Back to Journals » OncoTargets and Therapy » Volume 14
Bone Marrow Mesenchymal Stem Cells-Derived Exosomal miR-425-5p Inhibits Acute Myeloid Leukemia Cell Proliferation, Apoptosis, Invasion and Migration by Targeting WTAP
Authors Zhang L, Khadka B, Wu J, Feng Y, Long B, Xiao R, Liu J
Received 12 October 2020
Accepted for publication 22 May 2021
Published 24 September 2021 Volume 2021:14 Pages 4901—4914
DOI https://doi.org/10.2147/OTT.S286326
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Sanjay Singh
This paper has been retracted.
Ling Zhang, Bijay Khadka, Jieying Wu, Yashu Feng, Bing Long, Ruozhi Xiao, Jiajun Liu
Department of Hematology, The Third Affiliated Hospital of Sun Yat-sen University, Guangzhou City, Guangdong Province, 510630, People's Republic of China
Correspondence: Jiajun Liu
Department of Hematology, The Third Affiliated Hospital of Sun Yat-sen University, No. 600 Tianhe Avenue, Guangzhou City, Guangdong Province, 510630, People's Republic of China
Email [email protected]
Introduction: Acute myeloid leukemia (AML) is a predominant blood malignancy with high mortality and severe morbidity. AML is affected by microRNAs (miRNAs) loaded in exosomes derived from bone marrow mesenchymal stem cells (BM-MSCs). MiR-425-5p has been reported to participate in different cancer models. However, the function of BM-MSCs-derived exosomal miR-425-5p in AML is unclear.
Methods: The expression of miR-425-5p was measured by qRT-PCR in clinical AML samples. The immunophenotype of BM-MSCs was analyzed using antibodies against CD44, CD90, and CD105. The exosome was isolated from BM-MSCs. The effect of BM-MSCs-derived exosomal miR-425-5p on AML was analyzed by CCK-8 assay, Edu assay, transwell assay, flow cytometry in AML cells. qRT-PCR, luciferase reporter gene assay and Western blot analysis were also conducted in AML cells.
Results: The expression levels of miR-425-5p were decreased in CD34 + CD38-AML cells from primary AML patients compared to that from the bone marrow of healthy cases, and were reduced in exosomes from AML patients compared that from healthy cases. Similarly, miR-425-5p was also down-regulated in AML cell lines compared with BM-MSCs. MiR-425-5p was able to express in exosomes from BM-MSCs. CCK-8, Edu, transwell assay and flow cytometry analysis revealed that BM-MSCs-derived exosomal miR-425-5p significantly inhibited cell viability, Edu positive cells, invasion and migration, and induced apoptosis of AML cells. Meanwhile, the expression levels of cleaved PARP and cleaved caspase3 were increased by BM-MSCs-derived exosomal miR-425-5p in cells. MiR-425-5p inhibited the expression of Wilms tumor 1-associated protein (WTAP). Moreover, overexpression of WTAP could reverse the miR-425-5p-induced inhibition effect on AML cell proliferation, apoptosis, migration and invasion.
Conclusion: BM-MSCs-derived exosomal miR-425-5p inhibits proliferation, invasion and migration of AML cells and induced apoptosis of AML cells by targeting WTAP. Therapeutically, BM-MSCs-derived exosomal miR-425-5p may serve as a potential target for AML therapy.
Keywords: AML, BM-MSCs, exosome, miR-425-5p, WTAP
Introduction
Acute myeloid leukemia (AML) is a prevalent destructive cancer characterized by immature leukemic blast cell production and destroyed white blood cell maturation, which can develop into other organs, including gums, skin, and the central nervous system.1 AML patients regularly undergo easy bleeding, severe anemia, and infection because of reduced normal blood cells.2 Hazard factors for AML development contain exposure to carcinogenic agents, cigarette smoking, genetic mutations, chemotherapy, and radiation drugs, such as doxorubicin and cyclophosphamide.3,4 Even with great improvement in stem cell transplantation and chemotherapy, the survival incidence of AML cases is still unsatisfied due to frequent recurrence and chemoresistance.5 In recent years, anthracycline and cytarabine combined with intensive chemotherapy (IC) induction regimen, and marrow transplant, have become the principal therapy choices for AML patients, but the efficacy was generally restricted by high recurrence rates and poor prognosis.6,7 Therefore, it is of great importance to illustrate the mechanisms underlying AML progression to address these challenges.
Bone marrow mesenchymal stem cells (BM-MSCs) are a group of cells with differentiating potentials, which are able to differentiate into myoblasts, adipocytes, chondrocytes and osteoblasts.8 BM-MSCs play essential roles in modulating the hematopoietic capacity of bone marrow at physiological conditions and have been recognized as the foundation of the hematopoietic stem cell context.9 AML is featured by hematopoietic insufficiency, and BM-MSCs from AML patients present notable proliferation deficiency and reduced osteogenic differentiation ability.10 Studies have demonstrated the possible function of BM-MSCs transplantation for blood malignancies, including AML.11,12 In addition to the role of BM-MSCs, the bioactive substrates and paracrine agents delivered by BM-MSCs also have therapeutic and supportive impacts.13,14
Exosomes are nano-sized particles that can transport vesicles of biological loads, such as mRNAs, long non-coding RNAs (lncRNAs), proteins, and microRNAs (miRNAs), leading to the phenotypic effect in receiving cells.15 Exosomes are identified as crucial regulators of BM-MSCs activity.10 MiRNAs are small ncRNAs with 20–25 nucleotides in length, which regulate different targets that are involved in diverse physiological and pathological processes, such as cell apoptosis, differentiation, proliferation, invasion, metastasis, and tumorigenesis.16 Moreover, exosomes can shuttle certain miRNAs and exert critical functions in long-distance cell communication and cancer development.17 It has been well recognized that exosomes can promote cancer progression by regulating the expression of miRNAs in tumors and surrounding cells.18,19 Meanwhile, the BM-MSCs-derived exosomal miRNAs are involved in regulating AML pathogenesis.20 Furthermore, miR-425-5p is a well-studied miRNA in multiple cancer models, including prostate cancer, pancreatic cancer, and gastric cancer.21–23 And it has been reported that miR-425-5p is down-regulated in exosomes from clinical AML samples.20 However, the role of BM-MSCs-derived exosomal miR-425-5p in the development of AML remains elusive.
