Back to Journals » Biologics: Targets and Therapy » Volume 14
Blinatumomab for the Treatment of Adult B-Cell Acute Lymphoblastic Leukemia: Toward a New Era of Targeted Immunotherapy
Authors Franquiz MJ , Short NJ
Received 1 January 2020
Accepted for publication 31 January 2020
Published 14 February 2020 Volume 2020:14 Pages 23—34
DOI https://doi.org/10.2147/BTT.S202746
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Doris Benbrook
Miguel J Franquiz,1,2 Nicholas J Short2
1Baylor College of Medicine, Houston, TX, USA; 2Department of Leukemia, The University of Texas MD Anderson Cancer Center, Houston, TX, USA
Correspondence: Nicholas J Short
Department of Leukemia, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd. Unit 428, Houston, TX 77030, USA
Tel +1-713-563-4485
Email [email protected]
Abstract: Several therapeutic advancements in the treatment of B-cell acute lymphoblastic leukemia (ALL) have surfaced in the past decade, primarily driven by an increased understanding of the immunopathobiology of this disease. The clinical use of blinatumomab, a bispecific antibody that coordinates cytotoxic CD3+ T lymphocytes and CD19+ lymphoblasts, has resulted in improved outcomes in both relapsed/refractory and minimal residual disease-positive B-cell ALL. Promising emerging data also demonstrate the efficacy of this agent in the frontline setting and in combination regimens. Uncertainty remains regarding the optimal sequencing and combination of blinatumomab with cytotoxic chemotherapy and other emerging agents. The pharmacology and clinical data on blinatumomab for adult B-cell ALL, both as monotherapy and in combinations, will be reviewed herein.
Keywords: blinatumomab, bispecific antibodies, acute lymphoblastic leukemia, precursor cell lymphoblastic leukemia-lymphoma, adult, B-cell leukemia
Introduction
Acute lymphoblastic leukemia (ALL) is a hematologic malignancy caused by proliferation and accumulation of lymphoid progenitor cells in the bone marrow or in extramedullary sites. ALL can present with B-cell and T-cell phenotypic subgroups; however more than two-thirds of all cases in adults are B-cell phenotype.1 ALL affects children, adults, and older adults, with treatment outcomes differing among each age group. Over half of new B-cell ALL cases occur in children, with a second peak in incidence occurring at >60 years of age.1 In the past decades, significant progress has been made in the treatment of pediatric ALL, and modern multiagent chemotherapy regimens have resulted in cure rates >90% in many subgroups.2 However, these advances have not translated to similar outcomes in adult ALL. In adult ALL, multiagent chemotherapy has shown higher complete remission (CR) rates (80–95%), but high treatment-related mortality and frequent relapses leading to a cure rate of only 30–50%.3 Adults with ALL have poorer outcomes than their pediatric counterparts due to more frequent high-risk disease features (e.g. poor-risk molecular and cytogenetic abnormalities), higher rates of comorbidities, and less physiologic reserve to tolerate aggressive cytotoxic chemotherapy.4 Adults >60 years of age have consistently poorer outcomes when compared to younger individuals, with 5-year OS <20% even in patients treated at academic centers.5,6 For patients with relapsed/refractory disease, outcomes are dismal, with cure achieved in <10% in several studies when combination salvage chemotherapy is used.7,8
Recent years have witnessed the development of more sophisticated means of characterizing tumor biology,9 extremely sensitive assays used to assess residual disease burden,10,11 and accelerated development of several novel targeted therapies.12 These advancements have resulted in significant improvements in the treatment of adult ALL subtypes, particularly in the relapsed/refractory setting. This review will focus on one such improvement, the bispecific CD3-CD19 antibody blinatumomab. Blinatumomab currently carries US Food and Drug Administration (FDA) approvals for the treatment of relapsed/refractory B-cell ALL and minimal/measurable residual disease (MRD)-positive B-cell ALL in adults. Herein, we will review the pharmacology of blinatumomab and the clinical data for its use in these settings. We will also discuss the emerging data supporting the use of blinatumomab in the frontline setting and in combination with chemotherapy and other novel agents.
Blinatumomab
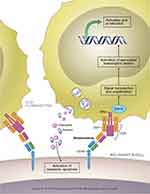
Blinatumomab is a 55-kilodalton fusion protein consisting of two recombinantly expressed single-chain variable fragments (murine anti-CD19 and anti-CD3) joined by a flexible glycine-serine linker.13 (Figure 1) CD19 is a cell surface antigen expressed ubiquitously on precursor B-cells, and has been implicated in the self-renewal capacity of leukemia cells.14 CD3 is a T-cell co-receptor involved in the activation of various types of T cells.15 The bispecificity of blinatumomab leads to the colocalization of cytotoxic T lymphocytes (CTLs) and B-cells expressing CD19, leading to the release of cytotoxic granules containing granzyme and perforin, and ultimately resulting in induction of apoptosis of the malignant B-cells.13,16 (Figure 2) Blinatumomab also results in the elaboration of various cytokines including interleukin (IL)-2, tumor necrosis factor-α, interferon-γ, IL-6, IL-10, and IL-4.13 The role of cytokine release is unclear with regards to its efficacy but is responsible for some of the common adverse events observed with blinatumomab.17 These on-target effects are achieved at a very low drug concentration (10–100 pg/mL).18 The high potency of blinatumomab has been attributed to costimulatory molecule-independent CTL expansion as well as its capacity for serial cytolysis at low effector-to-target ratios.19–21
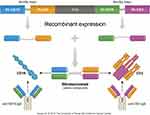 |
Figure 1 Blinatumomab structure. Variable regions of anti-CD19 and anti-CD3 joined by a serine-glycine linker are recombinantly expressed in Chinese hamster ovary cells. |
The pharmacokinetics of blinatumomab have been summarily described in an analysis of multiple studies including 387 patients exposed to blinatumomab at various doses.22 Table 1 details the pharmacokinetics of blinatumomab. Blinatumomab exhibits linear pharmacokinetics with first-order elimination. At clinically relevant doses, steady-state plasma concentrations range from 565 to 771 pg/mL. Blinatumomab exhibits minimal tissue distribution approximating the plasma compartment (VD 4.5L). On aggregate blinatumomab exhibits rapid clearance (mean CL 2.7 L/h) and has a very short elimination half-life (t1/2 1–2h). Population analysis of blinatumomab clearance reveals a bimodal distribution with significant interindividual variability. In this study, a minority (10%) of patients exhibited ultra-rapid clearance of blinatumomab (>5L/h). The mechanism of blinatumomab catabolism is incompletely described. Less than 0.2% of blinatumomab is recovered as a complete drug molecule in urinary assays. Age, sex, race, type of hematologic malignancy, renal function, and hepatic function have not been shown to significantly affect blinatumomab pharmacokinetics. Blinatumomab appears to exhibit minimal immunogenicity, with <1% of patients in this analysis developing neutralizing anti-drug antibodies. Because of its extremely short half-life, blinatumomab must be administered as a 24 hr continuous intravenous infusion, which can present practical challenges to drug delivery.
