Back to Journals » International Journal of General Medicine » Volume 15
Body Mass Index and New-Onset Atrial Fibrillation in Patients with Acute Myocardial Infarction
Authors Liu L, Liu X, Ding X , Chen H , Li H
Received 25 March 2022
Accepted for publication 7 June 2022
Published 21 June 2022 Volume 2022:15 Pages 5717—5728
DOI https://doi.org/10.2147/IJGM.S367868
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Lei Liu,1 Xiaoyan Liu,1 Xiaosong Ding,1 Hui Chen,1 Hongwei Li1– 3
1Department of Cardiology, Cardiovascular Center, Beijing Friendship Hospital, Capital Medical University, Beijing, People’s Republic of China; 2Beijing Key Laboratory of Metabolic Disorder Related Cardiovascular Disease, Beijing, People’s Republic of China; 3Department of Geriatrics, Cardiovascular Center, Beijing Friendship Hospital, Capital Medical University, Beijing, People’s Republic of China
Correspondence: Hongwei Li, Department of Cardiology, Cardiovascular Center, Beijing Friendship Hospital, Capital Medical University, 95 Yongan Road, Beijing, 100050, People’s Republic of China, Tel +8613801396679, Email [email protected]; [email protected]
Background: The “obesity paradox” has not been elucidated in the long-term outcomes in acute myocardial infarction (AMI) patients. This study sought to characterize the relationship between body mass index (BMI) and the risk of new-onset atrial fibrillation (NOAF).
Methods: A total of 4282 participants free from AF at baseline were identified at Beijing Friendship Hospital. Baseline body mass index (BMI) was categorized into four groups. Incidence of NOAF was observed at the follow-up visits. The associations between different BMI categories and the incidence of NOAF were assessed by multivariate Cox regression analysis.
Results: Over a median follow-up period of 42.0 months, 4282 participants (age 62.7 ± 6.6 years, 38.7% women) were enrolled, 23.0% were BMI < 23.0kg/m2, 22.5% were 23.0– 24.9 kg/m2, 44.3% were 25.0– 29.9 kg/m2 and 10.2% were ≥ 30.0 kg/m2. Compared with patients with the lowest BMI levels, those with BMI≥ 30 kg/m2 showed a younger, higher inflammatory response and a larger left atrium and were more likely to be combined with traditional cardiovascular risk factors. After adjustment for confounding variables, compared to BMI ≥ 30 kg/m2 group, patients with lower BMI (< 23 kg/m2) significantly increased the risk of NOAF in AMI patients (HR 2.884, 95% CI 1.302– 6.392). Moreover, the all-cause mortality and cardiac mortality in BMI < 23.0kg/m2 group was apparently higher than that in BMI≥ 30 kg/m2 group after a long-term follow-up.
Conclusion: In this AMI cohort study, the present finding of an inverse association between BMI and risk of NOAF supports the “obesity paradox”. Decreasing BMI was associated with an increased risk of NOAF.
Trial Registration: Prospective registered.
Keywords: atrial fibrillation, acute myocardial infarction, body mass index, obesity
Introduction
Obesity is a major public health problem all over the world, and it is also an important risk factor for a wide range of chronic diseases, such as coronary heart disease (CHD) or diabetes mellitus (DM). The management of cardiovascular disease (CVD) in patients with obesity undisputedly presents numerous challenges. Atrial fibrillation (AF) is the most common heart arrhythmia worldwide. Multiple risk factors for cardiovascular disease, including obesity, hypertension, diabetes mellitus and old age, can increase the incidence of AF. Once AF occurs, non-negligible risks such as stroke will exist, and treatments aimed at eliminating AF are associated with limited long-term success rate.1,2
Body mass index (BMI) is the most widely used measure of adiposity. Previous studies showed elevated BMI was associated with an increased risk of AF in the general population.1,3,4 However, a phenomenon termed the “obesity paradox” was existed that in patients with heart failure, stable coronary artery disease, and acute coronary syndrome (ACS), obesity was associated with lower mortality than normal weight.5–7
The presence of the obesity paradox in AMI and AF populations remains unclear.
