Back to Journals » OncoTargets and Therapy » Volume 13
Bone Marrow-Derived Myofibroblasts Promote Gastric Cancer Metastasis by Activating TGF-β1 and IL-6/STAT3 Signalling Loop
Authors Wang J , Li Q, Cheng X, Zhang B, Lin J, Tang Y, Li F, Yang CS, Wang TC , Tu S
Received 16 June 2020
Accepted for publication 9 September 2020
Published 16 October 2020 Volume 2020:13 Pages 10567—10580
DOI https://doi.org/10.2147/OTT.S266506
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Arseniy Yuzhalin
Jianzheng Wang,1,* Qingli Li,1,* Xiaojiao Cheng,1 Baiwen Zhang,1 Jiacheng Lin,1 Yao Tang,1 Fuli Li,1 Chung S Yang,2 Timothy C Wang,3 Shuiping Tu1
1Department of Oncology, Renji Hospital, School of Medicine, Shanghai Jiaotong University, Shanghai, 200127, People’s Republic of China; 2Department of Chemical Biology, Ernest Mario School of Pharmacy, Rutgers, The State University of New Jersey, Piscataway, NJ 08854, USA; 3Department of Medicine, College of Physicians & Surgeons, Columbia University, New York, NY 10032, USA
*These authors contributed equally to this work
Correspondence: Shuiping Tu; Jianzheng Wang
Department of Oncology, Renji Hospital, School of Medicine, Shanghai Jiaotong University, Shanghai 200127, People’s Republic of China
Tel +862168385623; +16696079698
Email [email protected]; [email protected]
Background: Murine bone marrow-derived myofibroblasts (BMFs) have previously been shown to promote gastric cancer growth. However, whether BMFs promote gastric cancer cell metastasis remains largely unknown.
Methods: Wound healing assay, Transwell invasion and migration assay and 3D organotypic co-culture systems were conducted to study the effects of BMFs on invasion and migration of gastric cancer cells and the invasion and migration ability of gastric cancer stem cell-like cells (CSC-LCs) induced by BMFs. We employed two animal model to study the role of BMFs on the in vivo metastasis of gastric cancer cells and the metastatic ability of gastric BMF-induced CSC-LCs. A human gastric cancer tissue microarray and TCGA gastric cancer database were analysed to study the relationship between the expression of IL-6 and TGF-β 1 and clinicopathological characteristics and survival in gastric cancer.
Results: We found that BMFs promoted the in vitro migration and invasion of gastric cancer cells. BMFs promoted liver, lung, subcutaneous, and splenic metastases of MKN28 cells in the spleen injection liver metastasis model and co-injection of caudal vein (IOCV) mouse model. BMFs reprogrammed non-gastric cancer stem cell (CSC) to CSC-LCs and enhanced CSC-LC migration and metastasis. BMF-derived IL-6 and gastric cancer cell-secreted TGF-β 1 mediated the interaction between BMFs and gastric cancer cells, promoting tumour metastasis. BMFs enhanced the expressions of STAT3 and p-STAT3 in co-cultured gastric cancer cells. A combination of Napabucasin and Galunisertib exhibited the strongest inhibition of cell migration compared to when administered alone. Gastric cancer tissue array and TCGA database indicated that the overexpression of IL-6 and TGF-β 1 was associated with gastric cancer metastasis.
Conclusion: Our results demonstrated that BMFs promote gastric cancer metastasis through the activation of the TGF-β 1 and IL-6/STAT3 signalling pathways. Targeting the inhibition of these interactions may be a potent therapeutic strategy for addressing gastric cancer metastasis.
Keywords: bone marrow-derived myofibroblasts, gastric cancer, metastasis, IL-6, TGF-β 1
Introduction
Gastric cancer is one of the most common cancers and the third leading cause of cancer-related death worldwide.1 Most gastric cancer patients are diagnosed at an advanced stage with distant metastasis.2 Although the past few decades have seen a progression in disease diagnosis and treatment, the overall prognosis for patients remains poor owing to local relapse and distant metastasis.3 Thus, improved understanding of the underlying mechanisms that promote gastric cancer metastasis will enable the development of new treatment strategies.
Gastric cancers are composed of cancer and stromal cells that include inflammatory, endothelial, bone marrow-derived myeloid cells, and myofibroblasts, such as bone marrow-derived myofibroblasts (BMFs).4–6 Myofibroblasts in tumour stroma play an important role in cancer progression, including promoting metastasis.7,8 Our previous studies have shown that BMFs isolated from gastric dysplastic tissue of IL-1β transgenic mice contribute to a niche of mesenchymal stem cells (MSCs) and promote gastric cancer development.9 BMFs or BMF-CM induced cancer stem cell-like spheroids, clone the formation of gastric and colon cancer cells through the activation of the interleukin-6/Janus kinase 2/signal transducer and activator of transcription 3 (IL-6/JAK2/STAT3) pathway, and promote gastric and colon cancer growth.10,11 We also found that the blockade of TGF-β1 and IL-6/JAK2/STAT3 pathways could inhibit BMF-induced lung cancer growth.12 However, whether BMF promotes tumour metastasis remains unknown.