 |
Table 1 Blinatumomab Pharmacokinetics |
Evidence Summary
Available clinical studies evaluating the efficacy of blinatumomab in the relapsed/refractory, MRD-positive, and frontline settings will be reviewed below. A tabulated summary of the safety and efficacy findings from these studies is listed in Table 2. Where ongoing clinical trials incorporating blinatumomab are referenced, readers are directed to Table 3.
 |
Table 2 Blinatumomab Clinical Trial Data in B-Cell ALL |
 |
Table 3 Ongoing Blinatumomab Clinical Trials |
Relapsed/refractory B-cell ALL
Ph-Negative B-Cell ALL: Monotherapy
In an early open label Phase I/II study, 36 patients with relapsed/refractory Ph-negative B-cell ALL received blinatumomab at either escalating doses or fixed-dose at trial entry.23 Fatal neurologic and cytokine release syndrome (CRS) events were observed in the fixed-dose group, and therefore this dosing strategy was abandoned. The remaining 18 patients enrolled received a ramp-up strategy with a maximum dose of 15 mcg/m2/day. Patients were permitted to receive up to 4 cycles of blinatumomab (4 weeks of continuous infusion followed by a 2-week off period). Among 36 patients treated, 25 patients (69%) achieved CR or CR with partial hematologic recovery (CRh). Among the responders, 22 (88%) achieved MRD negativity, with the majority after the first cycle of blinatumomab. Median OS was 9.8 months, and median relapse-free survival (RFS) was 7.6 months. Six patients had grade 3 or higher central nervous system (CNS) toxicity, and 2 patients had grade 4 cytokine release syndrome. Although the pathophysiology of CRS is incompletely understood, it is postulated that blinatumomab administration sometimes results in the activation of bystander immune and endothelial cells, resulting in systemic inflammation.24 In a follow-up long-term analysis of this study population, 10 of 36 patients (28%) were long-term responders (defined as OS >30 months).25 MRD response was strongly associated with long-term response (with all long-term responders achieving MRD negativity). In this analysis, T-cell expansion was more pronounced in MRD responders, confirming the relationship between the on-target effects of blinatumomab and clinical outcomes.25
A subsequent confirmatory Phase II study was conducted in which 189 patients with relapsed/refractory Ph-negative B-cell ALL were treated with blinatumomab 9 mcg/day for 1 week, followed by 28 mcg/day for 3 weeks, and then a 2-week drug-free interval.26 Seventy-four patients (39%) were in their second or greater relapse, and 64 (34%) had previously undergone HSCT. After 2 cycles, 43% of patients achieved CR/CRh. The overall response rate (ORR) was strongly associated with lower baseline blast percentage (73% if <50% blasts versus 29% if ≥50% blasts). Among evaluable responders, 82% also achieved an MRD response. Median OS for the entire study population was 6.1 months, and median RFS was 5.9 months. Twenty-four grade 3 or higher CNS adverse events occurred (12% rate), and three grade 3 or higher instances of CRS occurred (2% rate). The findings of this study led to the preliminary accelerated FDA approval of blinatumomab monotherapy for relapsed/refractory Ph-negative B-cell ALL in 2014, pending additional confirmatory studies. Notably, blinatumomab was the first FDA-approved bispecific antibody, representing a new class of effective antibody constructs in oncology.
The pivotal Phase III TOWER study was an international, multicenter, open-label trial that randomized 405 patients with relapsed/refractory Ph-negative B-cell ALL in a 2:1 fashion to either blinatumomab monotherapy by continuous infusion (administered as 9 mcg/day on week 1, followed by 28 mcg/day on weeks 2–4, followed by a 2-week drug-free period) or multiagent chemotherapy.27 Patients who were randomized to blinatumomab could receive up to 5 cycles as induction and consolidation, followed by maintenance blinatumomab for up to 1 year (i.e. 4-week blinatumomab infusion every 12 weeks). Overall, 45% of patients were in second or greater salvage, 34% had previously undergone HSCT, and 75% had ≥50% bone marrow blasts at enrollment. The chemotherapy regimens employed were FLAG-IDA (45%, fludarabine, high-dose cytarabine, and granulocyte colony-stimulating factor with or without an anthracycline), a high-dose cytarabine-based regimen (17%), a high-dose methotrexate-based regimen (20%), and a clofarabine-based regimen (17%). A higher CR rate was observed in the blinatumomab group as compared to the standard chemotherapy group (91/271 [34%] versus 21/134 [16%], p <0.001), and a higher ORR defined as CR/CRh/CR with incomplete hematologic recovery (CRi) was observed in patients treated with blinatumomab as well (119/271 [44%] versus 33/134 [25%], p <0.001). Among responders, MRD negativity rates were 76% for blinatumomab versus 48% for chemotherapy. Median OS, which was the primary outcome of the study, was significantly longer in patients treated with blinatumomab (7.7 versus 4.0 months, hazard ratio [HR] 0.71, p = 0.01). Grade 3 or higher adverse CNS events occurred in 9.4% of blinatumomab treatment patients and 8.3% of chemotherapy treatment patients, and grade 3 CRS occurred in 4.9% and 0%, respectively. The findings of this study resulted in the full FDA approval of blinatumomab monotherapy for relapsed/refractory B-cell ALL in 2017. The inclusion of patients with Ph-positive B-cell ALL was based on promising results in this subset from the parallel phase II ALCANTARA study which will be discussed below.