There have been limited investigations regarding the long-term prognosis into how BMI relates to new-onset atrial fibrillation (NOAF) risk in patients with AMI. Therefore, we examined the prognostic significance of BMI with NOAF in AMI patients in the study cohort.
Methods
Study Population
The study subjects were identified from the database at the Cardiovascular Center of Beijing Friendship Hospital. From December 2012 to December 2020, the exclusion criteria included 1. patients with missing clinical data (N = 161), 2. patients with AF or atrial flutter at baseline, or who had a prior history of AF (N = 408), and 3. patients who did not receive follow-up screening (N = 20). Finally, a total of 4282 consecutive patients with AMI were enrolled in this study (Supplementary Figure 1). The present study cohort included individuals who did not meet the exclusion criteria and subsequently received ≥1 annual examination. All patients were followed up to December 2021 with a median follow-up of 42.0 months (IQR: 18.7, 67.3 months). The primary endpoint was the incidence rate of new-onset AF (NOAF) during the clinical follow-up period. All-cause mortality and cardiac mortality were also observed as secondary endpoints in our study.
The local institutional review board at our hospital approved the study protocol, and this study was in accordance with the Declaration of Helsinki.
Ascertainment of AF
NOAF diagnosis was ascertained based on the European Society of Cardiology guidelines, and all the following 12-lead electrocardiogram (ECG) criteria were met: 1) irregular R-R intervals, 2) absence of repeating P waves, and 3) irregular atrial activity.8 ECG was reviewed by two cardiologists and the final diagnosis of NOAF was confirmed only when both cardiologists independently confirmed the same. The incidence time of NOAF was defined as the time of the first signs of AF.
Assessment of BMI
BMI was defined as weight (kg) divided by height in meter squared (m2) and categorized according to the classified according to the National Heart, Lung, and Blood Institute and World Health Organization defined categories.9,10 We classified BMI into four groups: BMI < 23.0kg/m2, BMI 23.0–24.9 kg/m2, BMI 25.0–29.9 kg/m2, and BMI ≥ 30.0 kg/m2.
Other Data Collection and Definitions
Patient demographic information, medical and medication history, and laboratory measurements were collected and confirmed through electronic medical records. The left atrium (LA), left ventricular end-diastolic dimension (LVEDD), E/A ratio, ventricular wall motion and left ventricular ejection fraction (LVEF) were determined using 2-dimensional echocardiography during the index hospitalization.
Statistical Analysis
Depending on the distribution of the data, continuous variables were expressed as mean value± SD or median and interquartile range (IQR). Frequencies and percentages were used to describe categorical data. Differences between continuous and categorical variables were assessed using Student’s t-test, analysis of variance, Chi-square test, and Wilcoxon signed rank test as appropriate.
The association of baseline BMI with incidence of NOAF was determined by calculation of hazard ratios (HR) and 95% confidence intervals (CI) with use of Cox hazard models, after verification of the proportional hazard assumption with schoenfeld residuals. Baseline variables that were significantly correlated with outcomes by univariate analysis and clinically relevant were entered into the multivariate model. In model 1, we adjusted for age and sex. In model 2, we further adjusted for LA, Killip (II–IV) and chronic total occlusion (CTO). In model 3, we further included estimated glomerular filtration rate (eGFR), peak value of N-terminal pro-B-type natriuretic peptide (pNT-proBNP), triglyceride and peak value of troponin I (pTNI).
All analyses were two-tailed and P value <0.05 was considered statistically significant. Data were analyzed using SPSS statistical package version 26.0 (SPSS Inc., Chicago, IL, USA).