Our previous results indicated that BMFs could reprogram non-CSC cells to cancer cells with features of stem cells (referred herein as cancer stem cell-like cells, CSC-LCs) in gastric cancer cell lines.11 CSCs or CSC-LCs have been shown to play a crucial role in tumourigenesis and cancer progression in several types of tumours.13,14 These CSC-LCs possess the traits of self-renewal and epithelial-mesenchymal transition (EMT), and exhibit an increased capacity for tumourigenesis and metastasis.15 The CSC-LCs also had an increased expression of the metastasis-related genes CD10 and KIAA1199,16,17 and a decreased expression of metastasis suppressor Kiss-1.18 However, there remain large gaps in knowledge regarding the mechanisms by which BMFs promote tumour metastasis, and the mechanisms underlying the interactions between BMFs and cancer cells that lead to the production of CSC-LCs and contribute to tumour metastasis.
The interactions between gastric cancer cells and BMFs were shown to promote tumour growth through the IL-6/JAK2/STAT3 pathway.11 IL-6 is a dynamic cytokine which plays a role not only in immune responses and inflammation, but also in various epithelial tumours.19 Another proinflammatory cytokine, the transforming growth factor-β (TGF-β), is closely related to various cancer activities such as tumour onset and migration.20 The JAK/STAT3 pathway is required for TGF-β-induced EMT and cancer cell migration and invasion via up-regulation of the expression of p-Smad3 and Snail. The IL-6/JAK/STAT3 and TGF-β/Smad signalling pathways synergistically enhance EMT in lung carcinomas.21 Previously, we demonstrated that BMFs could secrete higher levels of cytokines, chemokines and growth factors when compared to wild-type fibroblasts and possess greater tumour promotion and tumour invasion capabilities.9 However, we did not investigate whether blocking the related signalling pathways can inhibit BMF-induced cell metastasis.
Here, we found that BMFs promoted the invasion and metastasis of gastric cancer cells in vitro and in vivo. BMFs also reprogrammed non-gastric cancer stem cells to CSC-LCs and enhanced tumour metastasis. Targeted inhibition of the TGF-β and IL-6/STAT3 signalling loop mediated the interactions between BMFs and gastric cancer cells. This consequently suppressed BMF-promoted gastric cancer metastasis. Our results suggested that the targeted suppression of interactions between BMFs and cancer cells might be a potent treatment strategy for gastric cancer metastasis in the future.
Materials and Methods
Cell Lines and Cell Reagents
Human gastric cancer cell MKN45 (RIKEN, Japan), SGC-7901 (Cell Bank, Shanghai), and MKN28 (RIKEN, Japan) were maintained in RPMI-1640. BMFs within 12 generations were used. Napabucasin (STAT3 inhibitor; Cat.No. HY-13,919) was purchased from MedChemExpress, and Galunisertib (TGFβ receptor I inhibitor; Cat.No. S2230) was purchased from Selleck.cn.
Isolation and Culture Cells
Wild type (WT) MFs and BMFs were isolated from the stomachs of C57BL/6, IL-1b/aSMA-RFP mice. The stomachs were cut into small pieces and incubated with collagenase I at 37°C for 1 hour. Characteristic features of MFs (abundant myofibrils with dense bodies, indented nucleus, basal lamina-like structure, capacity to express aSMA, vimentin and laminin) were demonstrated in both primary and secondary cultures.
Wound Healing Migration Assay
Viable cells were plated in an Ibidi Culture-Insert. The application of 3–7 × 105 cells/mL (70 µL) resulted in a confluent layer within 24 h depending on different cell types. Six-well culture plates were filled with 2 mL serum-free medium (SFM) or bone marrow derived-fibroblast conditioned medium (BMF-CM).
Wound healing percentage = (initial area - area at a certain point in time)/initial area. Within each assay, the experiments were performed in triplicates. Data shown are representative of at least three independent experiments.
Transwell Migration and Invasion Assay
Cell migration and invasion ability were investigated using the Transwell assays with modifications. The migration of the gastric cancer cells was assayed in Corning Costar Transwell chambers (Corning Costar; Transwell Permeable Supports, 6.5 mm Insert, 24 Well Plate 8.0 µm Polycarbonate Membrane). The cell invasion was assayed in Corning Matrigel invasion chambers (24-well Plate 8.0 Micron). Gastric cancer cells were counted and seeded (1 x 105 cells) in to the upper chamber in a final volume of 200 μL with SFM, 1 x 105 BMFs were added to the lower chamber in a final volume of 500 µL. The migrated cells were randomly selected from five fields under a 400-fold microscope and counted. Each data point is the average number of cells in five random fields, and is the mean ± standard deviation (SD) of three individual wells.
3D Organotypic Co-Culture System
3D Collagen/Matrigel contained 1 x 105 BMFs or WT-MFs cells. After seven days of contraction, 5 × 105 MKN28 or MKN45 cells were implanted in the upper layer of the Matrigel. They were cultured for two days in Epidermalisation I medium, followed by Epidermalisation II medium for two days, and then in Epidermalisation III medium for 6–10 days. 3D collagen/matrix was mixed with 10% formalin, embedded in paraffin, and subjected to hematoxylin and eosin (H&E) staining. The invading cells were counted under a microscope.