Ph-Negative B-Cell ALL: Combination Therapy
After blinatumomab was used successfully as a single agent in relapsed/refractory B-cell ALL, several investigators have evaluated whether combining blinatumomab with cytotoxic agents or other novel therapies might further improve outcomes. Of particular interest is the combination of blinatumomab with inotuzumab ozogamicin (INO), an anti-CD22 antibody–drug conjugate that delivers ozogamicin (a calicheamicin derivative that induces DNA scission) to CD22-bearing malignant B-cell precursors. In a phase III randomized trial in patients with relapsed/refractory B-cell ALL, INO demonstrated highly superior CR/CRi rates when compared to standard chemotherapy (81% versus 30%, p <0.001), longer median progression-free survival (5.0 versus 1.8 months, HR 0.45, p <0.001), and longer median OS (7.7 versus 6.7 months, HR: 0.77, p = 0.04). The findings of this study lead to the full FDA approval of INO for relapsed/refractory B-cell ALL in 2017.28
An ongoing phase II trial (NCT01371630) conducted at the MD Anderson Cancer Center has employed a novel regimen of modified, dose-reduced hyper-CVAD (termed “mini” hyper-CVD) with concurrent INO, followed by consolidation with blinatumomab in patients with relapsed/refractory Ph-negative B-cell ALL.29 The first 49 patients enrolled received induction chemotherapy consisting of 8 alternating cycles of dose-reduced cyclophosphamide, vincristine, and dexamethasone (with omission of doxorubicin) and dose-reduced methotrexate and cytarabine. This was followed by 36 months of maintenance therapy or HSCT. Intrathecal central nervous system prophylaxis, the anti-CD20 antibody rituximab (for patients with CD20 expression >20%), and INO were administered during the first four cycles of mini hyper-CVD. After treatment-emergent cases of veno-occlusive disease (VOD) were observed, the dose of INO was reduced from 1.8 mg/m2 in cycle 1 and 1.3 mg/m2 in cycles 2–4 to 1.3 mg/m2 in cycle 1 and 1.0 mg/m2 in cycles 2–4. After initial safety and efficacy was established with this regimen, the protocol was amended again to administer only 4 cycles of hyper-CVD alternating with methotrexate and cytarabine, with lower, fractionated dosing of INO to achieve a total dose of 0.9 mg/m2 during cycle 1 and 0.6 mg/m2 during cycles 2–4, followed by 4 cycles of blinatumomab consolidation. The duration of maintenance therapy was reduced to 18 months and consists of 3 cycles of POMP chemotherapy (6-mercaptopurine, vincristine, methotrexate, and prednisone) alternating with 1 cycle of blinatumomab for 16 total cycles. The purpose of these changes is to decrease treatment-related toxicity by using fewer cycles of chemotherapy and using lower and fractionated INO dosing. The incorporation of blinatumomab is intended to distance the INO from subsequent transplant with a goal of reducing VOD and hopefully increasing depth of response by integrating both of these active monoclonal antibody constructs into the same treatment regimen.
In the most recent update, 84 patients with relapsed/refractory Ph-negative B-cell ALL have been treated with mini hyper-CVD and INO ± blinatumomab.30 Twenty-three percent had previously undergone HSCT and 42% of patients were in second or greater salvage. To date, only 17 patients (20%) had received the amended regimen with lower, fractionated dosing of INO and incorporation of blinatumomab. ORR defined as CR/CRi or complete response without platelet recovery (CRp) was 80% (92% for first salvage, 56% for second salvage, 60% for third or higher salvage) and 80% of responders achieved MRD negativity. Forty percent of patients underwent subsequent HSCT. Nine (15%) patients treated with the original unfractionated INO dosing schedule developed VOD, compared to 0/17 treated with fractionated, dose-reduced INO. Median OS was 25 months, 6 months, and 7 months for first salvage, second salvage, and third or greater salvage, respectively. Response rates and long-term survival, particularly for patients in first salvage, appear to be substantially better than historical outcomes of patients with relapsed/refractory ALL.7,8,31–33 This study continues to accrue patients. More patients and longer follow-up will be needed to confirm the additional benefit of adding blinatumomab to the hyper-CVD plus INO regimen.
Overall, these data from blinatumomab combination studies compare favorably to blinatumomab monotherapy for relapsed/refractory Ph-negative B-cell ALL, particularly in first salvage, although will require further verification in larger studies. Additional ongoing clinical trials are investigating various regimens incorporating blinatumomab in relapsed/refractory Ph-negative B-cell ALL (NCT03518112, NCT02997761, NCT03160079).
Ph-Positive B-Cell ALL
Approximately one-quarter of patients with B-cell ALL harbor the Philadelphia chromosome, or t(9;22), which results in constitutive expression of the fusion tyrosine kinase BCR-ABL, driving the proliferation of malignant B-lymphocytes.34 The presence of this translocation has historically been associated with poorer prognosis in ALL, although the advent of BCR-ABL directed tyrosine kinase inhibitors (TKI) has improved prognosis significantly.35 However, for patients relapsing after standard frontline chemotherapy plus TKI therapy, the outcomes remain poor, which has opened the potential for use of blinatumomab in this setting.
The ALCANTARA study was the confirmatory phase II clinical trial that resulted in FDA approval of blinatumomab monotherapy for relapsed/refractory Ph-positive B-cell ALL in 2017.36 This was the first indication expansion for blinatumomab, and occurred in concert with its full approval for relapsed/refractory Ph-negative B-cell ALL. In this study, 45 patients who had failed at least one second or greater generation TKI received blinatumomab for two cycles. Eighty-two percent of patients had failed at least 2 TKIs previously (including 51% who had received prior ponatinib), 75% had >50% bone marrow blasts at treatment initiation, and 27% harbored the T315I mutation. The CR/CRh rate was 36%, and 31% of patients achieved CR. Nearly all patients who responded did so after cycle 1. Among the 16 responders, MRD negativity was achieved in 88%. In subgroup analyses, CR/CRh rate appeared independent of number of prior TKIs, previous HSCT, or presence of the T315I mutation. Median OS was 7.1 months and 44% of responders were able to be bridged to HSCT. Three patients experienced grade 3 or higher CNS events, and no patients experienced grade 3 or higher CRS. Of note, a recently published propensity score analysis in patients with relapsed/refractory Ph-positive ALL also suggests that blinatumomab is superior to standard chemotherapy when compared to historical controls, with improvement in both ORR and OS.37
Given persistent dependence on BCR-ABL signaling in relapsed/refractory Ph-positive ALL, the combination of blinatumomab with TKIs is postulated to further improve outcome further. A retrospective report demonstrated the successful use of blinatumomab and concurrent TKI in 13 patients with relapsed/refractory Ph-positive B-cell ALL (n=10) or chronic myeloid leukemia in lymphoid blast phase (n=3).38,39 Six patients had an overt hematologic relapse and 7 had MRD-only disease. Patients received up to 4 cycles of blinatumomab, and most patients (62%) received ponatinib as the concomitant TKI. The complete hematologic, cytogenetic, and molecular response rates were 57% (4/7), 75% (6/8), and 77% (10/13), respectively. After 1 year of follow-up, median OS was not reached. Other subsequent retrospective studies including a total of 33 patients treated with blinatumomab plus a TKI further support these findings.40,41 To prospectively confirm these findings, ongoing clinical trials are evaluating blinatumomab plus TKI combinations in patients with relapsed/refractory Ph-positive ALL, including blinatumomab + ponatinib (NCT03263572), and chemotherapy + blinatumomab + ponatinib (NCT03147612).
