, are warranted to provide definitive evidence-based data regarding the efficacy and safety of these agents in the treatment of MBM. In the interim, the combination of BRAFi and radiation is feasible and appropriate, though clinicians are advised to hold the drug before and after radiotherapy. BRAFi may also increase the risk of RN from SRS; until prospective clinical trials report on the safety of this combination, hypofractionated SRS may be a method to mitigate this toxicity in situations where the risk of RN may already be higher (eg, larger lesions, resection cavities).
Disclosure
The authors report no conflicts of interest in this work.
References
DeAngelis LM, Posner JB. Intracranial metastases. In: DeAngelis LM, Posner JB, editors. Neurologic Complications of Cancer. New York, NY: Oxford University Press; 2009:141–193. | ||
Nayak L, Lee EQ, Wen PY. Epidemiology of brain metastases. Curr Oncol Rep. 2012;14(1):48–54. | ||
Fife KM, Colman MH, Stevens GN, et al. Determinants of outcome in melanoma patients with cerebral metastases. J Clin Oncol. 2004;22(7):1293–1300. | ||
Sampson JH, Carter JH Jr, Friedman AH, Seigler HF. Demographics, prognosis, and therapy in 702 patients with brain metastases from malignant melanoma. J Neurosurg. 1998;88(1):11–20. | ||
de la Monte SM, Moore GW, Hutchins GM. Patterned distribution of metastases from malignant melanoma in humans. Cancer Res. 1983;43(7):3427–3433. | ||
Fonkem E, Uhlmann EJ, Floyd SR, et al. Melanoma brain metastasis: overview of current management and emerging targeted therapies. Expert Rev Neurother. 2012;12(10):1207–1215. | ||
Sperduto PW, Berkey B, Gaspar LE, Mehta M, Curran W. A new prognostic index and comparison to three other indices for patients with brain metastases: an analysis of 1,960 patients in the RTOG database. Int J Radiat Oncol Biol Phys. 2008;70(2):510–514. | ||
Sperduto PW, Chao ST, Sneed PK, et al. Diagnosis-specific prognostic factors, indexes, and treatment outcomes for patients with newly diagnosed brain metastases: a multi-institutional analysis of 4,259 patients. Int J Radiat Oncol Biol Phys. 2010;77(3):655–661. | ||
Steeg PS, Camphausen KA, Smith QR. Brain metastases as preventive and therapeutic targets. Nat Rev Cancer. 2011;11(5):352–363. | ||
Kocher M, Soffietti R, Abacioglu U, et al. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952–26001 study. J Clin Oncol. 2011;29(2):134–141. | ||
Chang EL, Wefel JS, Hess KR, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol. 2009;10:1037–1044. | ||
Brown PD, Jaeckle K, Ballman KV, et al. Effect of radiosurgery alone vs radiosurgery with whole brain radiation therapy on cognitive function in patients with 1 to 3 brain metastases: a randomized clinical trial. JAMA. 2016;316(4):401–409. | ||
Aoyama H, Shirato H, Tago M, et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA. 2006;295(21):2483–2491. | ||
Yamamoto M, Serizawa T, Shuto T, et al. Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): a multi-institutional prospective observational study. Lancet Oncol. 2014;15(4):387–395. | ||
Fogarty GB, Hong A, Gondi V, et al. Debate: adjuvant whole brain radiotherapy or not? More data is the wiser choice. BMC Cancer. 2016;16:372. | ||
Mehta MP. The controversy surrounding the use of whole-brain radiotherapy in brain metastases patients. Neuro Oncol. 2015;17(7):919–923. | ||
Mathieu D, Kondziolka D, Cooper PB, et al. Gamma knife radiosurgery in the management of malignant melanoma brain metastases. Neurosurgery. 2007;60(3):471–481. [discussion 472–481]. | ||
Yu C, Chen J, Apuzzo ML, et al. Metastatic melanoma to the brain: prognostic factors after gamma knife radiosurgery. Int J Radiat Oncol Biol Phys. 2002;52(5):1277–1287. | ||
Neal MT, Chan MD, Lucas JT Jr, et al. Predictors of survival, neurologic death, local failure, and distant failure after gamma knife radiosurgery for melanoma brain metastases. World Neurosurg. 2014;82(6):1250–1255. | ||
Shaw E, Scott C, Souhami L, et al. Radiosurgery for the treatment of previously irradiated recurrent primary brain tumors and brain metastases: initial report of radiation therapy oncology group protocol (90–05). Int J Radiat Oncol Biol Phys. 2000;34(3):647–654. | ||
Chao ST, Ahluwalia MS, Barnett GH, et al. Challenges with the diagnosis and treatment of cerebral radiation necrosis. Int J Radiat Oncol Biol Phys. 2013;87(3):449–457. | ||