Western Blot Analysis
Cancer cells were lysed with the lysis buffer. Protein samples were subjected to SDS-polyacrylamide gels electrophoresis. The gels were transferred onto PVDF membranes (Millipore). Cell proteins were extracted and subjected to Western blot analysis using anti-STAT3 (Cat. 9139S) (Cell Signalling Technology), anti-phospho-STAT3-Tyr705 (Cat. 76,315) and anti- β-actin (Cat. ab6276) (Abcam).
Animal Model for Liver and Lung Tumour Metastases
We established an animal model of spleen injection for liver metastasis in SCID mice. Animal protocols were approved by the Animal Care and Facilities Committee (ACFC) of Rutgers, The State University of New Jersey (Protocol No. 09–050). The “Guide for the Care and Use of Laboratory Animals” (NIH) was followed when carrying out these experiments. The SCID mice were weighed and then divided into two groups of five mice each. Gastric cancer cells 0.1 mL 1 x 106 FL-MKN28 (Firefly-luciferase-MKN28 cell) or 0.1 mL of 1 x 105 FL-MKN45 (firefly-luciferase-MKN45 cell) were co-injected with 0.1 mL 1 x 106 BMFs or injected separately into mouse spleen. Xenogen IVIS mouse imaging system was adopted to detect the formation of liver metastases in mice every week.
Animal model of Lung metastasis was established by injection of caudal vein gastric cancer cells. Nude mice (5 Weeks) were weighed and divided into two groups mice (n=10) each 0.1 mL of 4 x 106 MKN28 cells were co-injected with 0.1 mL of 4 x 106 BMFs or injected alone. Mice were euthanised after three months. The pulmonary tissue was formalin-fixed and paraffin-embedded; H&E staining was performed according to a standard protocol.
Sphere Formation Assay
Stem cell medium (SCM) was a modified RPMI-1640 medium supplemented with epidermal growth factor (EGF; 20 ng/mL), basic fibroblast growth factor (bFGF; 10 ng/mL), and 0.3% bovine serum albumin (BSA). Cancer cells (1 x 104) were cultured either alone or with BMFs (1 x 104) in SCM in six-well plates for two weeks.
For the indirect co-culture system, BMFs and cancer cells were seeded in the upper Transwell inserts (0.4 μM in diameter, Corning) and lower wells in SCM for two weeks, respectively. In other experiments, cancer cells (1 x 102) were seeded in ultra-low attachment 96-well plates and incubated with BMF-CM or SCM for two weeks. A sphere is considered to be a three-dimensional (3D) spherical structure composed of cell colonies. The number of spheres in the entire well was calculated, and the percentage of total spheres to total seed cells was referred to as the “spherical ratio.”
Immunohistochemistry and Tissue Array
The human gastric cancer tissue array was from the Shanghai Ruijin Hospital, Shanghai Jiaotong University School of Medicine. The sample included 41 cases of non-metastatic and 82 cases of metastatic gastric cancer tissues. This study was approved and conducted in accordance with the guidelines (the Administration of Human Genetic Resources) of the Medical Ethics Committee of Shanghai Jiaotong University School of Medicine in China. All enrolled patients provided written informed consent at the start of the study. Tissue fixation, dehydration, paraffin embedding, and immunohistochemical staining was conducted as described previously. The sections were incubated with anti-IL-6 antibody (Abcam ab6672) and anti-TGF-β1 antibody (Abcam, Cat. ab66043) overnight at 4°C. Sample sections were incubated with a biotinylated secondary antibody, streptavidin-biotin complex (Dako, Glostrup, Denmark) and dyed with diaminobenzidine. Serial sections of mouse lung metastasis were incubated with anti-TGF-β1 antibody (GB14154), anti-IL-6-antibody (GB11117) and anti-p-STAT3-antibody (GB13168).
Statistical Analysis
All experiments were performed at least three times unless otherwise stated. Results were expressed as means ± SD and analysed for differences between two groups using two-tailed t-tests, assuming equal variances, with statistical significance set at p < 0.05. One-way ANOVA was adopted for comparing the results of three or more groups. All statistical analyses were performed using GraphPad Prism 7.0 software.
Results
BMFs and BMF-CM Promote Gastric Cancer Cell Migration
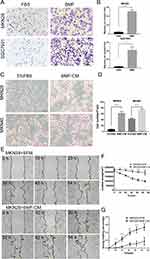
Our previous results have shown that BMFs promoted tumourigenesis and growth in gastric cancer cells.9,11 In this study, we further determined whether BMFs promote tumour migration, invasion, and metastasis in gastric cancer cells. Firstly, we found that BMFs promoted migration of MKN28, SGC7901 (Figure 1A and B) and MKN45 (Supplementary Figure 1A and B) cells. Secondly, to investigate whether BMFs promote cancer cell migration due to BMF-secreted cytokines or chemokines, we determined the effects of BMF-CM on the migration of gastric cancer cells. Transwell migration assay also showed that BMF-CM increased the migration of the MKN28 and MKN45 cells (Figure 1C and D). The wound healing assay demonstrated that BMF-CMs significantly enhanced the migration capabilities of MKN-28 (Figure 1E–G) and SGC-7901 cells (Supplementary Figure 1C–E) compared with the normal medium. These results demonstrated that BMFs promoted gastric cancer cell migration.