MRD-Positive B-Cell ALL
Up to 50% of adults with ALL have persistently detectable MRD after initial chemotherapy, which is highly predictive of eventual overt hematologic relapse.42–44 After the early clinical development of blinatumomab was hampered by significant treatment-emergent adverse effects thought to be related to high baseline tumor burden, it was hypothesized that blinatumomab may be efficacious with fewer instances of high-grade CRS in the setting of MRD-only disease. In a phase II study, 21 patients with MRD-positive ALL in CR received blinatumomab dosed at 15 mcg/m2/day for 4 weeks followed by a 2-week drug-free period.45 MRD was quantified by polymerase chain reaction (PCR) and an MRD load of >10−4 cells was required for trial entry. Eighty percent of evaluable patients achieved an MRD response (defined as no detectable MRD or MRD load <10−4 by PCR) after 1 cycle of blinatumomab. Response rates were high in patients with Ph-negative and Ph-positive disease, as well as in patients with high baseline MRD load defined as >10−2 cells (87%, 60%, 90%, respectively). Thirty-eight percent of patients underwent subsequent HSCT, and 1-year RFS was 78%. Four patients (19%) patients developed grade 3 or higher CNS adverse events and no patient developed CRS, consistent with the hypothesis that lower baseline disease burden would be associated with decreased blinatumomab-induced CRS. Conversely, baseline disease burden has not been shown to correlate with neurologic adverse events. In a long-term follow-up analysis of the entire study cohort, after a median of 33 months, RFS was 61%, which is better than would be historically expected in the setting of MRD-positive disease.46
The BLAST trial was conducted to build upon these promising but preliminary data.47 In this trial, 116 patients with persistent or recurrent MRD after initial chemotherapy (MRD defined as >10−3 by PCR) received blinatumomab 15 mcg/m2/day 4-weeks on, 2-weeks off for up to 4 cycles. MRD response was defined as no detectable MRD or MRD <10−4. Four percent of patients were Ph-positive, 36% of patients were in second or greater CR, and 47% had an MRD load >10−2 at the time of enrollment. Among evaluable patients, 78% achieved an MRD response, with nearly all responses (98%) occurring at the end of cycle 1; only 2 additional responses occurred after subsequent cycles. In subgroup analyses, MRD response did not vary with baseline MRD load or first versus later CR at enrollment. Fifteen patients (13%) experienced grade 3 or higher CNS adverse events and 2 (2%) experienced grade 3 or higher CRS. Seventy-six patients (66%) subsequently underwent HSCT, and median OS was 36.5 months. Not surprisingly, complete MRD responders had better outcomes than those who did not respond (median RFS: 23.6 versus 5.7 months, respectively, p=0.002; median OS: 38.9 versus 12.5 months, respectively, p=0.002). Thirty-six patients in the study cohort did not undergo HSCT or receive chemotherapy after completing the trial protocol, among whom 9 (25%) remained in continuous CR at a median follow up of 24 months, suggesting that long-term remissions may be possible for a subgroup of patients without the need for HSCT. In a post hoc analysis comparing OS in this non-transplanted group to those who underwent HSCT, no significant difference was observed (OR: 1.84, p=0.24). In a 5-year follow-up analysis of the study cohort median OS was not reached in patients who had a complete MRD response, with plateauing of survival curves suggesting possible cure.48 These data along with MRD response rates observed in clinical trials for overt hematologic disease established that blinatumomab is highly effective at eradicating MRD. Based on these results, blinatumomab gained FDA approval for the treatment of MRD-positive ALL in March 2018 (its most recent indication expansion).
One disadvantage of these prospective trials is that they contain a few patients who are Ph-positive. While it is generally assumed that blinatumomab should be effective in this setting, it is not definitely known whether blinatumomab should be combined with a TKI in this setting and also whether HSCT can be safely deferred for MRD responders. In a recent, small retrospective analysis of 9 patients with MRD-positive Ph-positive ALL, blinatumomab administered with TKIs (ponatinib; n=5, dasatinib; n=4, nilotinib; n=1, imatinib; n=1) resulted in MRD clearance in 8/9 (88%) patients, with no relapses among responders after a median follow up of 10.8 months.49
For patients who are MRD-positive after remission induction, the historical standard of care has been HSCT in first remission. While this approach is still often used, there is more uncertainty as to the necessity of HSCT in the era of blinatumomab.43 The 5-year analysis and post hoc analysis of the BLAST trial raise the question of whether or not all MRD-positive patients who “clear” MRD with blinatumomab require HSCT to achieve long-term survival or cure. The available data suggests that HSCT after MRD clearance with blinatumomab is associated with a lower rate of relapse but a higher rate of death in remission (due to transplant-related mortality), which may ultimately lead to similar long-term survival whether or not HSCT is performed in this setting. Thus, treatment decisions should be individualized for such patients, taking into account risk for transplant-related morbidity and mortality.
Newly Diagnosed B-Cell ALL
The current standard of care for the frontline treatment of B-cell acute lymphoblastic leukemia is multi-agent chemotherapy, with or without a BCR-ABL TKI depending on the presence or absence of t(9;22), or clinical trial. Most induction regimens contain a backbone of vincristine, corticosteroids, and an anthracycline, along with intrathecal prophylaxis. With cytotoxic chemotherapy, complete remission is achieved in >80% of adult patients and long-term survival in 30–50%.2,3 Given the success of blinatumomab in treating relapsed or refractory ALL, it has been proposed that addition of blinatumomab to chemotherapy in the frontline setting may further increase CR rates and improve long-term prognosis. The rationale is that early incorporation of active agents into frontline regimens (rather than reserving them for the relapsed/refractory setting when the disease is significantly less likely to be curable) will improve outcomes. Several ongoing clinical trials with preliminary results address the potential use of frontline blinatumomab.