Results
Baseline Characteristics
As shown in Supplementary Table 1, 4282 eligible patients (mean age 63.9 years, men 74.2%) included in our study, BMI was log-normally distributed, with a mean BMI of 25.5 ± 3.7kg/m2. More than half of patients (64.5%, N = 2762) were identified as having hypertension, 38.0% (N = 1629) had diabetes mellitus (DM), 27.4% (N = 1176) had CHD, 16% (N = 683) had a history of stroke and 11.0% (N = 470) had a history of myocardial infarction. AF was newly diagnosed in 132 patients, and the incidence of NOAF was 3.1%. Compared with the AF-free group, the NOAF group showed significantly older, lower BMI, total cholesterol (TC), triglyceride, estimated glomerular filtration rate (eGFR) and LVEF, larger LA, higher percent of Killip II–IV at admission and chronic total occlusion (CTO) after coronary angiogram.
We further evaluated the estimated infarction size by using serum peak value of troponin I (pTNI) levels. Peak value of N-terminal pro-B-type natriuretic peptide (pNT-proBNP) was also compared in our study. The NOAF patients showed higher pTNI and pNT-proBNP levels than AF-free patients.
All the patients were categorized into four groups according to their BMI categories as <23 kg/m2 (23.0%), 23–24.9 kg/m2 (22.5%), <25–29.9 kg/m2 (44.3%), or ≥30 kg/m2 (10.2%). Compared with patients with lower BMI, those with BMI ≥30 kg/m2 showed significantly younger, higher white blood cells (WBC), hypersensitivity C-reactive protein (hsCRP), eGFR, TC, triglyceride, low-density lipoprotein cholesterol (LDL-C), larger LA and LVEDD, higher E/A ratio, higher percent of hypertension and current/ex-smokers, lower percent of abnormal anterior wall motion and E/A<1. In addition, patients in BMI ≥30 kg/m2 group had lower percent of old myocardial infarction (OMI), killip II–IV and left main coronary artery (LM). In addition, patients in BMI ≥30 kg/m2 group had lower high-density lipoprotein cholesterol (HDL-C) than those in BMI <23.0 kg/m2 group (Table 1).
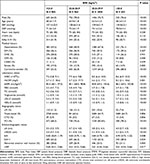 |
Table 1 Clinical Characteristics According to BMI Categories |
As shown in Table 2, we found that patients in BMI<23 kg/m2 group had the lowest pCKMB and pTnI levels (P = 0.059; P = 0.069, respectively). Patients with BMI<23 kg/m2 had significantly higher pNT-proBNP than other groups (P < 0.001).
 |
Table 2 Myocardial Injury Markers According to BMI Categories |
The Primary Endpoint During Follow-Up Period
During a median follow-up of 42.0 months (IQR: 18.7, 67.3 months), the incidence of NOAF was 3.1%. Figure 1 shows the unadjusted proportions of NOAF in different BMI categories. The BMI <23.0 kg/m2 group had a significantly higher incidence of NOAF during the long-term follow-up period, and the BMI≥30 kg/m2 group had a lowest incidence of NOAF (4.4% vs 3.8% vs 2.3% vs.1.8%, respectively, P = 0.004).
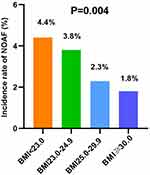 |
Figure 1 NOAF risk in different BMI categories. Abbreviations: NOAF, new-onset atrial fibrillation; BMI, body Mass Index. |
Association of BMI with NOAF in AMI Patients
A multivariable Cox regression analysis was conducted to determine the BMI associated with the incidence of NOAF during the follow-up period as shown in Table 3. On repeat analysis using the BMI ≥30.0kg/m2 group as reference, after adjusting for potential confounders including sex, age, LA, Killip (II–IV) and CTO, eGFR, triglyceride, pNT-proBNP and pTNI, BMI group of <23.0 kg/m2 and BMI group of 23.0–24.9 kg/m2 significantly increased the risk of NOAF in AMI patients. The HRs of NOAF across BMI categories (≥30 kg/m2, 25.0–29.9 kg/m2, 23.0–24.9 kg/m2, <23.0kg/m2) were 1 (reference); HR 1.281, 95% CI 0.597–2.750; HR 2.253, 95% CI 1.031–4.920; HR 2.884, 95% CI 1.302–6.392, respectively. When BMI was modeled as a continuous variable, we found a decreased risk of NOAF along with increasing BMI (P < 0.001) during the median follow-up period of 42.0 months.