BMFs Promote in vitro Gastric Cancer Cell Invasion
Next, we investigated the effects of BMFs on the invasion of gastric cancer. We found that BMFs promoted the invasion of MKN28 and SGC7901 (Figure 2A and B) cells using a Transwell Matrigel invasion assay. Furthermore, using the 3D organotypic co-culture system, we observed that BMFs enhanced the invasion of MKN28 and MKN45 cells compared with the normal gastric MFs (Figure 2C). MKN28 and MKN45 cells displayed higher levels of invasion into the extracellular matrix (ECM) gel when co-cultured with BMFs (Figure 2C). These results suggested that BMFs could promote gastric cancer cell invasion.
BMFs Promote Gastric Cancer Cell Metastases in Liver and Lung
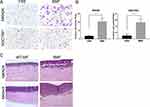
We further investigated the effects of BMFs on in vivo metastases of gastric cancer cells. MKN45 cells were highly tumourigenic, whereas MKN28 cells were weakly tumourigenic.11 The liver is one of the most common organs for gastric cancer metastasis.22,23 The intrasplenic (i.s.) implantation of tumour cells has been widely used in liver metastasis models.24 All cancer cell lines used in this project were engineered with a firefly-luciferase (FL) that enable them to be monitored in vivo using IVIS imaging, however, this labelling did not alter their biological properties. Immunofluorescence (IF) double staining confirmed EGFP+ a-SMA+BMFs in the liver tissues (data not shown). We first investigated whether BMFs can enhance the metastatic capacities of metastatic MKN45 cells. We found that co-injected MKN45 cells with BMFs into spleen developed larger liver metastatic tumours with stronger fluorescence in all 10 mice eight weeks after the injection of cells, whereas a single injection of MKN45 cells developed smaller tumours in 7/10 mice (Figure 3A). These results suggested that BMFs could remarkably enhance tumour metastasis in metastatic cancer cell lines.
Next, we investigated whether BMFs could induce metastasis in non-metastatic MKN28 cells. Our results showed that co-injection of MKN28 cells with BMFs caused the development of metastatic liver tumours in all five mice, whereas a single injection of MKN28 cells did not develop liver metastasis (Supplementary Figure 2A). The results suggested that BMFs can induce non-metastatic cancer cells to develop metastatic tumours.
To further confirm our findings, we determined whether BMFs could promote lung metastasis of MKN28 in an IOCV lung metastasis mouse model. The lung is also one of the most common metastatic sites of advanced gastric cancer, with lung metastasis occurring in 20–40% of metastasised gastric cancers.25 The results showed that 9/10 mice co-injected with MKN28 cells and BMFs developed lung metastasis three months after the injection (Figure 3C, D and F). Interestingly, 3/10 mice in the co-injection group developed subcutaneous metastasis (Figure 3B, E and F). Mice (1/10) developed both lung and splenic metastases in the co-injection group (Supplementary Figure 2B). However, no organ metastasis occurred in the MKN28 injection group (Figure 3B and F). These results demonstrated that BMFs had a strong capability to promote in vivo metastasis of gastric cancer cells.
BMF-Reprogrammed CSC-LCs Exhibit Increased Capacities of Migration, Invasion, and Metastasis
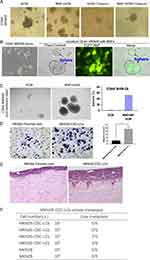
We previously identified CD44+ as a gastric CSC marker. We found that CD44+ and not CD44− MKN45 cells formed spheres in the SCM and developed tumourigenesis. MKN45 cells expressed CD44 (cell surface marker), whereas MKN28 cells did not express any known CSC markers (i.e., CD44−, CD133−, CD24−).11,26 Our previous studies indicated that BMFs accelerated tumour growth and exhibited stronger capability to enhance tumourigenesis than wild-type fibroblasts.11 In this study, we further found that BMFs enhanced the sphere clonal formation of CSC-LCs when CD44+MKN45 were co-cultured with BMFs in SCM compared with when they were cultured in SCM alone (Figure 4A). BMFs prevented CSC-LC differentiation in serum (Figure 4A). Furthermore, we found that CD44−MKN28 cells formed spheres when co-cultured with BMFs (EGFP+ cells) in SCM in attachment plates, whereas co-cultured BMFs did not form spheres (sphere were EGFP− cells) (Figure 4B). CD44−MKN28 cells formed more spheres when co-cultured with BMFs in SCM in unattachment plates, whereas CD44− MKN28 cells formed fewer spheres when cultured in SCM alone (Figure 4C). These results suggested that BMFs could reprogram non-CSC-LCs to CSC-LCs in gastric cancer MKN28 cells.