Wilms tumor 1 (WT1)-associated protein (WTAP) is a nuclear protein known as its particular interaction with WT1.24 WTAP also has a tight connection with malignant tumors. For instance, WTAP is upregulated in cholangiocarcinoma, particularly in patients with vascular invasion or lymph node metastasis.25 It was also reported that the expression levels of WTAP were closely correlated with tumor-node-metastasis stages.26 Besides, WTAP plays a crucial role in the invasion and migration of cholangiocarcinoma cells.26 Distinctly, WTAP is up-regulated in glioblastoma and is correlated with the prognosis of glioblastoma.26 The oncogenic role of WTAP has also been observed in AML.27 Overexpression of WTAP inhibits differentiation and enhances proliferation of AML cells.27 However, the correlation of WTAP with BM-MSCs-derived exosomal miR-425-5p in the regulation of AML is unclear.
In this study, we aimed to investigate the role and the underlying mechanism of BM-MSCs-derived exosomal miR-425-5p in AML. Our data showed that miR-425-5p was downregulated in the exosomes from AML patients and AML cell lines. BM-MSCs-derived exosomal miR-425-5p significantly inhibited cell proliferation, invasion and migration, and induced apoptosis of AML cells. MiR-425-5p inhibited the expression of WTAP by targeting its 3ʹ-untranslated regions (3ʹ-UTR). Overexpression of WTAP reversed the effect of miR-425-5p on AML. Thus, we discovered a novel function of AML in inhibiting AML development by regulating WTAP.
Materials and Methods
AML Clinical Samples
A total of 20 bone marrow (BM) samples from primary AML patients and healthy volunteers were obtained from the Third Affiliated Hospital of Sun Yat-Sen University. The samples were immediately frozen in liquid nitrogen and stored at −80 °C before use. All patients and healthy volunteers signed the written consent form. Informed consent from the patients and healthy volunteers Approval from the Ethics Committee of The Third Affiliated Hospital of Sun Yat-Sen University Compliance with the Declaration of Helsinki. The information of patients was shown in Table 1.
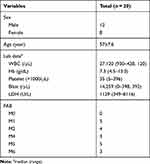 |
Table 1 The Information of Patients |
Patient Specimens, CD34 + CD38−cells, and AML Cell Line Separation
Human cord blood (hCB) specimens were collected after elective caesarean delivery following full-term pregnancy at Beijing Union Hospital. All experimental procedures were approved by the Ethics Committee of the Third Affiliated Hospital of Sun Yat-Sen University. Each participant signed the informed consent. CD38 and CD34 MicroBead kits (Miltenyi, Germany) were used to separate CD34 + CD38− cells from hCB and bone marrow.28
Identification of BM-MSCs
The human BM-MSCs were derived from normal subjects and purchased from BeNa Culture Collection (Beijing, China). BM-MSCs were maintained in high glucose Dulbecco’s modified Eagle’s medium (DMEM, Gibco, CA, USA) supplemented with 10% fetal bovine serum (FBS, Gibco), 4 mM L-glutamine and sodium pyruvate at 37 °C with 5% CO2. For identification of BM-MSCs, the morphology of BM-MSCs at passage 3 was observed under a microscope. The immunophenotype of BM-MSCs was analyzed using antibodies against CD44, CD90, and CD105 (ebioscience, Thermo Fisher Scientific, CA, USA) using a FACScalibur flow cytometer (Becton Dickinson, Bedford, MA, USA) as previously described.14
Exosome Isolation and Analysis
The culture medium and plasma and were centrifuged at 3000x g for 15 min to drop cells and cellular debris. Next, Exoquick exosome precipitation solution (System Biosciences, USA) was used to isolate exosomes. The characteristics of exosomes were analyzed by transmission electron microscopy (TEM) as previously described.29
Cell Culture and Treatment
The KG-1A, NB4, MV411, and THP-1 cell lines were obtained from American Type Tissue Culture Collection. The human BM-MSCs were purchased from BeNa Culture Collection (Beijing, China). The KG-1A, NB4, MV411, THP-1 cells, and BM-MSCs cells were cultured in DMEM medium (Gibco, USA) containing 100 units/mL penicillin (Gibco, USA), 0.1 mg/mL streptomycin (Gibco, USA), and 10% fetal bovine serum (Gibco, USA) at 37 °C with 5% CO2. The control mimic, miR-425-5p mimic, pcDNA3.1 and pcDNA3.1-WTAP overexpression vectors were obtained from GenePharma (China). Transfection of cells was performed using Liposome 3000 (Invitrogen, USA) according to the manufacturer’s instructions. To evaluate the effect of BM-MSCs-derived exosomal miR-425-5p on AML, the exosomes were extracted from BM-MSCs cells treated with control mimic or miR-425-5p mimic, and KG-1A and THP-1 cells were further treated with exosomes for further analysis.