Preliminary results from an ongoing phase II trial examining hyper-CVAD in sequential combination with blinatumomab for younger patients (<60 years old) with newly diagnosed Ph-negative B-cell ALL have recently been presented.50 Briefly, hyper-CVAD employs cycles of cyclophosphamide, vincristine, doxorubicin, and dexamethasone alternating with high-dose methotrexate and cytarabine and, historically, was given for 8 cycles, followed by 2–3 years of POMP maintenance.3 In this hyper-CVAD plus blinatumomab trial, the chemotherapy is decreased to four cycles of hyper-CVAD/methotrexate-cytarabine, followed by four cycles of blinatumomab. The duration of maintenance therapy has also been reduced to 18 months and consists of 3 cycles of POMP alternating with 1 cycle of blinatumomab for 15 total cycles. The goal of adding blinatumomab was to increase MRD response and reduce toxicity from chemotherapy. Twenty-seven patients (22 newly diagnosed and 5 in CR from first induction) have so far been treated. Median age was 38 years (range 18–59). Fifteen patients (56%) had high-risk features at baseline (e.g. Ph-like ALL, complex karyotype, t(4;11), near triploidy karyotype). All patients achieved CR. Twenty-six patients (96%) achieved MRD negativity by 6-color flow cytometry with a sensitivity of 0.01%. Eight patients (30%) have undergone HSCT. One-year RFS and OS were 76% and 89%, respectively. While still preliminary findings, these results compare favorably to current standard induction chemotherapy for newly diagnosed Ph-negative B-cell ALL. Longer follow-up and more patients will be needed to assess whether early incorporation of blinatumomab improves outcomes versus chemotherapy alone.
Another ongoing phase II trial examines mini hyper-CVD and INO ± blinatumomab (regimen described above) for older adults with newly diagnosed Ph-negative B-cell ALL.51 Adults aged >60 years with ALL have consistently poorer outcomes when compared to younger individuals, largely driven by poor tolerance of intensive chemotherapy regimens.4–6 Sixty-four patients aged >60 years have been treated. Median age was 68 years (range 60–81) and 27 (42%) were >70 years. Among evaluable patients, the ORR was 58/59 (98%) with 86% achieving CR. Fifty-nine (94%) patients achieved MRD negativity by 6-color flow cytometry. Twenty-one (33%) of patients died in CR/CRp, with a disproportionate rate of remission deaths in adults >70 years of age. Three-year OS was 54% and the 3-year continuous remission rate was 76%. Six patients (9%) developed VOD, of whom 3 died. These preliminary findings indicate a potential substantial improvement in the treatment of older adults with newly diagnosed ALL. A recently published propensity score analysis comparing this study cohort to historical controls who received traditional hyper-CVAD induction demonstrated the superiority of this novel regimen, with 3-year event-free survival rates of 64% and 34%, respectively (HR: 0.55, p = 0.02).52 Given the increased rate of death in remission in patients >70 years of age (most of which were due to infection), the investigators plan to amend the study in this >70 years of age population to further reduce the chemotherapy and rely more on less myelosuppressive agents such as INO and blinatumomab.
Novel regimens for the frontline treatment of Ph-positive B-cell are also being investigated. An ongoing phase II trial examines blinatumomab + dasatinib for adults with newly diagnosed Ph-positive B-cell ALL.53 In this trial, induction is with 85 days of single-agent dasatinib 140 mg followed by up to 5 cycles of blinatumomab. The study allowed adults of all ages to be enrolled, and the median age was 54.5 years (range: 24.1–81.7). At the end of dasatinib induction, 17 of 58 evaluable patients (29%) achieved complete molecular response or positive non-quantifiable disease. Of those who have completed the mandatory 2 cycles of blinatumomab 56% responded. The ORR increased to 80% in patients who have completed 4 cycles of blinatumomab. Eight patients underwent HSCT, and 1-year OS and DFS were 94% and 88%, respectively. This trial is unique in that no cytotoxic chemotherapy was used and patients of all ages were treated with this low-intensity regimen. It is likely that the lack of associated treatment-emergent adverse effects contributed to the encouraging survival outcomes reported.
Several ongoing clinical trials are evaluating blinatumomab for Ph-negative ALL in the frontline setting, including three blinatumomab + cytotoxic chemotherapy trials (NCT02003222, NCT02877303, NCT02143414), and one trial examining blinatumomab + INO without cytotoxic chemotherapy (NCT03739814). There are also several ongoing clinical trials evaluating blinatumomab for Ph-positive ALL in the frontline setting: including blinatumomab + dasatinib (NCT02143414), blinatumomab + ponatinib, and hyper-CVD + blinatumomab + ponatinib (NCT03263572, NCT03147612). The goal of all these studies is that with the addition of blinatumomab to TKI therapy, we can reduce (or eliminate) the reliance on intensive chemotherapy in Ph-positive ALL for all age groups.
Future Directions
Blinatumomab has improved the prognosis for many patients with relapsed/refractory B-cell ALL, has revolutionized the way we treat MRD, and is allowing for the development of more targeted, chemotherapy-sparing approaches in ALL. These achievements demonstrate the potential for major future treatment breakthroughs as our understanding of the basic immunobiology of leukemia improves. However, many unanswered questions remain regarding how to optimize the therapeutic impact of blinatumomab.
The place of blinatumomab in frontline ALL therapy remains a particularly important question, with multiple ongoing clinical trials as outlined above. How to best combine blinatumomab with cytotoxic chemotherapy and INO remains unknown. Another area of inquiry focuses on how much cytotoxic chemotherapy can be avoided if novel therapies are used in combination, without decreasing response rates or impairing long-term outcomes. The long duration of response observed in patients who achieve MRD negativity with blinatumomab suggests that not all patients with MRD require HSCT, which is revolutionizing our concept of ALL therapy. Additionally, lengthy disease-free survival has been observed in patients in first salvage who respond to blinatumomab, INO and cytotoxic chemotherapy, raising a similar question of the need for HSCT in the relapsed/refractory setting. How blinatumomab might be combined with chimeric antigen receptor (CAR) T cells is also a matter of debate, with some experts advocating for combination therapy and a head-to-head trial of blinatumomab versus CAR T cells ongoing (NCT03628053).