However, it is still unclear whether BMF-induced CSC-LCs possess greater capacities of invasion and metastasis. In this study, we found that BMF-reprogrammed MKN28-CSC-LCs exhibited increased capacities of migration (Figure 4D) and invasion (Figure 4E). Furthermore, although MKN28 cells are non-metastatic, the MKN28-CSC-LCs could still develop liver metastasis in an i.s. transplantation mouse model (Figure 4F). These results suggested that BMFs can reprogram cancer cells to CSC-LCs which then are thought to serve as initiators of cancer metastasis.
Blocking the Signalling Loop Between Cancer Cells and BMFs Inhibits BMF-Promoted Gastric Cancer Migration
Our previous study showed that BMF-derived IL-6 and cancer cell-derived TGF-β1 mediate the interactions between BMFs and tumour cells, as well contribute to the induction of CSC-LCs. As our previous reports,11 murine BMFs co-cultured with human gastric cancer cells secreted higher levels of mIL-6 when compared to murine BMFs cultured alone. Conversely, human gastric cancer cells co-cultured with murine BMFs expressed higher levels of hTGF-β1 than human gastric cancer cells cultured alone.
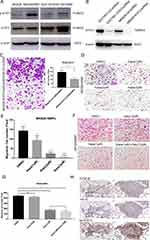
Whether the TGF-β1/IL-6 signalling loop mediates BMF-promoted metastasis remains unclear. Therefore, we determined the effects of blocking the signalling loop on BMF-induced migration in vitro. We found that the expression of STAT3 and p-STAT3 increased in MKN28 and SGC7901 cells when co-cultured with BMFs (Figure 5A). We first determined whether blocking IL-6/STAT3 signalling in cancer cells suppressed BMF-induced gastric cancer cell migration. We established STAT3-knockdown MKN28 stable cells and STAT3-knockdown SGC7901 stable cells (Figure 5B). We found that the migration cell number was significantly lower in MKN28-STAT3-knockdown cells co-cultured with BMFs when compared to that in MKN28-Ctrl-shRNA cells co-cultured with BMFs (Figure 5C). We observed similar results in SGC7901-STAT3-knockdown cells co-cultured with BMFs (Supplementary Figure 3A). Furthermore, treatment with Napabucasin (BBI608), a new specific small molecule inhibitor of STAT3 signalling,27–30 significantly inhibited BMF-induced migration of MKN28 (Figure 5D–E) and SGC7901 cells (Supplementary Figure 3B) compared with that of the control group.
Galunisertib is a TGF-β RI small molecular inhibitor.31,32 We observed that treatment with Galunisertib slightly decreased BMF-induced migration in MKN28 cells in a dose-independent manner (Supplementary Figure 4). However, there were no significant differences between the concentration groups. The combination of Napabucasin and Galunisertib synergistically enhanced the inhibition of BMF-induced migration in MKN28 cells (Figure 5F–G) compared with that of the single Napabucasin and Galunisertib treatments. These results demonstrated that the IL-6/STAT3 and TGF-β1 signalling loop played an important role in BMF-promoted migration.
To confirm the role of the IL-6/STAT3 and TGF-β1 signalling in BMF-promoted metastasis, we determined the expression of IL-6, p-STAT3, and TGF-β1 in lung metastatic BMF-promoted gastric tumours (Figure 3). We observed that the expressions of hTGF-β1, IL-6, and p-STAT3 were also remarkably higher in cancer tissues when compared to adjacent non-cancerous tissues with co-localisation (Figure 5H). These results suggested that mIL-6/STAT3 and hTGF-β1 mediated the interaction between gastric cancer cells and BMFs to promote metastasis.
High Expressions of IL-6 and TGF-β1 are Associated with Poor Clinical Prognosis
To investigate the clinical relevance of the expressions of TGF-β1 and IL-6 identified in mouse lung metastatic tumours, we further determined their expression in human gastric cancer tissues using a tissue array. We found that TGF-β1 overexpression was detected in gastric cancer cells in 57/82 of gastric cancer tissues with metastatic lesions, and only in 15/41 gastric cancer tissues without them. No expression was detected in normal gastric tissues (Figure 6A and B). IL-6 expression was detected in both stromal fibroblasts and cancer cells in 66/82 gastric cancer tissues with metastatic lesions and in 12/41 non-metastatic gastric cancer tissues, whereas no expression was detected in normal gastric tissues (Figure 6A and B). These results suggested that the expression of TGF-β1 and IL-6 might be associated with the development of gastric cancer metastasis. Our results were consistent with previous reports that cancer cell-derived TGF-β1 facilitates human colon cancer metastatic engraftment and expansion,33 and that IL-6 expression is associated with human gastric cancer lymph node and/or hepatic metastasis.34 To further determine the relationship between the expression of IL-6 and TGF-β1 and clinicopathological features in gastric cancer, we analysed data from stomach adenocarcinoma (STAD) in The Cancer Genome Atlas. The expressions of TGF-β1 and IL-6, both correlated with the T1 and T2-4 stages (p<0.05) (Figure 6C and D) and the M0 and M1 stages (p<0.05) (Figure 6E and F). The high expressions of TGF-β1 and IL-6 were associated with short survival (p<0.05) (Figure 6G and H). In conclusion, high expressions of IL-6 and TGF-β1 were associated with poor clinical prognosis.