Colony Formation Assay
Cell proliferation was evaluated by colony formation assay. About 1×103 cells were plated in 6 wells and incubated in DMEM at 37 °C. After 2 weeks, cells were cleaned with PBS Buffer, precipitated in methanol for 30 min, and dyed with 1% crystal violet dye. The number of colonies was then calculated.
Edu Assay
Cell proliferation was evaluated by EdU assay using EdU detecting kit (RiboBio, China). Briefly, KG-1A and THP-1 cells were cultured with EdU for 2 h, followed by fixing using 4% paraformaldehyde at room temperature for 30 min. Then, cells were permeabilized with 0.4% Triton X-100 for 10 min and stained with staining cocktail of EdU at room temperature for 30 min in the dark. Next, the nuclear of the cells was stained with Hoechst at room temperature for 30 min. Images were analyzed using a fluorescence microscope.30
Transwell Assay
Migration and invasion of AML cells were measured using Transwell assay with a Transwell plate (Corning, USA) following the manufacturer’s instructions. Briefly, the upper chamber was plated with about 1×105 cells, then solidified with 4% paraformaldehyde and dyed with crystal violet. The invaded and migrated cells were recorded and calculated.30
Cell Apoptosis Assay
About 2×105 cells were plated on 6-well dishes. Cell apoptosis was measured using the Annexin V-FITC Apoptosis Detection Kit (CST, USA) following the manufacturer’s instructions. Briefly, about 2×106 cells were collected and washed by binding buffer and dyed at 25 °C, followed by flow cytometry analysis.31
Quantitative Reverse Transcription-PCR (qRT-PCR)
Total RNAs were extracted from the tissues and cells using TRIZOL (Invitrogen, USA). The first-strand cDNA was synthesized using Stand cDNA Synthesis Kit (Thermo, USA). The qRT-PCR was carried out using SYBR Real-time PCR I kit (Takara, Japan). The standard control for mRNA and miRNA was GAPDH and U6, respectively. Quantitative determination of the RNA levels was conducted by SYBR GreenPremix Ex TaqTM II Kit (TaKaRa, Japan). The experiments were independently repeated for three times. The primer sequences were as follows:
miR-425-5p F: 5′-TGCGGAATGACACGATCACTCCCG-3′
miR-21-5p R: 5′-CCAGTGCAGGGTCCGAGGT-3′
WTAP F: 5′-TGCGACTAGCAACCAAGGAA-3′
WTAP R: 5′-ATCTCAGTTGGGCAACGCTC-3′
GAPDH F: 5′-AAGAAGGTGGTGAAGCAGGC-3′
GAPDH R: 5′-TCCACCACCCAGTTGCTGTA-3′
U6 F: 5′-GCTTCGGCAGCACATATACTAA-3′
U6 R: 5′-AACGCTTCACGAATTTGCGT-3′
Luciferase Reporter Gene Assay
Luciferase reporter gene assay was conducted using a Dual-luciferase Reporter Assay System (Promega, USA). Briefly, KG-1A and THP-1 cells were treated with the control mimic or miR-425-5p mimic, the vector containing WTAP and WTAP mutant fragment were transfected in cells using Lipofectamine 3000 (Invitrogen, USA), followed by the analysis of luciferase activities. Renilla was used as a normalized control.30
Western Blot Analysis
Total proteins were extracted from cells or mice tissues with RIPA buffer (CST, USA). Protein concentrations were measured using the BCA Protein Quantification Kit (Abbkine, USA). The same amount of protein samples were divided by SDS-PAGE (12% polyacrylamide gels), followed by transferring to PVDF membranes (Millipore, USA). The membranes were blocked with 5% milk and incubated with the primary antibodies for WTAP (1:1000) (Abcam, USA), Tsg101 (1:1000) (Abcam, USA), CD63 (1:1000) (Abcam, USA), Grp94 (1:1000) (Abcam, USA), caspase3 (1:1000) (Abcam, USA), PARP (1:1000) (Abcam, USA), and β-actin (1:1000) (Abcam, USA) at 4 °C overnight, in which β-actin served as the control. Then, the corresponding second antibodies (1:1000) (Abcam, USA) were used for hatching the membranes at room temperature for 1 h, followed by visualization using an Odyssey CLx Infrared Imaging System.
Statistical Analysis
Data were presented as mean ± standard deviation (SD). All statistical analyses were conducted using Prism software v.7 (GraphPad, La Jolla, CA, USA). The unpaired Student’s t-test was used to compare two groups, and the one-way ANOVA was used to compare multiple groups. P < 0.05 was considered as statistically significant.
Results
The Expression Levels of miR-425-5p are Decreased in the Exosome of AML Patients
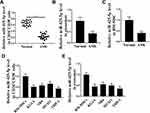
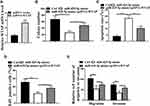
To assess the potential correlation of miR-425-5p with AML, the expression of miR-425-5p in AML patients was detected. It showed that the expression levels of miR-425-5p were significantly decreased in the CD34+CD38− AML cells from primary AML patients (n = 20) compared to that from bone marrow of healthy cases (n = 20) (P < 0.01, Figure 1A). Moreover, the exosomes were extracted, and the expression levels of miR-425-5p were reduced in primary AML patients (n = 20) compared with that in the normal cases (n = 20) (P < 0.01, Figure 1B), indicating that miR-425-5p may be correlated with the clinical development of AML. Importantly, the expression levels of miR-425-5p were also decreased in BM-MSCs from AML patients compared with that from the controls (P < 0.01, Figure 1C). Meanwhile, miR-425-5p was also down-regulated in the AML cell lines, including KG-1A, NB4, MV411, and THP-1 cells, compared with BM-MSCs (P < 0.01, Figure 1D). Similarly, the expression levels of miR-425-5p were decreased in the exosomes from KG-1A, NB4, MV411, and THP-1 cells compared to that from BM-MSCs (P < 0.01, Figure 1E). Taken together, these data suggest that the expression of miR-425-5p is inhibited in the exosomes of AML patients and cells lines.