 |
Table 3 Cox Proportional Hazard Regression Analysis of NOAF |
Subgroup Analysis of NOAF
The subgroup analyses stratified by sex, age, diabetes mellitus, STEMI, hypertension and LVEF are shown in Figure 2. In all subgroups, patients with lower BMI had a trend of higher risk of NOAF, except for LVEF ≤0.45 group. However, the patients with BMI <23.0kg/m2 in subgroup of age ≥65 years, age <65 years, male, no diabetes mellitus, NSTEMI, hypertension, no hypertension, LVEF >0.45 showed significantly higher risk of NOAF (all P < 0.05). Figure 2 Continue.
Kaplan-Meier Analysis of Secondary Endpoints
During a median follow-up period of 42.0 months, the total all-cause mortality was 12.7% (545/4282), the total cardiac mortality was 7.8% (335/4282) in all AMI patients. The Kaplan–Meier analysis showed the mortalities in different BMI categories. Figure 3 indicates that patients with BMI <23.0 kg/m2 had significantly higher all-cause mortality and cardiac mortality risk (all Log-Rank P < 0.001).
 |
Figure 3 Kaplan–Meier curve for all-cause mortality (A) and cardiac mortality (B). Abbreviations: NOAF, new-onset atrial fibrillation; BMI, body Mass Index. |
Discussion
In this study cohort of AMI patients, we found that the patients with higher BMI were more likely to be combined with traditional cardiovascular risk factors such as hypertension, smoking and hyperlipidemia, and more likely to be combined with severe inflammatory response. The “obesity paradox” existed in the relationship between BMI and NOAF, and lower BMI increased the risk of NOAF in AMI patients, which was inconsistent with previous studies. Furthermore, patients with lower BMI had higher all-cause mortality and cardiac mortality, which were consistent with previous studies.
Obesity is a chronic metabolic disease characterized by an increase in body fat stores. It can increase plasma triglyceride levels, reduce HDL-C levels, increase systemic inflammation, and increase prevalence of type 2 DM.11,12 Adipose tissue is also recognized as a rich source of proinflammatory mediators.13 Moreover, obesity can produce haemodynamic changes and cardiac morphology alterations, left atrial enlargement is a common feature in patients who are obese, and left ventricular systolic function is usually normal.12 These were all harmful to health and promoted the development of chronic disease. In clinical practice, the body fatness is estimated by BMI, and BMI can reflect the degree of obesity.14 Consistent with previous studies, in our study we also found the patients with BMI >30 kg/m2 had higher TC, triglyceride, LDL-C levels, lower HDL-C levels and a larger LA and LVEDD than those with lower BMI. Moreover, the obese patients had higher inflammatory response due to the higher WBC and hsCRP, which reacted to the degree of inflammatory response.
According to AF, previous studies determined that obesity was an important, potential risk factor, and the association appeared to be mediated by left atrial dilatation.15 A meta-analysis demonstrated that obesity increased the risk of developing AF in the general population, and the risk escalated in parallel with increased BMI.16 Therefore, obesity had long been considered as a risk factor for AF development. However, in some cases, “Obesity paradox” was existed. “Obesity paradox” in coronary heart disease was first introduced in 2002.6 The current studies showed “obesity paradox” in the overall population, overweight or obese patients had a lower incidence of main adverse cardiac events (MACEs) compared with the normal-weight group, and the risk of stroke was also lowered in obese patients.17 CB Jong et al18 also found the relationship between BMI and mortality rate was U, class I obesity represented the nadir and normal weight was the peak in patients with type 2 diabetes mellitus and acute coronary syndrome. Previous studies paid more attention to the relationship between mortality and MACEs and obesity, and the presence of the obesity paradox with NOAF in this very high-risk population such as AMI remains unclear. In our study cohort, we found that the lowest BMI conferred a more than 2-fold increased risk of NOAF during median 42.0 months follow-up period. Obesity is not a risk factor for NOAF development. The results support the phenomenon of “the obesity paradox”, but it was inconsistent with previous studies.