Discussion
Tumour stromal myofibroblasts play critical roles in tumour initiation and progression in mouse tumour models and human cancers.7,8,35 Using the unique BMFs established by our research team, for the first time to our knowledge, we systematically investigated the roles of BMFs in tumour metastasis. We first found that BMFs could promote gastric cancer cells migration and invasion in vitro. Secondly, we had shown that BMFs could promote liver, lung, spleen, and subcutaneous metastasis of gastric cancer cells in vivo using two tumour metastatic animal models. We also found that Napabucasin inhibited BMF-promoted metastasis. These results demonstrated that BMFs had strong capacities to promote gastric cancer metastasis. This also suggested that targeting the inhibition of STAT3 signalling might be a novel strategy for the inhibition of BMF-promoted metastasis.
CSCs play critical roles in tumour initiation and tumour progression including in invasion, metastasis and tumour recurrence.36 However, whether BMF-reprogrammed CSC-LCs (CD44+ cells) could metastasise remains unknown. In our study, we found that BMF-reprogrammed MKN28-CSC-LCs exhibited enhanced capacities of migration and invasion in vitro and increased in vivo metastasis compared with parental MKN28 cells. The BMF-reprogrammed CSC-LCs are a major mechanism by which BMFs promote tumour metastasis. These results provided new evidence to support the notion that CSCs are the seeds of cancer metastasis.36 In our previous study, we found that murine IL-6 levels were much higher in the BMFs than the wild type fibroblasts.9 Murine IL-6 levels were higher in co-cultured-CM medium compared with BMF-CM, and blocking the IL-6 signalling reduced BMF-induced CSC-LCs.10 We found that BMF-CM could promote cell migrations, and blocking IL-6/STAT3 signalling reduced BMF-CM or BMF-induced cell migration and invasion in gastric cancer cells. These results demonstrate that BMF-derived IL-6 is a major cytokine that contributes to cell migration. To our knowledge, we are the first to demonstrate that BMFs could promote tumour cell migration and metastasis in gastric cancer. However, the study of whether other cytokines in BMF-CM also play an important role in tumour metastasis is still warranted.
Here, one of the interesting findings was that BMFs could promote metastasis of gastric cancer cells in multiple sites. Tail vein injection of tumour cells is a typical animal model to determine lung metastasis. We co-injected mice with MKN-28 cells and BMFs through the tail vein. These mice subsequently developed lung, subcutaneous, and splenic metastases, whereas mice (n=10) injected with only MKN-28 cells did not develop any metastasis. Our results contrasted previous reports that tumour cells by tail vein injection developed only lung metastasis.37 Our findings demonstrated that BMFs promote in vivo tumour metastasis. It is plausible that co-injected BMFs interact with cancer cells in vivo, and provide a microenvironment that maintains the features of CSCs and supports cancer cell survival. However, the detailed mechanisms by which BMFs promote the formation of subcutaneous and spleen metastases of gastric cancer cells requires further investigation.
We also identified that the IL-6/STAT3 and TGF-β1 signalling pathways played a critical role in mediating BMF-promoting tumour metastasis. We blocked the IL-6/STAT3 pathways and TGF-β1 signalling with Napabucasin and Galunisertib, respectively. This inhibited BMFs or BMF-CM-induced migration and invasion in gastric cancer cells and knockdown of the STAT3 significantly reduced BMFs-induced migration and invasion. Furthermore, analysis of clinical samples from the human gastric cancer tissue array and TCGA database also showed that the overexpression of TGF-β1 and IL-6 were associated with the development of gastric cancer metastasis and poor prognosis. The clinical relevance supports the notion that TGF-β1 and IL-6 contribute to metastasis of gastric cancer.
Another interesting finding was that Napabucasin exhibited stronger inhibition of migration and invasion of gastric cancer cells promoted by BMFs than Galunisertib.38–40 These results suggested that BMF-activated IL-6/STAT3 signalling might play a more important role in cancer metastasis. Our previous study demonstrated that BMF-derived IL-6/STAT3 signalling plays a critical role in CSC-LC induction.11 Our current study has shown that BMF-induced CSC-LCs can metastasise. Therefore, targeted inhibition of the IL-6/STAT3 signalling may be a novel strategy to inhibit the BMF-promoted metastasis. In fact, we have shown that Napabucasin significantly inhibited BMF-promoted lung metastasis of gastric cancer cells. Napabucasin has been considered a novel small molecule inhibitor of the cancer stem cell pathway and is undergoing clinical trials.28 Napabucasin has been increasingly shown to exhibit potent inhibition of tumour growth in a variety of human cancer mouse xenograft models including colon and pancreatic cancers and inhibition of liver metastasis of colon cancer cells.41 Our results showed that Galunisertib alone showed a minor inhibition of in vitro cancer cell migration induced by BMFs. However, the combination of Napabucasin and Galunisertib exhibited synergistic in vitro inhibition of BMF-induced migration gastric cancer cells. It remains to be investigated whether the combination of both can synergistically inhibit in vivo lung metastasis of gastric cancer.