Minniti G, Clarke E, Lanzetta G, et al. Stereotactic radiosurgery for brain metastases: analysis of outcome and risk of brain radionecrosis. Radiat Oncol. 2011;6:48. | ||
Eaton BR, Gebhardt B, Prabhu RS, Shu HK, Curran W, Crocker I. Hypofractionated radiosurgery for intact or resected brain metastases: defining the optimal dose and fractionation. Radiat Oncol. 2013;8:135. | ||
Lito P, Rosen N, Solit DB. Tumor adaptation and resistance to RAF inhibitors. Nat Med. 2013;19(11):1401–1409. | ||
Hoeflich KP, Gray DC, Eby MT, et al. Oncogenic BRAF is required for tumor growth and maintenance in melanoma models. Cancer Res. 2006;66(2):999–1006. | ||
Yang H, Higgins B, Kolinsky K, et al. RG7204 (PLX4032), a selective BRAFV600E inhibitor, displays potent antitumor activity in preclinical melanoma models. Cancer Res. 2010;70(13):5518–5527. | ||
Sosman JA, Kim KB, Schuchter L, et al. Survival in BRAF V600-mutant advanced melanoma treated with vemurafenib. N Engl J Med. 2012;366(8):707–714. | ||
Chapman PB, Hauschild A, Robert C, et al; BRIM-3 Study Group. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364(26):2507–2516. | ||
Falchook GS, Long GV, Kurzrock R, et al. Dabrafenib in patients with melanoma, untreated brain metastases, and other solid tumours: a phase 1 dose-escalation trial. Lancet. 2012;379(9829):1893–1901. | ||
Ascierto PA, Minor D, Ribas A, et al. Phase II trial (BREAK-2) of the BRAF inhibitor dabrafenib (GSK2118436) in patients with metastatic melanoma. J Clin Oncol. 2013;31(26):3205–3211. | ||
Hauschild A, Grob JJ, Demidov LV, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomized controlled trial. Lancet. 2012;380:358–365. | ||
Van Allen EM, Wagle N, Sucker A, et al; Dermatologic Cooperative Oncology Group of Germany (DeCOG). The genetic landscape of clinical resistance to RAF inhibition in metastatic melanoma. Cancer Discov. 2014;4(1):94–109. | ||
Shi H, Hugo W, Kong X, et al. Acquired resistance and clonal evolution in melanoma during BRAF inhibitor therapy. Cancer Discov. 2014;4(1):80–93. | ||
Solit DB, Rosen N. Resistance to BRAF inhibition in melanomas. N Engl J Med. 2011;364:772–774. | ||
Flaherty KT, Infante JR, Daud A, et al. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N Engl J Med. 2012;367(18):1694–1703. | ||
Long GV, Stroyakovskiy D, Gogas H, et al. Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. N Engl J Med. 2014;371(20):1877–1888. | ||
Larkin J, Ascierto PA, Dreno B, et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N Engl J Med. 2014;371(20):1867–1876. | ||
Robert C, Karaszewska B, Schachter J, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med. 2015;372(1):30–39. | ||
Long GV, Stroyakovskiy D, Gogas H, et al. Dabrafenib and trametinib versus dabrafenib and placebo for Val600 BRAF-mutant melanoma: a multicentre, double-blind, phase 3 randomised controlled trial. Lancet. 2015;386(9992):444–451. | ||
Carson MJ, Doose JM, Melchior B, Schmid CD, Ploix CC. CNS immune privilege: hiding in plain sight. Immunol Rev. 2006;213:48–65. | ||
Rochet NM, Kottschade LA, Markovic SN. Vemurafenib for melanoma metastases to the brain. N Engl J Med. 2011;365(25):2439–2441. | ||
Dummer R, Goldinger SM, Turtschi CP, et al. Vemurafenib in patients with BRAF(V600) mutation-positive melanoma with symptomatic brain metastases: final results of an open-label pilot study. Eur J Cancer. 2014;50(3):611–621. | ||
Dzienis MR, Atkinson VG. Response rate to vemurafenib in patients with B-RAF-positive melanoma brain metastases: a retrospective review. Melanoma Res. 2014;24(4):349–353. | ||
Harding JJ, Catalanotti F, Munhoz RR, et al. A retrospective evaluation of vemurafenib as treatment for BRAF-mutant melanoma brain metastases. Oncologist. 2015;20(7):789–797. | ||
Long GV, Trefzer U, Davies MA, et al. Dabrafenib in patients with Val600Glu or Val600Lys BRAF-mutant melanoma metastatic to the brain (BREAK-MB): a multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13(11):1087–1095. | ||
Azer MW, Menzies AM, Haydu LE, Kefford RF, Long GV. Patterns of response and progression in patients with BRAF-mutant melanoma metastatic to the brain who were treated with dabrafenib. Cancer. 2014;120(4):530–536. | ||
Stevens G, McKay MJ. Dispelling the myths surrounding radiotherapy for treatment of cutaneous melanoma. Cancer Res. 2010;70(13):5518–5527. | ||
Rofstad EK, Brustad T. Broad-shouldered survival curves of a human melanoma xenograft. Implications for radiation therapy in the absence and presence of misonidazole. Acta Radiol. 1981;20(4):261–265. | ||