miR-425-5p is Expressed in the Exosomes from BM-MSCs
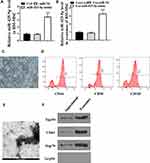
Then, whether miR-425-5p was loaded in the exosome of BM-MSCs was explored. BM-MSCs were derived from normal subjects. For identification of BM-MSCs, the morphology of BM-MSCs at passage 3 was observed under a microscope (Figure 2C). The immunophenotype of BM-MSCs was analysed using antibodies against CD44, CD90, and CD105 (Figure 2D). BM-MSCs were treated with miR-425-5p mimic and the treatment efficiency was validated (P < 0.001, Figure 2A). The expression of miR-425-5p was inhibited in the exosomes from miR-425-5p mimic-treated BM-MSCs (P < 0.001, Figure 2B). In addition, the characteristic of the exosome was identified by transmission electron microscopy (TEM) (Figure 2E). The expression of the exosome markers, such as CD63 and Tsg101, was enriched in the exosomes (Figure 2F). Together, these results indicate that miR-425-5p is expressed in the exosomes from BM-MSCs.
BM-MSCs-Derived Exosomal miR-425-5p Inhibits Viability and Induces Apoptosis of AML Cells
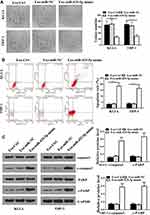
Since miR-425-5p was incorporated in the exosomes from BM-MSCs, we assessed the effect of BM-MSCs-derived exosomal miR-425-5p on AML. The exosomes were extracted from BM-MSCs treated with control mimic or miR-425-5p mimic, and KG-1A and THP-1 cells were further treated with the exosomes. Colony formation assay results showed that miR-425-5p mimic significantly inhibited cell proliferation of KG-1A and THP-1 cells (P < 0.05, Figure 3A). Meanwhile, cell apoptosis of KG-1A and THP-1 cells was enhanced by miR-425-5p mimic (P < 0.001, Figure 3B). Similarly, the expression levels of cleaved PARP and cleaved caspase3 were significantly increased by miR-425-5p mimic in KG-1A and THP-1 cells (P < 0.001, Figure 3C). These results indicate that BM-MSCs-derived exosomal miR-425-5p inhibits viability and induces apoptosis of AML cells.
BM-MSCs-Derived Exosomal miR-425-5p Inhibits Proliferation, Invasion, and Migration of AML Cells
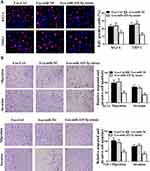
Next, we further assessed the role of BM-MSCs-derived exosomal miR-425-5p in modulating proliferation, invasion, and migration of AML cells. The exosomes were extracted from BM-MSCs treated with control mimic or miR-425-5p mimic, and KG-1A and THP-1 cells were further treated with the exosomes. Edu assay results showed that cell proliferation was inhibited by miR-425-5p mimic in KG-1A and THP-1 cells (P < 0.01, Figure 4A). Meanwhile, invasion and migration of KG-1A and THP-1 cells were reduced by miR-425-5p mimic (P < 0.01, Figure 4B). Together, these data indicate that BM-MSCs-derived exosomal miR-425-5p inhibits proliferation, invasion and migration of KG-1A and THP-1 cells.
miR-425-5p Targets WTAP in AML Cells
Then, we aimed to investigate the mechanism underlying the miR-425-5p-mediated AML. The miR-425-5p-targeted site in WTAP 3ʹ-UTR was identified by bioinformatics analysis using Targetscan (http://www.targetscan.org/vert_72/) (Figure 5A). Notably, miR-425-5p mimic treatment inhibited luciferase activities of the wild type WTAP but did not affect WTAP with the mutated miR-425-5p-binding site (P < 0.01, Figure 5B). Exosomes were extracted from BM-MSCs treated with the control mimic or miR-425-5p mimic, and KG-1A and THP-1 cells were further treated with the exosomes. The expression of WTAP was inhibited by miR-425-5p in the cells (P < 0.01, Figure 5C). Similarly, miR-21-5p mimic could decrease the expression levels of WTAP in the cells (P < 0.01, Figure 5D). The expression levels of WTAP were increased in CD34+CD38− AML cells from primary AML patients (n = 20) compared to that from bone marrow of healthy cases (n = 20) (P < 0.001, Figure 5E). The WTAP was up-regulated in KG-1A, NB4, MV411, and THP-1 cells compared with that in BM-MSCs (P < 0.001, Figure 5F). Taken together, these results indicate that miR-425-5p targets WTAP in AML cells.