Blinatumomab resistance remains incompletely understood. Originally thought to be driven by an isolated molecular event involving disordered membrane trafficking of CD19,54 recent data suggest that the majority of patients who relapse after responding to blinatumomab do not lose CD19 expression.55 Some data suggest that resistance may be mediated by the alternative splicing of the CD19 locus resulting in truncated, non-immunogenic CD19.56 Non-response to blinatumomab has also been attributed to an exaggerated regulatory T cell (Treg) response seen in some patients, and more investigation is warranted into the cause of this Treg expansion and potential mitigating strategies.57 Several ongoing clinical trials are examining the ability of antibodies directed against programmed death receptor-1 to attenuate Treg expansion (NCT03160079, NCT03512405, NCT02879695).
The administration of blinatumomab is a cumbersome process for some patients in that a 24 hr continuous infusion is required, with a minimal delay during bag changes. This is particularly a challenge in resource-limited settings where outpatient blinatumomab administration is not feasible due to the required frequency of bag changes. Polymer-based depot formulation of a bispecific antibody has been reported previously,58 and a current Phase I trial is investigating the potential for intermittent subcutaneous administration of blinatumomab, albeit in lymphoma (NCT02961881). Future advancements in the drug product formulation of blinatumomab may produce an increased plasma half-life with less frequent dosing.
Conclusion
Blinatumomab represents a significant treatment advancement for patients with relapsed/refractory or MRD-positive B-cell ALL. Compared to other available therapies, it is particularly effective at eradicating MRD, which is a well-established endpoint that correlates with improved long-term survival. Emerging data indicate that combinations with blinatumomab, including with cytotoxic chemotherapy and/or INO, are highly effective for the frontline treatment of B-cell ALL in both older and younger adults, as well as for those with relapsed/refractory disease. The degree to which blinatumomab will permit a reduction in the use of cytotoxic chemotherapy when incorporated into traditional induction or salvage regimens is currently being investigated. Additional insight into the cellular and molecular events which contribute to blinatumomab treatment failure may further improve treatment outcomes for patients with ALL.
Disclosure
NJS reports consulting fees from Takeda Oncology and AstraZeneca, research funding from Takeda Oncology and Astellas Pharma Inc., and honoraria from Amgen. The authors report no other conflicts of interest in this work.
References
1. Dores GM, Devesa SS, Curtis RE, et al. Acute leukemia incidence and patient survival among children and adults in the United States, 2001–2007. Blood. 2012;119(1):34–43. doi:10.1182/blood-2011-04-347872
2. Pui CH, Evans WE. Treatment of acute lymphoblastic leukemia. N Engl J Med. 2006;354(2):166–178. doi:10.1056/NEJMra052603
3. Kantarjian HM, O’Brien S, Smith TL, et al. Results of treatment with hyper-CVAD, a dose-intensive regimen, in adult acute lymphocytic leukemia. J Clin Oncol. 2000;18(3):547–561. doi:10.1200/JCO.2000.18.3.547
4. Short NJ, Kantarjian H, Jabbour E, et al. Novel therapies for older adults with acute lymphoblastic leukemia. Curr Hematol Malig Rep. 2018;13(2):91–99. doi:10.1007/s11899-018-0440-3
5. Sive JI, Buck G, Fielding A, et al. Outcomes in older adults with acute lymphoblastic leukaemia (ALL): results from the international MRC UKALL XII/ECOG2993 trial. Br J Haematol. 2012;157(4):463–471.
6. O’Brien S, Thomas DA, Ravandi F, et al. Results of the hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone regimen in elderly patients with acute lymphocytic leukemia. Cancer. 2008;113(8):2097–2101. doi:10.1002/cncr.v113:8
7. Gökbuget N, Stanze D, Beck J, et al. Outcome of relapsed adult lymphoblastic leukemia depends on response to salvage chemotherapy, prognostic factors, and performance of stem cell transplantation. Blood. 2012;120(10):2032–2041. doi:10.1182/blood-2011-12-399287
8. Fielding AK, Richards SM, Chopra R, et al. Outcome of 609 adults after relapse of acute lymphoblastic leukemia (ALL); an MRC UKALL12/ECOG 2993 study. Blood. 2007;109(3):944–950. doi:10.1182/blood-2006-05-018192
9. Coccaro N, Anelli L, Zagariaet A, et al. Next-generation sequencing in acute lymphoblastic leukemia. Int J Mol Sci. 2019;20(12):2929. doi:10.3390/ijms20122929
10. Beldjord K, Chevret S, Asnafi V, et al. Oncogenetics and minimal residual disease are independent outcome predictors in adult patients with acute lymphoblastic leukemia. Blood. 2014;123(24):3739–3749. doi:10.1182/blood-2014-01-547695
11. Bassan R, Spinelli O, Oldani E, et al. Improved risk classification for risk-specific therapy based on the molecular study of minimal residual disease (MRD) in adult acute lymphoblastic leukemia (ALL). Blood. 2009;113(18):4153–4162. doi:10.1182/blood-2008-11-185132
12. Phelan KW, Avandi AS. Novel therapies in acute lymphoblastic leukemia. Curr Hematol Malig Rep. 2018;13(4):289–299. doi:10.1007/s11899-018-0457-7
13. Löffler A, Kufer P, Lutterbüse R, et al. A recombinant bispecific single-chain antibody, CD19× CD3, induces rapid and high lymphoma-directed cytotoxicity by unstimulated T lymphocytes. Blood. 2000;95(6):2098–2103. doi:10.1182/blood.V95.6.2098
14. Kong Y, Yoshida S, Saito Y, et al. CD34+CD38+CD19+ as well as CD34+CD38-CD19+ cells are leukemia-initiating cells with self-renewal capacity in human B-precursor ALL. Leukemia. 2008;22(6):1207–1213. doi:10.1038/leu.2008.83
