Back to Journals » Journal of Pain Research » Volume 11
Botulinum toxin-A for the treatment of neuralgia: a systematic review and meta-analysis
Authors Meng F , Peng K , Yang JP, Ji FH , Xia F, Meng XW
Received 22 March 2018
Accepted for publication 24 July 2018
Published 12 October 2018 Volume 2018:11 Pages 2343—2351
DOI https://doi.org/10.2147/JPR.S168650
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Katherine Hanlon
Fan Meng, Ke Peng, Jian-Ping Yang, Fu-Hai Ji, Fan Xia, Xiao-Wen Meng
Department of Anesthesiology, First Affiliated Hospital of Soochow University, Suzhou, Jiangsu, China
Aim: This meta-analysis was performed to evaluate the efficacy and safety of botulinum toxin-A (BTX-A) for the treatment of neuralgia.
Methods: We searched PubMed, EMBASE, and Cochrane databases to identify randomized controlled trials (RCTs) comparing BTX-A treatment with saline for alleviating neuropathic pain. Primary outcome measures were pain scores up to 24 weeks after treatment. Secondary outcomes were hours of sleep, Short Form-36 (SF-36) life quality questionnaire, and adverse events. We used Review Manager 5.3 for the data analyses.
Results: Twelve RCTs were included (n=495). Pain scores in the BTX-A group were significantly lower compared to the saline group at 4 weeks (mean difference [MD] =–1.64, 95% CI [–3.21, –0.07], P=0.04), 12 weeks (MD =–1.49, 95% CI [−2.05, –0.93], P<0.00001), and 24 weeks (MD =–1.61, 95% CI [−2.81, –0.40], P=0.009). There were no significant differences in hours of sleep, SF-36 questionnaire, or the incidence of injection pain or hematoma between the two groups. No serious adverse events associated with BTX-A were noted. Fourteen out of 108 patients (12.9%) with trigeminal neuralgia experienced mild facial asymmetry after the BTX-A treatment.
Conclusion: Based on the current evidence, BTX-A may be an effective and safe option for the treatment of neuralgia. Due to the limited number of patients included in this meta-analysis, more trials are still needed to confirm these results.
Keywords: botulinum toxin, neuralgia, neuropathic pain, meta-analysis
Introduction
Clinical examples of neuropathic pain include trigeminal neuralgia, diabetic neuropathic pain, postherpetic neuralgia, and postsurgical neuralgia. Of note, neuropathic pain is often difficult to treat and can lead to anxiety and depression, which seriously compromise patients’ quality of life.1
Over the years, the pharmacotherapies used for neuropathic pain include pregabalin, gabapentin, antidepressants, anticonvulsants, carbamazepine, lamotrigine, opioids, and so on. However, the evidence for these interventions is often inconclusive.2 In addition, side effects associated with the medications such as dizziness, ataxia, nausea, vomiting, somnolence, and rash can be troublesome and debilitating.3
Botulinum toxin-A (BTX-A), a potent neurotoxin produced by Clostridium botulinum, blocks acetylcholine release at neuromuscular junctions and causes muscle relaxation.4 Animal studies have indicated several possible mechanisms for the analgesic effects of BTX-A: 1) it inhibits the release of pain mediators from both motor and sensory neurons, and blocks the release of calcitonin gene-related peptide and other neuropeptide;5 2) it reduces chronic inflammation and acute injury by inhibiting neurotransmitter release;6 and 3) it deactivates the sodium channel in central nerve system neurons.7
Recent studies have shown promising analgesic effects of BTX-A on postherpetic neuralgia, trigeminal neuralgia, and other types of neuralgia.8–19 However, sample sizes of the previous studies are relatively small. In this systematic review and meta-analysis, we aim to evaluate the safety and efficacy of BTX-A in the treatment of neuralgia based on the evidence from randomized controlled trials (RCTs).
Methods
Search strategy
This study adheres to the guidelines of PRISMA statement and the recommendations of the Cochrane Collaboration (Table S1).20,21
FM and KP independently searched PubMed, EMBASE, and Cochrane databases without language or publication date restrictions. The search strategies are shown in Table S2. Additional articles in the references and reviews were manually searched.