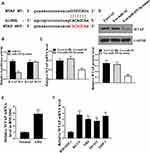 |
Figure 5 miR-425-5p targets WTAP in AML cells. (A) The interaction of miR-425-5p and WTAP 3ʹ-UTR was identified by bioinformatic analysis using Targetscan (http://www.targetscan.org/vert_72/). (B) The KG-1A and THP-1 cells were treated with miR-425-5p mimic or the control mimic. The luciferase activities of wild type WTAP (WTAP WT) and WTAP with the miR-425-5p-binding site mutant (WTAP MUT) were determined by luciferase reporter gene assays in the cells. (C and D) The exosomes were extracted from the BM-MSCs treated with miR-425-5p mimic or the control mimic, and the KG-1A and THP-1 cells were further treated with the exosomes. (C) The mRNA expression of WTAP was measured by qPCR assays in the cells. (D) The protein expression of WTAP was tested by Western blot analysis in the cells. The results of Western blot analysis were quantified by ImageJ software. (E) The expression levels of WTAP were measured by qPCR assays in the CD34+CD38− AML cells from primary AML patients (n = 20) and bone marrow of healthy cases (n = 20). (F) The expression levels of miR-425-5p were assessed by qPCR assays in the KG-1a, NB4, MV411, THP-1 cells, and BM-MSCs. Data are presented as mean ± SD. Statistic significant differences were indicated: **P < 0.01, ***P < 0.001. |
miR-425-5p Inhibits AML by Targeting WTAP
Next, we explored whether miR-425-5p inhibits AML by targeting WTAP. The efficiency of overexpression of WTAP was confirmed in KG-1A cells (P < 0.01, Figure 6A). Colony formation assay revealed that overexpression of WTAP could rescue miR-425-5p mimic-inhibited cell proliferation in the cells (P < 0.01, Figure 6B). Meanwhile, the miR-425-5p mimic-induced apoptosis of KG-1A cells was blocked by overexpression of WTAP (P < 0.01, Figure 6C). Edu assay results showed that overexpression of WTAP could enhance proliferation decreased by miR-425-5p mimic in \KG-1A cells (P < 0.01, Figure 6D). Moreover, overexpression of WTAP rescued the miR-425-5p mimic-inhibited invasion and migration of KG-1A cells (P < 0.05, Figure 6E). Together, these data indicate that miR-425-5p inhibits AML by targeting WTAP.
Discussion
AML is the predominant type of blood cancer with severe morbidity and high mortality.32 The mechanism of the progression of AML remains unclear, limiting the development of biomarkers of diagnosis, prognosis and effective therapies for AML patients.33 During the past decade, BM-MSCs miRNAs have been demonstrated to play crucial roles in the modulation of AML. In this study, we aimed to investigate the role of BM-MSCs-derived exosomal miR-425-5p in the development of AML. We observed abnormal expression of miR-425-5p in AML patients and AML cell lines. BM-MSCs-derived exosomal miR-425-5p attenuated cell proliferation, invasion and migration, and induced apoptosis of AML cells by inhibiting the expression of WTAP by targeting the 3ʹ-UTR of WTAP. Our findings demonstrated that BM-MSCs-derived exosomal miR-425-5p inhibited AML by regulating WTAP.
It has been reported that aberrantly expressed exosomal miRNAs participate in the development of AML. For example, exosomal miRNA-4532 of AML cells regulates regular hematopoiesis in hematopoietic stem cells through stimulating the LDOC1-related STAT3 signaling.34 High plasma exosomal miR-125b levels serve as the possible biomarker for the bad prognosis of intermediate-risk AML.35 Serum exosome-derived miRNA-532 is a new prognostic biomarker for patients with AML.36 Elevated plasma extracellular vesicle-derived miR-10b foretells the poor prognosis of AML patients.37 Exosomal miR-486 controls hypoxia-related erythroid differentiation by regulating Sirt1 in erythroleukemia cells.38 Moreover, as a well-studied miRNA, miR-425-5p is abnormally expressed in multiple cancer models and contributes to cancer development. For instance, the clinic significance of miR-425-5p and its association with apoptosis and proliferation of gastric cancer cells has been reported.39 Down-regulation of miR-425-5p represses cervical cancer progression by regulating AIFM1.40 MiR-425-5p inhibits the expressions of TUG1 and lncRNA MALAT1, and represses osteosarcoma development by regulating the Wnt/β-catenin signaling.41 Prader-Willi region non-protein coding RNA 1 suppresses gastric cancer progression by targeting miR-425-5p.42 MiR-425-5p modulates chemoresistance by regulating programmed cell death 10 in colorectal cancer cells.43 MiR-425-5p is involved in the lncRNA LINC-PINT/PTCH1/SHH axis-regulated chemoresistance and laryngeal carcinoma cell stemness.44 Meanwhile, miRNA profiling showed that miR-425-5p is reduced in exosomes from clinical AML samples.20 Furthermore, BM-MSCs play critical roles in the regulation of AML. It has been reported that AML converts BM-MSCs in the leukemia-permitted microenvironment by exosomes secretion.45 Human BM-MSCs-derived exosomal miR-222-3p inhibits the proliferation of AML cells by regulating IRF2 and INPP4B.46 MiR-7977 represses the Hippo-YAP signaling in BM-MSCs during AML progression.47 In this study, we found that the expression levels of miR-425-5p were decreased in CD34+CD38− AML cells from primary AML patients compared to that from the bone marrow of healthy cases. The expression levels of miR-425-5p were reduced in the exosomes from primary AML patients compared to those from normal cases. It implies that the abnormally expressed miR-425-5p in the exosomes may be involved in the progression of ovarian cancer in a clinical context, serving as a potential biomarker of ovarian cancer. Meanwhile, miR-425-5p was down-regulated in the AML cell lines compared with BM-MSCs. We also found that miR-425-5p was expressed in the exosomes from normal subject derived BM-MSCs, and BM-MSCs-derived exosomal miR-425-5p inhibited proliferation, invasion, and migration and induced apoptosis of AML cells. These data present a novel function of BM-MSCs-derived exosomal miR-425-5p in AML progression, providing valuable evidence for the fundamental role of BM-MSCs-derived exosomal miRNAs in the development of AML. However, the effect of BM-MSCs-derived exosomal miR-425-5p on the proliferation invasion, migration, and apoptosis of AML cells was only investigated in vitro, and the lacking of in vivo evidence may limit the profound role of BM-MSCs-derived exosomal miR-425-5p in the regulation of AML progression. It needs to be further investigated in future study.