15. Smith-Garvin JE, Koretzky GA, Jordan MS. T cell activation. Annu Rev Immunol. 2009;27:591–619. doi:10.1146/annurev.immunol.021908.132706
16. Haas C, Krinner E, Brischwein K, et al. Mode of cytotoxic action of T cell-engaging BiTE antibody MT110. Immunobiology. 2009;214(6):441–453. doi:10.1016/j.imbio.2008.11.014
17. Stein A, Franklin JL, Chia VM, et al. Benefit-risk assessment of blinatumomab in the treatment of relapsed/refractory B-cell precursor acute lymphoblastic leukemia. Drug Saf. 2019;42(5):587–601. doi:10.1007/s40264-018-0760-1
18. Dreier T, Lorenczewsk G, Brandl C, et al. Extremely potent, rapid and costimulation‐independent cytotoxic T‐cell response against lymphoma cells catalyzed by a single‐chain bispecific antibody. Int j Cancer. 2002;100(6):690–697. doi:10.1002/(ISSN)1097-0215
19. Zhu M, Kratzer A, Johnson J, et al. Blinatumomab pharmacodynamics and exposure–response relationships in relapsed/refractory acute lymphoblastic leukemia. J Clin Pharmacol. 2018;58(2):168–179. doi:10.1002/jcph.v58.2
20. Löffler A, Gruen M, Wuchter C, et al. Efficient elimination of chronic lymphocytic leukaemia B cells by autologous T cells with a bispecific anti-CD19/anti-CD3 single-chain antibody construct. Leukemia. 2003;17(5):900. doi:10.1038/sj.leu.2402890
21. Hoffmann P, Hofmeister R, Brischwein K, et al. Serial killing of tumor cells by cytotoxic T cells redirected with a CD19‐/CD3‐bispecific single‐chain antibody construct. Int j Cancer. 2005;115(1):98–104. doi:10.1002/(ISSN)1097-0215
22. Zhu M, Wu B, Brandl C, et al. Blinatumomab, a bispecific T-cell engager (BiTE®) for CD-19 targeted cancer immunotherapy: clinical pharmacology and its implications. Clin Pharmacokinet. 2016;55(10):1271–1288. doi:10.1007/s40262-016-0405-4
23. Topp MS, Gökbuget N, Zugmaier G, et al. Phase II trial of the anti-CD19 bispecific T cell-engager blinatumomab shows hematologic and molecular remissions in patients with relapsed or refractory B-precursor acute lymphoblastic leukemia. J Clin Oncol. 2014;32(36):4134–4140. doi:10.1200/JCO.2014.56.3247
24. Shimabukuro-Vornhagen A, Göde P, Subklewe M, et al. Cytokine release syndrome. J Immunother Cancer. 2018;6:56. doi:10.1186/s40425-018-0343-9
25. Zugmaier G, Gökbuget N, Klinger M, et al. Long-term survival and T-cell kinetics in relapsed/refractory ALL patients who achieved MRD response after blinatumomab treatment. Blood. 2015;126(24):2578–2584. doi:10.1182/blood-2015-06-649111
26. Topp MS, Gökbuget N, Stein AS, et al. Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: a multicentre, single-arm, Phase 2 study. Lancet Oncol. 2015;16(1):57–66. doi:10.1016/S1470-2045(14)71170-2
27. Kantarjian H, Stein A, Gökbuget N, et al. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N Engl J Med. 2017;376(9):836–847. doi:10.1056/NEJMoa1609783
28. Kantarjian H, DeAngelo D, Stelljes M, et al. Inotuzumab ozogamicin versus standard therapy for acute lymphoblastic leukemia. N Engl J Med. 2016;375(8):740–753. doi:10.1056/NEJMoa1509277
29. Jabbour E, Ravandi F, Kebriaei P, et al. Salvage chemoimmunotherapy with inotuzumab ozogamicin combined with mini-hyper-CVD for patients with relapsed or refractory philadelphia chromosome-negative acute lymphoblastic leukemia: a Phase 2 clinical trial. JAMA Oncol. 2018;4(2):230–234. doi:10.1001/jamaoncol.2017.2380
30. Sasaki K, Kantarjian H, Ravandi F, et al. Sequential combination of low-intensity chemotherapy (mini-hyper-CVD) plus inotuzumab ozogamicin with or without blinatumomab in patients with relapsed/refractory Philadelphia chromosome-negative acute lymphoblastic leukemia (ALL): a phase 2 trial. Blood. 2018;132(Suppl 1):553. doi:10.1182/blood-2018-99-115162
31. Sasaki K, Kantarjian H, Ravandi F, et al. Sequential combination of Inotuzumab Ozogamicin (InO) with low-intensity chemotherapy (mini-hyper-CVD) with or without blinatumomab is highly effective in Patients (pts) with philadelphia chromosome-negative Acute Lymphoblastic Leukemia (ALL) in first relapse. Blood. 2019;134(Suppl 1):3806.
32. Tavernier E, Boiron JM, Huguet F, et al. Outcome of treatment after first relapse in adults with acute lymphoblastic leukemia initially treated by the LALA-94 trial. Leukemia. 2007;21(9):1907–1914. doi:10.1038/sj.leu.2404824
33. Jabbour E, Sasaki K, Ravandi F, et al. Chemoimmunotherapy with inotuzumab ozogamicin combined with mini-hyper-CVD, with or without blinatumomab, is highly effective in patients with Philadelphia chromosome-negative acute lymphoblastic leukemia in first salvage. Cancer. 2018;124(20):4044–4055. doi:10.1002/cncr.31720
34. El Fakih R, Jabbour E, Ravandi F, et al. Current paradigms in the management of Philadelphia chromosome positive acute lymphoblastic leukemia in adults. Am J Hematol. 2018;93(2):286–295. doi:10.1002/ajh.24926
35. Ravandi F. How I treat Philadelphia chromosome-positive acute lymphoblastic leukemia. Blood. 2019;133(2):130–136. doi:10.1182/blood-2018-08-832105
36. Martinelli G, Boissel N, Chevallier P, et al. Complete hematologic and molecular response in adult patients with relapsed/refractory Philadelphia chromosome-positive b-precursor acute lymphoblastic leukemia following treatment with blinatumomab: results from a Phase II, single-arm, Multicenter study. J Clin Oncol. 2017;35(16):1795–1802. doi:10.1200/JCO.2016.69.3531
37. Rambaldi A, Ribera JM, Kantarjian H, et al. Blinatumomab compared with standard of care for the treatment of adult patients with relapsed/refractory Philadelphia chromosome-positive B-precursor acute lymphoblastic leukemia. Cancer. 2019;126(2):304–310. [Epub ahead of print].