Inclusion criteria
The studies that fulfilled the following criteria were included in the meta-analysis:
- Study design: RCT;
- participants: adult patients with neuralgia;
- interventions: use of BTX-A compared to saline;
- outcome measures: pain scores, hours of sleep, the Short Form-36 (SF-36) life quality questionnaire, and adverse events.
Exclusion criteria
The studies that met the following criteria were excluded:
- Insufficient data;
- case reports, reviews, abstracts, editorials, or letters;
- in vitro or animal experiments.
Data extraction
FM and KP independently screened the articles and extracted the following data: first author, publication date, country, sample size, groups and interventions, dose of BTX-A, and main outcomes. Any disagreement over study selection or data extraction was resolved by group discussion.
Primary and secondary outcomes
The primary outcome was the pain scores in visual analog scale (VAS) or numerical rating scale (NRS)22 at 4, 12, and 24 weeks after treatment (0= no pain, 10= maximum pain imaginable). The secondary outcomes were changes in hours of sleep and SF-36 at 12 weeks, and adverse events associated with the procedure.
Quality assessment
FM and KP independently assessed the risk of bias of the included studies using the Cochrane Collaboration tool.21 This tool includes seven areas: the generation of random sequence, allocation concealment, participant blind method, result evaluation blind method, data of incomplete results, report of selected results, and other potential sources of bias. For each area, the risk was rated as “high”, “low”, or “unclear”. Any disagreement over quality assessment was resolved by group discussion.
Statistical analysis
Data analyses were performed using Review Manager 5.3 (The Cochrane Collaboration, Copenhagen, Denmark). For continuous variables, mean differences (MDs) with 95% CIs were calculated, and for dichotomous variables, ORs with 95% CIs were calculated. I2 was used to evaluate heterogeneity, with I2>50% indicating significant heterogeneity.23 In view of the clinical heterogeneity, we applied a random-effects model in this meta-analysis.24 A P-value of <0.05 denoted statistical significance.
Results
Literature search and study characteristics
The PRISMA flow diagram is shown in Figure 1. Of the 211 articles initially identified, 56 duplicates were excluded. After reviewing the titles and abstracts, 38 articles remained. Finally, 12 RCTs were included in this meta-analysis.8–19 The study characteristics are presented in Table 1. A total of 495 patients (266 in the BTX-A group and 229 in the saline group) were followed up for 8–24 weeks after the treatment.
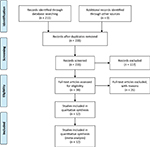  | Figure 1 PRISMA flow diagram. Note: Copyright © 2009. PLOS. Adapted from Moher D, Liberati A, Tetziaff J, Altman DG. The PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. PLoS Med. 2009;6(6):e1000097.33 |
Risk-of-bias assessment
The risk-of-bias assessment is summarized in Figure 2. All trials were randomized and double-blind. Two studies did not detail the random sequence generation,11,17 and two did not adequately report the allocation concealment.11,15
  | Figure 2 Cochrane risk-of-bias assessment: (A) risk-of-bias graph; and (B) risk-of-bias summary. |
Pain scores
As shown in Figure 3, the BTX-A group had significantly lower pain scores than the saline group at 4 weeks (MD =–1.64, 95% CI [−3.21, –0.07], P=0.04), 12 weeks (MD =–1.49, 95% CI [–2.05, –0.93], P<0.00001), and 24 weeks (MD =–1.61, 95% CI [–2.81, –0.40], P=0.009).
  | Figure 3 BTX-A vs saline for the treatment of neuralgia: pain scores at 4, 12, and 24 weeks. Abbreviations: BTX-A, botulinum toxin-A; IV, inverse variance. |
Secondary outcomes
As shown in Figure 4, there were no significant differences in changes in hours of sleep (MD =0.37, 95% CI [–0.28, 1.02], P=0.27) or SF-36 scores (MD =52.50, 95% CI [–35.88, 140.87], P=0.24) at 12 weeks between the two groups.