WTAP has been shown to function as an essential regulator in the development of AML. For example, WTAP promotes hepatocellular carcinoma progression by m6A-HuR-related epigenetic inhibiting of ETS1.48 WTAP serves as a prognosis biomarker for ovarian cancer and controls the ovarian cancer cell progression.49 WTAP forms a complex with BCL6 through Hsp90 and exerts an oncogenic function in diffuse large B-cell lymphoma.50 The up-regulation of WTAP is associated with poor prognosis of gastric cancer through regulating tumor-associated T lymphocyte infiltration.51 WTAP increases chemoresistance to gemcitabine and metastasis in pancreatic cancer by up-regulating Fak mRNA.52 WTAP enhances the proliferation of renal cell carcinoma cells by stabilizing CDK2.53 Moreover, several studies have identified the malignant role of WTAP in AML progression. It has been reported that WTAP serves as a new oncogenic factor in AML.27 WTAP is up-regulated in AML samples and contributes to AML progression by modulating m6A methylation.54 Our findings further demonstrated that miR-425-5p could target WTAP in AML cells and inhibited AML progression by targeting WTAP. These data identify the new downstream target WTAP of miR-425-5p, identifying the correlation of WTAP with miR-425-5p in AML. Previous studies have identified the role of miR-425-5p and WTAP in cancer cells, but there were few studies investigating the relationship between the two. Based on this, the novelty of this work is recognized. The chemoresistance is a severe clinical challenge of AML, leading to relapse of AML patients. The function of miR-425-5p in the modulation of chemotherapy resistance of AML cells will need to be further explored in the future.
Conclusion
In conclusion, we discovered that M-MSCs-derived exosomal miR-425-5p inhibited proliferation, invasion and migration of AML cells and induced apoptosis of AML cells by targeting WTAP. Our finding provides new insights into the mechanism by which BM-MSCs-derived exosomal miR-425-5p attenuates the AML development, enhancing understanding of the correlation of BM-MSCs-derived exosomal miRNA with AML. BM-MSCs-derived exosomal miR-425-5p may serve as a potential target for AML therapy.
Disclosure
The authors declare no conflicts of interest for this work.
References
1. Forte D, Garcia-Fernandez M, Sanchez-Aguilera A, et al. Bone marrow mesenchymal stem cells support acute myeloid leukemia bioenergetics and enhance antioxidant defense and escape from chemotherapy. Cell Metab. 2020;32(5):829–843.e9. doi:10.1016/j.cmet.2020.09.001
2. Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391–2405. doi:10.1182/blood-2016-03-643544
3. Mozessohn L, Cheung MC, Mittmann N, Earle CC, Liu N, Buckstein R. Real-world costs of azacitidine treatment in patients with higher-risk myelodysplastic syndromes/low blast-count acute myeloid leukemia. JCO Oncol Pract. 2020;OP2000446. doi:10.1200/OP.20.00446
4. Goldman SL, Hassan C, Khunte M, et al. Epigenetic modifications in acute myeloid leukemia: prognosis, treatment, and heterogeneity. Front Genet. 2019;10:133. doi:10.3389/fgene.2019.00133
5. Shi W, Jin W, Xia L, Hu H. Novel agents targeting leukemia cells and immune microenvironment for prevention and treatment of relapse of acute myeloid leukemia after allogeneic hematopoietic stem cell transplantation. Acta Pharm Sin B. 2020;10(11):2125–2139. doi:10.1016/j.apsb.2020.06.012
6. Cerrano M, Itzykson R. New treatment options for acute myeloid leukemia in 2019. Curr Oncol Rep. 2019;21(2):16. doi:10.1007/s11912-019-0764-8
7. O’Dwyer K, Freyer DR, Horan JT. Treatment strategies for adolescent and young adult patients with acute myeloid leukemia. Blood. 2018;132(4):362–368. doi:10.1182/blood-2017-12-778472
8. He JG, Li HR, Li BB, Xie QL, Yan D, Wang XJ. Bone marrow mesenchymal stem cells overexpressing GATA-4 improve cardiac function following myocardial infarction. Perfusion. 2019;34(8):696–704. doi:10.1177/0267659119847442
9. Lee GY, Jeong SY, Lee HR, Oh IH. Age-related differences in the bone marrow stem cell niche generate specialized microenvironments for the distinct regulation of normal hematopoietic and leukemia stem cells. Sci Rep. 2019;9(1):1007. doi:10.1038/s41598-018-36999-5
10. Geyh S, Rodriguez-Paredes M, Jager P, et al. Functional inhibition of mesenchymal stromal cells in acute myeloid leukemia. Leukemia. 2016;30(3):683–691. doi:10.1038/leu.2015.325
11. Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8(9):726–736. doi:10.1038/nri2395
12. Ciciarello M, Corradi G, Loscocco F, et al. The Yin and Yang of the bone marrow microenvironment: pros and cons of mesenchymal stromal cells in acute myeloid leukemia. Front Oncol. 2019;9:1135. doi:10.3389/fonc.2019.01135
13. Liu A, Zhang X, He H, et al. Therapeutic potential of mesenchymal stem/stromal cell-derived secretome and vesicles for lung injury and disease. Expert Opin Biol Ther. 2020;20(2):125–140. doi:10.1080/14712598.2020.1689954
14. Abbaszade Dibavar M, Soleimani M, Atashi A, Rassaei N, Amiri S. The effect of simultaneous administration of arsenic trioxide and microvesicles derived from human bone marrow mesenchymal stem cells on cell proliferation and apoptosis of acute myeloid leukemia cell line. Artif Cells, Nanomed Biotechnol. 2018;46(sup3):S138–S146. doi:10.1080/21691401.2018.1489821
15. Xie Y, Dang W, Zhang S, et al. The role of exosomal noncoding RNAs in cancer. Mol Cancer. 2019;18(1):37. doi:10.1186/s12943-019-0984-4
16. Rupaimoole R, Slack FJ. MicroRNA therapeutics: towards a new era for the management of cancer and other diseases. Nat Rev Drug Discov. 2017;16(3):203–222. doi:10.1038/nrd.2016.246
17. Nahand JS, Vandchali NR, Darabi H, et al. Exosomal microRNAs: novel players in cervical cancer. Epigenomics. 2020;12(18):1651–1660.