Chang DT, Amdur RJ, Morris CG, Mendenhall WM. Adjuvant radiotherapy for cutaneous melanoma: comparing hypofractionation to conventional fractionation. Int J Radiat Oncol Biol Phys. 2006;66(4):1051–1055. | ||
Henderson MA, Burmeister BH, Ainslie J, et al. Adjuvant lymph-node field radiotherapy versus observation only in patients with melanoma at high risk of further lymph-node field relapse after lymphadenectomy (ANZMTG 01.02/TROG 02.01): 6-year follow-up of a phase 3, randomised controlled trial. Lancet Oncol. 2015;16(9):1049–1060. | ||
Danish HH, Patel KR, Switchenko JM, et al. The influence of postoperative lymph node radiation therapy on overall survival of patients with stage III melanoma, a National Cancer Database analysis. Melanoma Research. 2016;26(6):595–603. | ||
Sambade MJ, Peters EC, Thomas NE, Kaufmann WK, Kimple RJ, Shields JM. Melanoma cells show a heterogeneous range of sensitivity to ionizing radiation and are radiosensitized by inhibition of B-RAF with PLX-4032. Radiother Oncol. 2011;98(3):394–399. | ||
Hecht M, Zimmer L, Loquai C, et al. Radiosensitization by BRAF inhibitor therapy-mechanism and frequency of toxicity in melanoma patients. Ann Oncol. 2015;26(6):1238–1244. | ||
Narayana A, Mathew M, Tam M, et al. Vemurafenib and radiation therapy in melanoma brain metastases. J Neurooncol. 2013;113(3):411–416. | ||
Gaudy-Marqueste C, Carron R, Delsanti C, et al. On demand Gamma-Knife strategy can be safely combined with BRAF inhibitors for the treatment of melanoma brain metastases. Ann Oncol. 2014;25(10):2086–2091. | ||
Ahmed KA, Freilich JM, Sloot S, et al. LINAC-based stereotactic radiosurgery to the brain with concurrent vemurafenib for melanoma metastases. J Neurooncol. 2015;122(1):121–126. | ||
Ly D, Bagshaw HP, Anker CJ, et al. Local control after stereotactic radiosurgery for brain metastases in patients with melanoma with and without BRAF mutation and treatment. J Neurosurg. 2015;123(2):395–401. | ||
Patel KR, Chowdhary M, Switchenko JM, et al. BRAF inhibitor and stereotactic radiosurgery is associated with an increased risk of radiation necrosis. Melanoma Res. 2016;26(4):387–394. | ||
Xu Z, Lee CC, Ramesh A, et al. BRAF V600E mutation and BRAF kinase inhibitors in conjunction with stereotactic radiosurgery for intracranial melanoma metastases. J Neurosurg. Epub 2016 May 20. | ||
Patel BG, Ahmed KA, Johnstone PA, Yu HH, Etame AB. Initial experience with combined BRAF and MEK inhibition with stereotactic radiosurgery for BRAF mutant melanoma brain metastases. Melanoma Res. 2016;26(4):382–386. | ||
Boussemart L, Boivin C, Claveau J, et al. Vemurafenib and radiosensitization. JAMA Dermatol. 2013;149(7):855–857. | ||
Anker CJ, Ribas A, Grossmann AH, et al. Severe liver and skin toxicity after radiation and vemurafenib in metastatic melanoma. J Clin Oncol. 2013;31(17):e283–e287. | ||
Harding JJ, Barker CA, Carvajal RD, Wolchok JD, Chapman PB, Lacouture ME. Cutis verticis gyrata in association with vemurafenib and whole-brain radiotherapy. J Clin Oncol. 2014;32(14):e54–e56. | ||
Schulze B, Meissner M, Wolter M, Rodel C, Weiss C. Unusual acute and delayed skin reactions during and after whole-brain radiotherapy in combination with the BRAF inhibitor vemurafenib. Two case reports. Strahlenther Onkol. 2014;190(2):229–232. | ||
Liebner DA, Walston SA, Cavaliere R, et al. Radiation necrosis mimicking rapid intracranial progression of melanoma metastasis in two patients treated with vemurafenib. Melanoma Res. 2014;24(2):172–176. | ||
Peuvrel L, Ruellan AL, Thillays F, et al. Severe radiotherapy-induced extracutaneous toxicity under vemurafenib. Eur J Dermatol. 2013;23(6):879–881. | ||
Lee LM, Feun L, Tan Y. A case of intracranial hemorrhage caused by combined dabrafenib and trametinib therapy for metastatic melanoma. Am J Case Rep. 2014;15:441–443. | ||
Anker CJ, Grossmann KF, Atkins MB, Suneja G, Tarhini AA, Kirkwood JM. Avoiding severe toxicity from combined BRAF inhibitor and radiation treatment: consensus guidelines from the Eastern Cooperative Oncology Group (ECOG). Int J Radiat Oncol Biol Phys. 2016;95(2):632–646. | ||
Khan N, Khan MK, Almasan A, Singh AD, Macklis R. The evolving role of radiation therapy in the management of malignant melanoma. Int J Radiat Oncol Biol Phys. 2011;80(3):645–654. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