Possible mechanisms for the inverse relationship were not mentioned in the literature. There are many explanations for the obesity paradox in AMI population. One is that most obese patients are younger, which usually implies less severe coronary artery disease, larger coronary vessel diameter and a higher rate of guideline-based medication use.18,19 Emre et al20 also found that obesity was associated with better coronary characteristics in the elderly patients (age ≥65 years) treated for ST-elevation myocardial infarction. In our study, we similarly found that the obese patients had a lower percent of left main lesion after coronary angiograph, which was consistent with previous studies. In addition, higher BMI was associated with increased liver lipase activity which may accelerate the breakdown of lipids.21 The membrane-stabilizing effect of cholesterol on lipid rafts and caveolae indirectly determines the localization of ion channels including K+, Na+ and Ca2+ subunits, which can induce prolongation of QT interval.22,23 Furthermore, cholesterol depletion also increased intracellular Ca2+ concentration and triggered a signaling cascade, culminating with contraction impairment and myofibril disruption in cardiomyocytes.24 That was a mechanism of AF development. Thirdly, adiponectin produced in adipose tissue and had a protective effect against atherosclerosis,13 which may potentially improve the myocardial ischemia and reduce AF risk in AMI patients. Adipose tissue can also produce a soluble tumor necrosis factor receptor, neutralizing the deleterious effects of tumor necrosis factor-alpha on the myocardium.25 Fourthly, other confounding factors, different sample size, or the varying wide study populations in different regions may be reasons too. Lastly, BMI itself has been questioned as the optimal measurement to use for assessing obesity. The measurement does not differentiate between fat, muscle, and skeletal weight.25–27 Thus, BMI may not be a good indicator to elevate the risk of NOAF. Evaluating visceral adiposity, such as pericardial fat or abdominal fat, may be more significant than BMI in controlling the risk of NOAF. Pericardial fat volume, which correlated with left atrial enlargement, was associated with increased risk of AF.28 It serves as a pro-inflammatory source and disrupts the myocardial architecture, causing conduction slowing.29 The accumulation of intra-abdominal fat is a marker for higher metabolic and cardiovascular disease risk, and it can be assessed by waist circumference.27
In fact, BMI is an easily regulated factor; therefore, controlling BMI levels in patients with AMI may obtain greater clinical benefits in decreasing NOAF risk. The association between BMI and AF should be reevaluated in AMI disease.
Study Limitations
First, this was a single-center study with a limited sample size. Second, although many potential interfering covariables were adjusted by our models, we cannot rule out residual confounding such as thyroid hormone levels or medication. The manner and frequency of the evaluation for the diagnosis of AF may lead to an underestimation of the incidence of NOAF. Third, we did not analyze the patients with BMI<18.5 kg/m2 separately in the cohort; therefore, we did not determine the effect of these populations on the risk of NOAF; these populations may influence our results.
Conclusion
In this AMI cohort study, the present finding of an inverse association between BMI and risk of NOAF supports the “obesity paradox” but was inconsistent with precious literature. Decreasing BMI was associated with an increased risk of NOAF. According to all-cause mortality and cardiac mortality, lower BMI may increase mortality.
Abbreviations
BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; ACS, acute coronary syndrome; AMI, acute myocardial infarction; CHD, coronary heart disease; OMI, old myocardial infarction; GABG, coronary artery bypass graft; DM, diabetes mellitus; WBC, white blood cells; hsCRP, hypersensitivity C-reactive protein; eGFR, estimated glomerular filtration rate; FBG, fasting blood glucose; TC, total cholesterol; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; pCKMB, peak value of creatine kinase isoenzyme-MB; pTNI, peak value of troponin I; pNT-proBNP, peak value of N-terminal pro-B-type natriuretic peptide; LVEDD, left ventricular end-diastolic dimension; LVEF, left ventricular ejection fraction; LM, left main trunk; PCI, percutaneous coronary intervention; CTO, chronic total occlusions; NOAF, new-onset atrial fibrillation; STEMI, ST-segment elevation myocardial infarction; NSTEMI, non-ST-segment elevation myocardial infarction.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author Hongwei Li on reasonable request.