The incidences of adverse events are presented in Figure 5. No significant difference in injection pain (OR =0.89, 95% CI [0.48, 1.64], P=0.71) or hematoma (OR =0.93, 95% CI [0.24, 3.67], P=0.92) was found between the groups. However, 14 out of 108 (12.9%) patients with trigeminal neuralgia reported mild facial asymmetry after BTX-A treatment (OR =7.5, 95% CI [1.64, 34.21], P=0.009).
  | Figure 5 BTX-A vs saline for the treatment of neuralgia: adverse events. Abbreviations: BTX-A, botulinum toxin-A; M-H, Mantel–Haenszel. |
Discussion
In this study, we comprehensively summarized the current evidence and found lower pain scores up to 24 weeks after BTX-A treatment compared with saline in patients with neuropathic pain. Additionally, there were no serious adverse events associated with BTX-A injections.
Recently, there are several published meta-analyses regarding the use of BTX-A in neuralgia. Morra et al25 evaluated BTX-A therapy in trigeminal neuralgia, suggesting that BTX-A may be an effective and safe option. However, only four RCTs were included in their study. Shackleton et al3 analyzed six RCTs and showed that BTX-A alleviated trigeminal neuralgia and postherpetic neuralgia. Another meta-analysis by Lakhan et al26 indicated that BTX-A treatment improved diabetic neuropathic pain with the results of only two trials. In our study, we included 12 RCTs to determine the effects of BTX-A treatment at three different time points (4, 12, and 24 weeks). Our results suggested that BTX-A treatment could relieve neuropathic pain both in the short term and in the long run.
This meta-analysis included studies of trigeminal neuralgia, postherpetic neuralgia, diabetic neuropathic pain, and other types of chronic neuropathic pain (peripheral neuropathic pain, thoracic outlet syndrome, piriformis syndrome, and spinal cord injury). Of these, piriformis syndrome is a myofascial condition caused by a nerve entrapment where BTX-A injections reduce the pain by relieving muscle spasms. In cases of other conditions responsible for neuropathic pain including occipital neuralgia, carpal tunnel syndrome, and phantom limb pain, BTX-A was also reported to help reduce the pain. For six patients with occipital neuralgia, BTX-A improved the sharp/shooting type of pain.27 One report showed that BTX-A relieved symptoms in five women with carpal tunnel syndrome suggesting the long-lasting antinociceptive effects of BTX-A,28 while another study found it did not provide significant relief of carpal tunnel syndrome symptoms.29 For amputee patients, BTX-A resulted in improvement of residual limb pain and pain tolerance.30 However, no RCT for these types of neuropathic pain could be identified through our literature search.
The injection dose of BTX-A varied among the included studies. Most patients received a dose of 100–200 U, subcutaneously, intradermally, or intramuscularly. For patients with postherpetic neuralgia and trigeminal neuralgia, subcutaneous or intradermal injections of BTX-A were used, which mainly acted by blocking peripheral nerve endings. For other types of chronic neuropathic pain, intramuscular injections were carried out to help relieve muscle spasms.4
In our study, BTX-A injections failed to improve hours of sleep or quality of life surveyed by SF-36 questionnaire in patients with neuropathic pain. These results did not parallel with the improvement of pain scores after BTX-A treatment. Apart from the limited number of studies included for these two measurements, the complexity of contributory factors in life quality should also be noted for this inconsistency.
Regarding the safety of BTX-A, no significant adverse effects were noted. Overall, 59 out of 142 (41.5%) patients in the BTX-A group and 59 out of 137 (43.1%) patients in the saline group reported injection pain. Besides, there were seven out of 128 (5.5%) and seven out of 92 (7.6%) patients who exhibited hematoma at injection site. Out of 108 patients with trigeminal neuralgia, 14 (12.9%) developed mild facial asymmetry after the BTX-A treatment. However, this symptom was self-limited without the need for other interventions. Compared to trigeminal neuralgia attacks, patients generally reported this complication tolerable.