18. Hoshino A, Costa-Silva B, Shen T-L, et al. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527(7578):329–335. doi:10.1038/nature15756
19. Mathivanan S, Ji H, Simpson RJ. Exosomes: extracellular organelles important in intercellular communication. J Proteomics. 2010;73(10):1907–1920. doi:10.1016/j.jprot.2010.06.006
20. Barrera-Ramirez J, Lavoie JR, Maganti HB, et al. Micro-RNA profiling of exosomes from marrow-derived mesenchymal stromal cells in patients with acute myeloid leukemia: implications in leukemogenesis. Stem Cell Rev Rep. 2017;13(6):817–825. doi:10.1007/s12015-017-9762-0
21. Liu S, Wang Q, Liu Y, Xia ZY. miR-425-5p suppresses tumorigenesis and DDP resistance in human-prostate cancer by targeting GSK3beta and inactivating the Wnt/beta-catenin signaling pathway. J Biosci. 2019;44:4. doi:10.1007/s12038-019-9920-4
22. Lu Y, Wu X, Wang J. Correlation of miR-425-5p and IL-23 with pancreatic cancer. Oncol Lett. 2019;17(5):4595–4599. doi:10.3892/ol.2019.10099
23. Yan YF, Gong FM, Wang BS, Zheng W. MiR-425-5p promotes tumor progression via modulation of CYLD in gastric cancer. Eur Rev Med Pharmacol Sci. 2017;21(9):2130–2136.
24. Little NA, Hastie ND, Davies RC. Identification of WTAP, a novel wilms’ tumour 1-associating protein. Hum Mol Genet. 2000;9(15):2231–2239. doi:10.1093/oxfordjournals.hmg.a018914
25. Jo HJ, Shim HE, Han ME, et al. WTAP regulates migration and invasion of cholangiocarcinoma cells. J Gastroenterol. 2013;48(11):1271–1282. doi:10.1007/s00535-013-0748-7
26. Jin DI, Lee SW, Han ME, et al. Expression and roles of wilms’ tumor 1-associating protein in glioblastoma. Cancer Sci. 2012;103(12):2102–2109. doi:10.1111/cas.12022
27. Bansal H, Yihua Q, Iyer SP, et al. WTAP is a novel oncogenic protein in acute myeloid leukemia. Leukemia. 2014;28(5):1171–1174. doi:10.1038/leu.2014.16
28. Peng D-Y, Song H, Liu L-B. Resveratrol-downregulated phosphorylated liver kinase B1 is involved in senescence of acute myeloid leukemia stem cells. J Huazhong Univ Sci Technolog Med Sci. 2015;35(4):485–489. doi:10.1007/s11596-015-1457-7
29. Pan R, Zhou H. Exosomal transfer of lncRNA H19 promotes erlotinib resistance in non-small cell lung cancer via miR-615-3p/ATG7 axis. Cancer Manag Res. 2020;12:4283–4297. doi:10.2147/CMAR.S241095
30. Zhao W, Han T, Li B, Ma Q, Yang P, Li H. miR-552 promotes ovarian cancer progression by regulating PTEN pathway. J Ovarian Res. 2019;12(1):121. doi:10.1186/s13048-019-0589-y
31. Zou YT, Gao JY, Wang HL, Wang Y, Wang H, Li PL. Downregulation of microRNA-630 inhibits cell proliferation and invasion and enhances chemosensitivity in human ovarian carcinoma. Genet Mol Res. 2015;14(3):8766–8777. doi:10.4238/2015.July.31.25
32. Ley TJ, Miller C, et al; Cancer Genome Atlas Research N. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N Engl J Med. 2013;368(22):2059–2074.