However, our research still has some limitations. The peritoneum is the most common metastasis site of gastric cancer. In our study, we did not construct a gastric cancer peritoneal metastasis model, because we mainly want to prove that BMF has a strong role in promoting the distant metastasis of gastric cancer. We have reason to believe that BMF should have the ability to promote gastric cancer peritoneal metastasis. We will further explore this point in the future research.
Given that the IL-6/STAT3 and TGF-β1 signalling pathways constitute the key signalling loop that promotes metastasis of gastric cancer, we proposed a new model of how BMFs contribute to gastric cancer metastasis (Supplementary Figure 5). Our research into BMFs, CSCs and tumour metastasis enhanced our knowledge and understanding of the role of bone marrow-derived cells in tumour metastasis. These findings will enable the development of novel approaches for effective prevention and treatment of tumour metastasis.
Acknowledgments
The project was supported by NSFC 81472727, NSFC 91029718, NSFC 91429307; Shanghai Education Committee Key Discipline and Specialty Foundation (J50208), Science and Technology Commission of Shanghai Municipality (15JC1403100). National laboratory of Oncogene and Cancer-related Genes foundation (90-15-05).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors have no conflicts of interest for this work.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
2. Van Cutsem E, Sagaert X, Topal B, Haustermans K, Prenen H. Gastric cancer. Lancet. 2016;388(10060):2654–2664. doi:10.1016/S0140-6736(16)30354-3
3. Catalano V, Labianca R, Beretta GD, Gatta G, de Braud F, Van Cutsem E. Gastric cancer. Crit Rev Oncol Hematol. 2009;71(2):127–164. doi:10.1016/j.critrevonc.2009.01.004
4. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi:10.1016/j.cell.2011.02.013
5. Joyce JA, Fearon DT. T cell exclusion, immune privilege, and the tumor microenvironment. Science. 2015;348(6230):74–80. doi:10.1126/science.aaa6204
6. Hui L, Chen Y. Tumor microenvironment: sanctuary of the devil. Cancer Lett. 2015;368(1):7–13. doi:10.1016/j.canlet.2015.07.039
7. Nguyen DX, Bos PD, Massague J. Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer. 2009;9(4):274–284. doi:10.1038/nrc2622
8. Bhowmick NA, Neilson EG, Moses HL. Stromal fibroblasts in cancer initiation and progression. Nature. 2004;432(7015):332–337. doi:10.1038/nature03096
9. Quante M, Tu SP, Tomita H, et al. Bone marrow-derived myofibroblasts contribute to the mesenchymal stem cell niche and promote tumor growth. Cancer Cell. 2011;19(2):257–272. doi:10.1016/j.ccr.2011.01.020
10. Zhu L, Cheng X, Ding Y, et al. Bone marrow-derived myofibroblasts promote colon tumorigenesis through the IL-6/JAK2/STAT3 pathway. Cancer Lett. 2014;343(1):80–89. doi:10.1016/j.canlet.2013.09.017
11. Zhu L, Cheng X, Shi J, et al. Crosstalk between bone marrow-derived myofibroblasts and gastric cancer cells regulates cancer stemness and promotes tumorigenesis. Oncogene. 2016;35(41):5388–5399. doi:10.1038/onc.2016.76
12. Shi J, Feng J, Xie J, et al. Targeted blockade of TGF-β and IL-6/JAK2/STAT3 pathways inhibits lung cancer growth promoted by bone marrow-derived myofibroblasts. Sci Rep. 2017;7(1):8660. doi:10.1038/s41598-017-09020-8
13. Nguyen LV, Vanner R, Dirks P, Eaves CJ. Cancer stem cells: an evolving concept. Nat Rev Cancer. 2012;12(2):133–143.
14. Beck B, Blanpain C. Unravelling cancer stem cell potential. Nat Rev Cancer. 2013;13(10):727–738. doi:10.1038/nrc3597
15. Thiery JP. Epithelial–mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2(6):442–454.
16. Sasaki T, Kuniyasu H, Luo Y, et al. Serum CD10 is associated with liver metastasis in colorectal cancer. J Surg Res. 2014;192(2):390–394. doi:10.1016/j.jss.2014.05.071
17. Matsuzaki S, Tanaka F, Mimori K, Tahara K, Inoue H, Mori M. Clinicopathologic significance ofKIAA1199Overexpression in human gastric cancer. Ann Surg Oncol. 2009;16(7):2042–2051.
18. Dhar DK, Naora H, Kubota H, et al. Downregulation ofKiSS-1 expression is responsible for tumor invasion and worse prognosis in gastric carcinoma. Int J Cancer. 2004;111(6):868–872. doi:10.1002/ijc.20357
19. Kishimoto T. INTERLEUKIN-6: from basic science to medicine—40 years in immunology. Annu Rev Immunol. 2005;23(1):1–21. doi:10.1146/annurev.immunol.23.021704.115806
20. Biswas S, Chytil A, Washington K, Romero-Gallo J, Gorska AE. Transforming growth factor β receptor type II inactivation promotes the establishment and progression of colon cancer. Cancer Res. 2004;64(14):4687–4692. doi:10.1158/0008-5472.CAN-03-3255
21. Liu R-Y, Zeng Y, Lei Z, et al. JAK/STAT3 signaling is required for TGF-β-induced epithelial-mesenchymal transition in lung cancer cells. Int J Oncol. 2014;44(5):1643–1651.