Ethics Approval and Consent to Participate
The study data collections were approved by the Institutional Review Board of Beijing Friendship Hospital, Capital Medical University, and written informed consent was obtained from all patients.
Acknowledgments
The authors gratefully acknowledge the contributions of all staff who work at the Cardiovascular Center of Beijing Friendship Hospital Data Bank (CBD BANK).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by the National Key R&D Program of China (2021ZD0111004), Natural Science Foundation of China (No. 82070357), Beijing Municipal Administration of Hospital Incubating Program (No. PX2018002), and Beijing Key Clinical Subject Program.
Disclosure
The authors declare that they have no competing interests.
References
1. Tedrow UB, Conen D, Ridker PM, et al. The long- and short-term impact of elevated body mass index on the risk of new atrial fibrillation the WHS (women’s health study). J Am Coll Cardiol. 2010;55(21):2319–2327. doi:10.1016/j.jacc.2010.02.029
2. Waldo AL. A perspective on antiarrhythmic drug therapy to treat atrial fibrillation: there remains an unmet need. Am Heart J. 2006;151(4):771–778. doi:10.1016/j.ahj.2005.06.014
3. Aune D, Sen A, Schlesinger S, et al. Body mass index, abdominal fatness, fat mass and the risk of atrial fibrillation: a systematic review and dose-response meta-analysis of prospective studies. Eur J Epidemiol. 2017;32(3):181–192. doi:10.1007/s10654-017-0232-4
4. Singleton MJ, German CA, Soliman EZ, et al. Body mass index, sex, and incident atrial fibrillation in diabetes: the ACCORD trial. JACC Clin Electrophysiol. 2020;6(13):1713–1720. doi:10.1016/j.jacep.2020.08.008
5. Horwich TB, Fonarow GC, Hamilton MA, MacLellan WR, Woo MA, Tillisch JH. The relationship between obesity and mortality in patients with heart failure. J Am Coll Cardiol. 2001;38(3):789–795. doi:10.1016/S0735-1097(01)01448-6
6. Gruberg L, Weissman NJ, Waksman R, et al. The impact of obesity on the short-term and long-term outcomes after percutaneous coronary intervention: the obesity paradox? J Am Coll Cardiol. 2002;39(4):578–584. doi:10.1016/S0735-1097(01)01802-2
7. Angerås O, Albertsson P, Karason K, et al. Evidence for obesity paradox in patients with acute coronary syndromes: a report from the Swedish Coronary Angiography and Angioplasty Registry. Eur Heart J. 2013;34(5):345–353. doi:10.1093/eurheartj/ehs217
8. Hindricks G, Potpara T, Dagres N, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021;42(5):373–498. doi:10.1093/eurheartj/ehaa612
9. Report of a WHO consultation. Obesity: preventing and managing the global epidemic. World Health Organ Tech Rep Ser. 2000;894:
10. NHLBI Obesity Education Initiative. The practical guide identification, evaluation, and treatment of overweight and obesity in adults. Available from: https://www.nhlbi.nih.gov/files/docs/guidelines/prctgd_c.pdf. Accesed June 15, 2022.