33. Davila J, Slotkin E, Renaud T. Relapsed and refractory pediatric acute myeloid leukemia: current and emerging treatments. Paediatr Drugs. 2014;16(2):151–168. doi:10.1007/s40272-013-0048-y
34. Zhao C, Du F, Zhao Y, Wang S, Qi L. Acute myeloid leukemia cells secrete microRNA-4532-containing exosomes to mediate normal hematopoiesis in hematopoietic stem cells by activating the LDOC1-dependent STAT3 signaling pathway. Stem Cell Res Ther. 2019;10(1):384. doi:10.1186/s13287-019-1475-7
35. Jiang L, Deng T, Wang D, Xiao Y. Elevated serum exosomal miR-125b level as a potential marker for poor prognosis in intermediate-risk acute myeloid leukemia. Acta Haematol. 2018;140(3):183–192. doi:10.1159/000491584
36. Lin X, Ling Q, Lv Y, et al. Plasma exosome-derived microRNA-532 as a novel predictor for acute myeloid leukemia. Cancer Biomark. 2020;28(2):151–158. doi:10.3233/CBM-191164
37. Fang Z, Wang X, Wu J, Xiao R, Liu J. High serum extracellular vesicle miR-10b expression predicts poor prognosis in patients with acute myeloid leukemia. Cancer Biomark. 2020;27(1):1–9. doi:10.3233/CBM-190211
38. Shi XF, Wang H, Kong FX, et al. Exosomal miR-486 regulates hypoxia-induced erythroid differentiation of erythroleukemia cells through targeting Sirt1. Exp Cell Res. 2017;351(1):74–81. doi:10.1016/j.yexcr.2016.12.023
39. Zhang Z, Wen M, Guo J, et al. Clinical value of miR-425-5p detection and its association with cell proliferation and apoptosis of gastric cancer. Pathol Res Pract. 2017;213(8):929–937. doi:10.1016/j.prp.2017.05.009
40. Zhang Y, Yang Y, Liu R, Meng Y, Tian G, Cao Q. Downregulation of microRNA-425-5p suppresses cervical cancer tumorigenesis by targeting AIFM1. Exp Ther Med. 2019;17(5):4032–4038. doi:10.3892/etm.2019.7408
41. Yang G, Zhang C, Wang N, Chen J. miR-425-5p decreases LncRNA MALAT1 and TUG1 expressions and suppresses tumorigenesis in osteosarcoma via Wnt/beta-catenin signaling pathway. Int J Biochem Cell Biol. 2019;111:42–51. doi:10.1016/j.biocel.2019.04.004
42. Chen Z, Ju H, Yu S, et al. Prader-Willi region non-protein coding RNA 1 suppressed gastric cancer growth as a competing endogenous RNA of miR-425-5p. Clin Sci. 2018;132(9):1003–1019. doi:10.1042/CS20171588
43. Zhang Y, Hu X, Miao X, et al. MicroRNA-425-5p regulates chemoresistance in colorectal cancer cells via regulation of programmed cell death 10. J Cell Mol Med. 2016;20(2):360–369. doi:10.1111/jcmm.12742
44. Yuan Z, Xiu C, Liu D, et al. Long noncoding RNA LINC-PINT regulates laryngeal carcinoma cell stemness and chemoresistance through miR-425-5p/PTCH1/SHH axis. J Cell Physiol. 2019;234(12):23111–23122. doi:10.1002/jcp.28874
45. Kumar B, Garcia M, Weng L, et al. Acute myeloid leukemia transforms the bone marrow niche into a leukemia-permissive microenvironment through exosome secretion. Leukemia. 2018;32(3):575–587. doi:10.1038/leu.2017.259
46. Zhang F, Lu Y, Wang M, et al. Exosomes derived from human bone marrow mesenchymal stem cells transfer miR-222-3p to suppress acute myeloid leukemia cell proliferation by targeting IRF2/INPP4B. Mol Cell Probes. 2020;51:101513. doi:10.1016/j.mcp.2020.101513
47. Yoshida M, Horiguchi H, Kikuchi S, et al. miR-7977 inhibits the Hippo-YAP signaling pathway in bone marrow mesenchymal stromal cells. PLoS One. 2019;14(3):e0213220. doi:10.1371/journal.pone.0213220
48. Chen Y, Peng C, Chen J, et al. WTAP facilitates progression of hepatocellular carcinoma via m6A-HuR-dependent epigenetic silencing of ETS1. Mol Cancer. 2019;18(1):127. doi:10.1186/s12943-019-1053-8
49. Yu HL, Ma XD, Tong JF, Li JQ, Guan XJ, Yang JH. WTAP is a prognostic marker of high-grade serous ovarian cancer and regulates the progression of ovarian cancer cells. Onco Targets Ther. 2019;12:6191–6201. doi:10.2147/OTT.S205730
50. Kuai Y, Gong X, Ding L, et al. Wilms’ tumor 1-associating protein plays an aggressive role in diffuse large B-cell lymphoma and forms a complex with BCL6 via Hsp90. Cell Commun Signal. 2018;16(1):50. doi:10.1186/s12964-018-0258-6
51. Li H, Su Q, Li B, et al. High expression of WTAP leads to poor prognosis of gastric cancer by influencing tumour-associated T lymphocyte infiltration. J Cell Mol Med. 2020;24(8):4452–4465. doi:10.1111/jcmm.15104
52. Li BQ, Liang ZY, Seery S, et al. WT1 associated protein promotes metastasis and chemo-resistance to gemcitabine by stabilizing Fak mRNA in pancreatic cancer. Cancer Lett. 2019;451:48–57. doi:10.1016/j.canlet.2019.02.043
53. Tang J, Wang F, Cheng G, et al. Wilms’ tumor 1-associating protein promotes renal cell carcinoma proliferation by regulating CDK2 mRNA stability. J Exp Clin Cancer Res. 2018;37(1):40. doi:10.1186/s13046-018-0706-6
54. Sorci M, Ianniello Z, Cruciani S, et al. METTL3 regulates WTAP protein homeostasis. Cell Death Dis. 2018;9(8):796. doi:10.1038/s41419-018-0843-z
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.