Limitations
There are several limitations of this study. First, the current literature was limited, and the sample size was relatively small. Second, we were not able to explore the potential publication bias through funnel plot due to the insufficient number of studies. Third, some studies suggested that NRS and VAS for pain are not necessarily interchangeable in some clinical situations;31,32 thus, potential bias may exist by pooling and analyzing these data. Next, heterogeneity exists for some of the results, so they should be interpreted with caution. Last, our study was underpowered to detect any effect of BTX-A on patients’ quality of life.
Conclusion
This study suggests that BTX-A may be a good choice for the treatment of neuralgia. More well-designed RCTs with large sample sizes are needed to confirm these findings.
Acknowledgments
This work was supported, in part, by grants from the National Natural Science Foundation of China (81601659 to KP, 81471835 and 81671880 to FHJ) and the Jiangsu Provincial Medical Youth Talents Program (QNRC2016741 to KP).
Disclosure
The authors report no conflicts of interest in this work.
References
Baron R, Binder A, Wasner G, Diagnosis Neuropathic Pain: Pathophysiological mechanisms, and treatment. Lancet Neurol. 2010;9(8):807–819. | ||
Dosenovic S, Jelicic Kadic A, Miljanovic M, et al. Interventions for Neuropathic Pain: An Overview of Systematic Reviews. Anesth Analg. 2017;125(2):643–652. | ||
Shackleton T, Ram S, Black M, Ryder J, Clark GT, Enciso R. The efficacy of botulinum toxin for the treatment of trigeminal and postherpetic neuralgia: a systematic review with meta-analyses. Oral Surg Oral Med Oral Pathol Oral Radiol. 2016;122(1):61–71. | ||
Sandrini G, de Icco R, Tassorelli C, Smania N, Tamburin S. Botulinum neurotoxin type A for the treatment of pain: not just in migraine and trigeminal neuralgia. J Headache Pain. 2017;18(1):38. | ||
Durham PL, Cady R. Insights into the mechanism of onabotulinumtoxinA in chronic migraine. Headache. 2011;51(10):1573–1577. | ||
Lucioni A, Bales GT, Lotan TL, Mcgehee DS, Cook SP, Rapp DE. Botulinum toxin type A inhibits sensory neuropeptide release in rat bladder models of acute injury and chronic inflammation. BJU Int. 2008;101(3):366–370. | ||
Shin MC, Wakita M, Xie DJ, et al. Inhibition of membrane Na+ channels by A type botulinum toxin at femtomolar concentrations in central and peripheral neurons. J Pharmacol Sci. 2012;118(1):33–42. | ||
Apalla Z, Sotiriou E, Lallas A, Lazaridou E, Ioannides D. Botulinum toxin A in postherpetic neuralgia: a parallel, randomized, double-blind, single-dose, placebo-controlled trial. Clin J Pain. 2013;29(10):857–864. | ||
Attal N, de Andrade DC, Adam F, et al. Safety and efficacy of repeated injections of botulinum toxin A in peripheral neuropathic pain (BOTNEP): a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2016;15(6):555–565. | ||
Finlayson HC, O’Connor RJ, Brasher PM, Travlos A. Botulinum toxin injection for management of thoracic outlet syndrome: a double-blind, randomized, controlled trial. Pain. 2011;152(9):2023–2028. | ||
Fishman LM, Wilkins AN, Rosner B. Electrophysiologically identified piriformis syndrome is successfully treated with incobotulinum toxin a and physical therapy. Muscle Nerve. 2017;56(2):258–263. | ||
Han ZA, Song DH, Oh HM, Chung ME. Botulinum toxin type A for neuropathic pain in patients with spinal cord injury. Ann Neurol. 2016;79(4):569–578. | ||
Ranoux D, Attal N, Morain F, Bouhassira D. Botulinum toxin type A induces direct analgesic effects in chronic neuropathic pain. Ann Neurol. 2008;64(3):274–283. | ||