11. Lavie CJ, De Schutter A, Parto P, et al. Obesity and prevalence of cardiovascular diseases and prognosis-the obesity paradox updated. Prog Cardiovasc Dis. 2016;58(5):537–547. doi:10.1016/j.pcad.2016.01.008
12. Lavie CJ, Arena R, Alpert MA, Milani RV, Ventura HO. Management of cardiovascular diseases in patients with obesity. Nat Rev Cardiol. 2018;15(1):45–56. doi:10.1038/nrcardio.2017.108
13. Lau DC, Dhillon B, Yan H, Szmitko PE, Verma S. Adipokines: molecular links between obesity and atherosclerosis. Am J Physiol Heart Circ Physiol. 2005;288(5):H2031–2041. doi:10.1152/ajpheart.01058.2004
14. Yumuk V, Tsigos C, Fried M, et al. European guidelines for obesity management in adults. Obes Facts. 2015;8(6):402–424. doi:10.1159/000442721
15. Wang TJ, Parise H, Levy D, et al. Obesity and the risk of new-onset atrial fibrillation. JAMA. 2004;292(20):2471–2477. doi:10.1001/jama.292.20.2471
16. Wanahita N, Messerli FH, Bangalore S, Gami AS, Somers VK, Steinberg JS. Atrial fibrillation and obesity–results of a meta-analysis. Am Heart J. 2008;155(2):310–315. doi:10.1016/j.ahj.2007.10.004
17. Park SJ, Ha KH, Kim DJ. Body mass index and cardiovascular outcomes in patients with acute coronary syndrome by diabetes status: the obesity paradox in a Korean national cohort study. Cardiovasc Diabetol. 2020;19(1):191. doi:10.1186/s12933-020-01170-w
18. Jong CB, Li HY, Pan SL, et al. Relationship between body mass index, antidiabetic agents, and midterm mortality in patients with both type 2 diabetes mellitus and acute coronary syndrome. J Am Heart Assoc. 2019;8(7):e011215. doi:10.1161/JAHA.118.011215
19. Lancefield T, Clark DJ, Andrianopoulos N, et al. Is there an obesity paradox after percutaneous coronary intervention in the contemporary era? An analysis from a multicenter Australian registry. JACC Cardiovasc Interv. 2010;3(6):660–668. doi:10.1016/j.jcin.2010.03.018
20. Emre E, Ural E, Aktas M, et al. The existence of obesity paradox and effect of obesity on in-hospital-outcomes on elderly patients treated with primary percutaneous coronary intervention. Int J Gerontol. 2018;12(1):17–21. doi:10.1016/j.ijge.2017.07.008
21. Wang P, Xu TY, Guan YF, Su DF, Fan GR, Miao CY. Perivascular adipose tissue-derived visfatin is a vascular smooth muscle cell growth factor: role of nicotinamide mononucleotide. Cardiovasc Res. 2009;81(2):370–380. doi:10.1093/cvr/cvn288
22. Maguy A, Hebert TE, Nattel S. Involvement of lipid rafts and caveolae in cardiac ion channel function. Cardiovasc Res. 2006;69(4):798–807. doi:10.1016/j.cardiores.2005.11.013
23. Dart C. SYMPOSIUM REVIEW: lipid microdomains and the regulation of ion channel function. J Physiol. 2010;588(17):3169–3178. doi:10.1113/jphysiol.2010.191585
24. Hissa B, Oakes PW, Pontes B, Ramírez-san juan G, Gardel ML. Cholesterol depletion impairs contractile machinery in neonatal rat cardiomyocytes. Sci Rep. 2017;7:43764. doi:10.1038/srep43764
25. Uretsky S, Messerli FH, Bangalore S, et al. Obesity paradox in patients with hypertension and coronary artery disease. Am J Med. 2007;120(10):863–870. doi:10.1016/j.amjmed.2007.05.011
26. De Schutter A, Lavie CJ, Arce K, Menendez SG, Milani RV. Correlation and discrepancies between obesity by body mass index and body fat in patients with coronary heart disease. J Cardiopulm Rehabil Prev. 2013;33(2):77–83. doi:10.1097/HCR.0b013e31828254fc
27. De Schutter A, Lavie CJ, Patel DA, Milani RV. Obesity paradox and the heart: which indicator of obesity best describes this complex relationship? Curr Opin Clin Nutr Metab Care. 2013;16(5):517–524. doi:10.1097/MCO.0b013e328363bcca
28. Baek YS, Yang PS, Kim TH, et al. Associations of abdominal obesity and new-onset atrial fibrillation in the general population. J Am Heart Assoc. 2017;6:6. doi:10.1161/JAHA.116.004705
29. Karam BS, Chavez-Moreno A, Koh W, Akar JG, Akar FG. Oxidative stress and inflammation as central mediators of atrial fibrillation in obesity and diabetes. Cardiovasc Diabetol. 2017;16(1):120. doi:10.1186/s12933-017-0604-9
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