38. Assi R, Kantarjian H, Short NJ, et al. Safety and efficacy of blinatumomab in combination with a tyrosine kinase inhibitor for the treatment of relapsed Philadelphia chromosome-positive leukemia. Clin Lymphoma Myeloma Leuk. 2017;17(12):897–901. doi:10.1016/j.clml.2017.08.101
39. Assi R, Kantarjian H, Short NJ, et al. Safety and efficacy of blinatumomab in combination with a tyrosine kinase inhibitor for the treatment of relapsed Philadelphia chromosome-positive leukemia. Blood. 2017;130(Suppl 1):2598.
40. Sokolov A, Parovichnikova E, Troitskaya V, et al. Targetable blinatumomab + tyrosine kinase inhibitors treatment in relapsed/refractory acute lymphoblastic leukemia patients: clinical effectiveness and peripheral lymphocytes subpopulations kinetics. Haematologica. 2017;102(S2):354–355.
41. Couturier MA, Thomas X, Raffoux E, et al. Blinatumomab + ponatinib for relapsed Ph1-positive acute lymphoblastic leukemia: the French experience. HemaSphere. 2019;3:426–427. doi:10.1097/01.HS9.0000562056.77454.d3
42. Raff T, Gokbuget N, Luschen S, et al. Molecular relapse in adult standard-risk ALL patients detected by prospective MRD monitoring during and after maintenance treatment: data from the GMALL 06/99 and 07/03 trials. Blood. 2007;109(3):910–915. doi:10.1182/blood-2006-07-037093
43. Berry DA, Zhou S, Higley H, et al. Association of minimal residual disease with clinical outcome in pediatric and adult acute lymphoblastic leukemia: a meta-analysis. JAMA Oncol. 2017;3(7):e170580. doi:10.1001/jamaoncol.2017.0580
44. Short NJ, Jabbour E, Albitar M, et al. Recommendations for the assessment and management of measurable residual disease in adults with acute lymphoblastic leukemia: a consensus of North American experts. Am J Hematol. 2019;94(2):257–265. doi:10.1002/ajh.v94.2
45. Topp MS, Kufer P, Gökbuget N, et al. Targeted therapy with the T-cell-engaging antibody blinatumomab of chemotherapy-refractory minimal residual disease in B-lineage acute lymphoblastic leukemia patients results in high response rate and prolonged leukemia-free survival. J Clin Oncol. 2011;29(18):2493–2498. doi:10.1200/JCO.2010.32.7270
46. Topp MS, Gökbuget N, Zugmaier G, et al. Long-term follow-up of hematologic relapse-free survival in a phase 2 study of blinatumomab in patients with MRD in B-lineage ALL. Blood. 2012;120(26):5185–5187. doi:10.1182/blood-2012-07-441030
47. Gökbuget N, Dombret H, Bonifacio M, et al. Blinatumomab for minimal residual disease in adults with B-cell precursor acute lymphoblastic leukemia. Blood. 2018;131(14):1522–1531. doi:10.1182/blood-2017-08-798322
48. Gökbuget N, Dombret H, Zugmaier G, et al. Blinatumomab for minimal residual disease in adults with B-cell precursor acute lymphoblastic leukemia (BCP-ALL): median overall survival (OS) is not reached in complete MRD responders at a median follow up of 53.1 months. Blood. 2018;132(Suppl 1):554. doi:10.1182/blood-2018-99-111516
49. King AC, Pappacena JJ, Tallman MS, et al. Blinatumomab administered concurrently with oral tyrosine kinase inhibitor therapy is a well-tolerated consolidation strategy and eradicates measurable residual disease in adults with Philadelphia chromosome positive acute lymphoblastic leukemia. Leuk Res. 2019;79:27–33. doi:10.1016/j.leukres.2019.02.009
50. Richard-Carpentier G, Kantarjian H, Short NJ, et al. Updated results from the Phase II study of hyper-CVAD in sequential combination with blinatumomab in newly diagnosed adults with B-Cell Acute Lymphoblastic Leukemia (B-ALL). Blood. 2019;134(Suppl 1):3807. doi:10.1182/blood-2019-129657
51. Kantarjian H, Ravandi F, Short NJ, et al. Inotuzumab ozogamicin in combination with low-intensity chemotherapy for older patients with Philadelphia chromosome-negative acute lymphoblastic leukaemia: a single-arm, phase 2 study. Lancet Oncol. 2018;19(2):240–248. doi:10.1016/S1470-2045(18)30011-1
52. Jabbour EJ, Sasaki K, Ravandi F, et al. Inotuzumab ozogamicin in combination with low-intensity chemotherapy (mini-HCVD) with or without blinatumomab versus standard intensive chemotherapy (HCVAD) as frontline therapy for older patients with Philadelphia chromosome-negative acute lymphoblastic leukemia: a propensity score analysis. Cancer. 2019;125(15):2579–2586. doi:10.1002/cncr.32139
53. Chiaretti S, Bassan R, Vitale A, et al. Dasatinib-blinatumomab combination for the front-line treatment of adult Ph+ ALL patients. updated results of the gimema LAL2116 D-alba trial. Blood. 2019;134(Suppl 1):740.
54. Braig F, Brandt A, Goebeler M, et al. Resistance to anti-CD19/CD3 BiTE in acute lymphoblastic leukemia may be mediated by disrupted CD19 membrane trafficking. Blood. 2017;129(1):100–104. doi:10.1182/blood-2016-05-718395
55. Jabbour E, Düll J, Yilmaz M, et al. Outcome of patients with relapsed/refractory acute lymphoblastic leukemia after blinatumomab failure: no change in the level of CD19 expression. Am J Hematol. 2018;93(3):371–374. doi:10.1002/ajh.v93.3
56. Sotillo E, Barrett DM, Black KL, et al. Convergence of acquired mutations and alternative splicing of CD19 enables resistance to CART-19 immunotherapy. Cancer Discov. 2015;5(12):1282–1295. doi:10.1158/2159-8290.CD-15-1020
57. Duell J, Dittrich M, Bedke T, et al. Frequency of regulatory T cells determines the outcome of the T-cell-engaging antibody blinatumomab in patients with B-precursor ALL. Leukemia. 2017;31(10):2181. doi:10.1038/leu.2017.41
58. Leconet W, Liu H, Gu M, et al. Anti-PSMA/CD3 bispecific antibody delivery and antitumor activity using a polymeric depot formulation. Mol Cancer Ther. 2018;17(9):1927–1940. doi:10.1158/1535-7163.MCT-17-1138
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.