Shehata HS, El-Tamawy MS, Shalaby NM, Ramzy G. Botulinum toxin-type A: could it be an effective treatment option in intractable trigeminal neuralgia? J Headache Pain. 2013;14:92. | ||
Wu CJ, Lian YJ, Zheng YK, et al. Botulinum toxin type A for the treatment of trigeminal neuralgia: results from a randomized, double-blind, placebo-controlled trial. Cephalalgia. 2012;32(6):443–450. | ||
Xiao L, Mackey S, Hui H, Xong D, Zhang Q, Zhang D. Subcutaneous injection of botulinum toxin a is beneficial in postherpetic neuralgia. Pain Med. 2010;11(12):1827–1833. | ||
Yuan RY, Sheu JJ, Yu JM, et al. Botulinum toxin for diabetic neuropathic pain: a randomized double-blind crossover trial. Neurology. 2009;72(17):1473–1478. | ||
Zhang H, Lian Y, Ma Y, et al. Two doses of botulinum toxin type A for the treatment of trigeminal neuralgia: observation of therapeutic effect from a randomized, double-blind, placebo-controlled trial. J Headache Pain. 2014;15(1):65. | ||
Zúñiga C, Piedimonte F, Díaz S, Micheli F. Acute treatment of trigeminal neuralgia with onabotulinum toxin A. Clin Neuropharmacol. 2013;36(5):146–150. | ||
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group, Group P, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8(5):336–341. | ||
Higgins J, Green S. Cochrane handbook for systematic reviews of interventions. Cochrane Collaboration. 2011; Version 5.1.0. Available from: http://handbook-5-1.cochrane.org/. Accessed March 19, 2018. | ||
Heller GZ, Manuguerra M, Chow R. How to analyze the Visual Analogue Scale: Myths, truths and clinical relevance. Scand J Pain. 2016;13:67–75. | ||
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. | ||
Peng K, Chen WR, Meng XW, Zhang J, Ji FH, Fh J. Intra-articular dexmedetomidine in knee arthroscopy: A systematic review and meta-analysis. Sci Rep. 2018;8(1):4089. | ||
Morra ME, Elgebaly A, Elmaraezy A, et al. Therapeutic efficacy and safety of Botulinum Toxin A Therapy in Trigeminal Neuralgia: a systematic review and meta-analysis of randomized controlled trials. J Headache Pain. 2016;17(1):63. | ||
Lakhan SE, Velasco DN, Tepper D. Botulinum Toxin-A for Painful Diabetic Neuropathy: A Meta-Analysis. Pain Med. 2015;16(9):1773–1780. | ||
Taylor M, Silva S, Cottrell C. Botulinum toxin type-A (BOTOX) in the treatment of occipital neuralgia: a pilot study. Headache. 2008;48(10):1476–1481. | ||
Tsai CP, Liu CY, Lin KP, Wang KC. Efficacy of botulinum toxin type a in the relief of Carpal tunnel syndrome: A preliminary experience. Clin Drug Investig. 2006;26(9):511–515. | ||
Breuer B, Sperber K, Wallenstein S, et al. Clinically significant placebo analgesic response in a pilot trial of botulinum B in patients with hand pain and carpal tunnel syndrome. Pain Med. 2006;7(1):16–24. | ||
Wu H, Sultana R, Taylor KB, Szabo A. A prospective randomized double-blinded pilot study to examine the effect of botulinum toxin type A injection versus Lidocaine/Depomedrol injection on residual and phantom limb pain: initial report. Clin J Pain. 2012;28(2):108–112. | ||
Hartrick CT, Kovan JP, Shapiro S. The numeric rating scale for clinical pain measurement: a ratio measure? Pain Pract. 2003;3(4):310–316. | ||
Lund I, Lundeberg T, Sandberg L, Budh CN, Kowalski J, Svensson E. Lack of interchangeability between visual analogue and verbal rating pain scales: a cross sectional description of pain etiology groups. BMC Med Res Methodol. 2005;5:31. | ||
Moher D, Liberati A, Tetziaff J, Altman DG. The PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. PLoS Med. 2009;6(6):e1000097. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