22. Kakeji Y, Morita M, Maehara Y. Strategies for treating liver metastasis from gastric cancer. Surg Today. 2010;40(4):287–294.
23. Surgical treatment of liver metastasis of gastric cancer: a retrospective multicenter cohort study (KSCC1302).
24. Xue KX, Jin G. Study of liver metastasis formation and its mechanism using intrasplenic inoculation of cancer cells. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 1987;9(3):217–222.
25. Yoshida Y, Imakiire T, Yoneda S, Obuchi T, Inada K, Iwasaki A. Ten cases of resected solitary pulmonary metastases arising from gastric cancer. Asian Cardiovasc Thorac Ann. 2014;22(5):578–582. doi:10.1177/0218492313513777
26. Zhu L, Shi J, Jin H, et al. 335 the IL-6/STAT3/HGF and TGF-β1 signaling loop mediates crosstalk between cancer cells and bone marrow-derived myofibroblasts that regulate cancer stem cells and gastric tumorigenesis. Gastroenterology. 2013;144(5):S–70.
27. Zhang Y, Jin Z, Zhou H, et al. Suppression of prostate cancer progression by cancer cell stemness inhibitor napabucasin. Cancer Med. 2016;5(6):1251–1258. doi:10.1002/cam4.675
28. Hubbard JM, Grothey A. Napabucasin: an update on the first-in-class cancer stemness inhibitor. Drugs. 2017;77(10):1091–1103. doi:10.1007/s40265-017-0759-4
29. Macdonagh L, Gray SG, Breen E, Cuffe S, Finn SP. BBI608 inhibits cancer stemness and reverses cisplatin resistance in NSCLC. Cancer Lett. 2018;428:117–126. doi:10.1016/j.canlet.2018.04.008
30. Zhou Q, Peng C, Du F, et al. Design, synthesis and activity of BBI608 derivatives targeting on stem cells. Eur J Med Chem. 2018;151:39–50
31. Holmgaard RB, Schaer DA, Li Y, et al. Targeting the TGFβ pathway with galunisertib, a TGFβRI small molecule inhibitor, promotes anti-tumor immunity leading to durable, complete responses, as monotherapy and in combination with checkpoint blockade. J Immunother Cancer. 2018;6(1):47. doi:10.1186/s40425-018-0356-4
32. Maier A, Peille A-L, Vuaroqueaux V, Lahn M. Anti-tumor activity of the TGF-β receptor kinase inhibitor galunisertib (LY2157299 monohydrate) in patient-derived tumor xenografts. Cell Oncol. 2015;38(2):131–144. doi:10.1007/s13402-014-0210-8
33. Calon A, Espinet E, Palomoponce S, et al. Dependency of colorectal cancer on a TGF-beta-driven programme in stromal cells for metastasis initiation. Cancer Cell. 2013;22(2):571–584.
34. Ashizawa T, Okada R, Suzuki Y, et al. Clinical significance of interleukin-6 (IL-6) in the spread of gastric cancer: role of IL-6 as a prognostic factor. Gastric Cancer. 2005;8(2):124–131. doi:10.1007/s10120-005-0315-x
35. Tsujino T, Seshimo I, Yamamoto H, et al. Stromal myofibroblasts predict disease recurrence for colorectal cancer. Clin Cancer Res. 2007;13(7):2082–2090. doi:10.1158/1078-0432.CCR-06-2191
36. Li S, Li Q. Cancer stem cells and tumor metastasis (Review). Int J Oncol. 2014;44(6):1806–1812.
37. Bos PD, Nguyen DX, Massagué J. Modeling metastasis in the mouse. Curr Opin Pharmacol. 2010;10(5):571–577.
38. Herbertz S, Sawyer JS, Stauber AJ, Gueorguieva I, Lahn MM. Clinical development of galunisertib (LY2157299 monohydrate), a small molecule inhibitor of transforming growth factor-beta signaling pathway. Drug Des Devel Ther. 2015;9(default):4479–4499.
39. Seeger RC, Tran HC, Wan Z, Sheard MA, Asgharzadeh S. R1 TGF blockade with galunisertib (LY2157299) enhances anti-neuroblastoma activity of anti-GD2 antibody dinutuximab (ch14.18) with natural killer cells. Clin Cancer Res. 2016;23(3):804.
40. David C, Andreas VD, Alba B, et al. Biomarker and histopathology evaluation of patients with recurrent glioblastoma treated with galunisertib, lomustine, or the combination of galunisertib and lomustine. Int J Mol Sci. 2017;18(5):995.
41. Li Y, Rogoff HA, Keates S, Gao Y, Li CJ. Suppression of cancer relapse and metastasis by inhibiting cancer stemness. Proc Natl Acad Sci U S A. 2015;112(6):1839–1844. doi:10.1073/pnas.1424171112
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.