Back to Journals » International Journal of Women's Health » Volume 12
Bleeding Pattern and Management of Unexpected Bleeding/Spotting with an Extended Regimen of a Combination of Ethinylestradiol 20 mcg and Drospirenone 3 mg
Authors Bonassi Machado R, Pompei LM , Andrade R , Nahas E, Guazzelli C , Wender MC , Cruz AM
Received 12 November 2019
Accepted for publication 7 March 2020
Published 30 March 2020 Volume 2020:12 Pages 235—242
DOI https://doi.org/10.2147/IJWH.S238294
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Everett Magann
Rogerio Bonassi Machado,1 Luciano de Melo Pompei,2 Rosires Andrade,3 Eliana Nahas,4 Cristina Guazzelli,5 Maria Celeste Wender,6 Achilles Machado Cruz7
1Department of Gynecology and Obstetrics, Jundiaí School of Medicine, Jundiaí, São Paulo 13202-550, Brazil; 2Department of Gynecology and Obstetrics, ABC School of Medicine, Santo André, São Paulo 09060-650, Brazil; 3Department of Gynecology and Obstetrics, Federal University of Parana, Curitiba, Paraná 80060-240, Brazil; 4Department of Gynecology and Obstetrics, Botucatu Medical School (Unesp), Botucatu, São Paulo 18618970, Brazil; 5Department of Obstetrics, Federal University of Sao Paulo, São Paulo 04024-002, Brazil; 6Department of Gynecology and Obstetrics, Federal University of Rio Grande do Sul, Porto Alegre, Rio Grande do Sul 90035-903, Brazil; 7Department of Clinical Research, Libbs Farmacêutica Ltda, São Paulo 01140-050, Brazil
Correspondence: Rogerio Bonassi Machado
Departamento de Ginecologia e Obstetrícia, Faculdade de Medicina de Jundiaí, R. Francisco Telles, 250, Jundiaí, São Paulo 12202-550, Brazil
Tel/Fax +55 11 4586 0660
Email [email protected]
Objective: To compare the bleeding pattern in women using ethinylestradiol 20 mcg/drospirenone 3 mg (EE 20 mcg/DRSP 3 mg) in a 24/4-day cyclic regimen with an extended regimen. Unexpected bleeding/spotting in the extended regimen group was managed by allowing a 4-day hormone-free interval (HFI).
Methods: This was a randomized, prospective, open-label, multicenter study. Participants (N = 348) were randomized to receive EE 20 mcg/DRSP 3 mg in either an extended regimen (EE/DRSPes group) or a 24/4-day cyclic regimen (EE/DRSP24/4 group) and followed for 168 days. In the EE/DRSPes group, a 4-day HFI was allowed whenever unexpected bleeding/spotting persisted for ≥ 7 consecutive days. The participants assessed their bleeding daily as “no bleeding,” “spotting,” or “light,” “moderate,” or “heavy” bleeding according to a predefined scale.
Results: EE/DRSPes group experienced fewer days of bleeding than those using a 24/4 cyclic regimen (P < 0.001). After 168 days, 57.5% of women in the EE/DRSPes group achieved complete amenorrhea (i.e., neither bleeding nor spotting) and 73.9% achieved “no bleeding” (i.e., no bleeding with or without spotting) during the final 28-day interval of the study period. Women in the extended group who instituted the 4-day HFI experienced a 94.1% rate of successful management of unexpected bleeding/spotting.
Conclusion: The use of EE 20 mcg/DRSP 3 mg in an extended regimen resulted in high rates of amenorrhea and “no bleeding”. Unexpected bleeding/spotting in the EE/DRSPes group could be managed effectively with a 4-day HFI.
Clinical Trial Registration: International Standard Randomised Controlled Trial Number (ISRCTN57661673): http://www.controlled-trials.com/isrctn/pf/57661673.
Keywords: bleeding profile, combined oral contraceptives, drospirenone, extended regimen, low dose oral contraceptive
Introduction
Monthly contraceptive interval has been questioned in recent years because the artificial bleeding resulting from hormonal withdrawal can be associated with menstrual-related symptoms.1 Some women wish to avoid monthly bleeding not only because of the symptoms experienced during this period but also for personal reasons such as convenience and practicality.2 In response, alternative regimens of birth control pills, such as extended or even continuous administration of active pills, are being used in order to avoid occasional problems related to the hormone-free interval (HFI).3 Extended regimens for oral contraception refer to the use of active pills for more than 28 days, without an HFI, including the continuous use of oral contraceptives and variations such as inclusion of an HFI every 3 months.4
The occurrence of unexpected bleeding/spotting in users of extended or continuous regimens is one of the most common problems associated with this administration scheme. Although the ideal outcome of these regimens is amenorrhea, the experience both in clinical practice and in trials is that the bleeding pattern can be erratic, especially during the first 6 months of use, but tends to improve as the duration of treatment increases.5,6 The extended use of different hormonal combinations produces mean rates of amenorrhea of 60% to 80% after 6 months of uninterrupted use.3,7-10
Management of any unexpected bleeding/spotting that occurs is greatly important for the patient’s adherence to treatment, and higher rates of amenorrhea are associated with higher rates of satisfaction.3 Paying special attention to the possibility of bleeding, particularly during the first months of the extended regimen, in the initial consultation is quite effective at minimizing premature discontinuation of the treatment. However, a therapeutic approach can be used in situations in which the bleeding persists.
The present study aimed to compare the bleeding pattern during a 168-day course of the combination of ethinylestradiol 20 mcg and drospirenone 3 mg (EE 20 mcg/DRSP 3 mg) in an extended regimen with that during a 24/4 cyclic regimen of the same formulation and to examine the effectiveness of an HFI for the management of unexpected bleeding/spotting in women using the extended regimen.
Methods
This was a randomized, open-label, multicenter study constituting part of the main study with the primary objective of evaluating menstrual-related symptoms in women using a contraceptive containing ethinylestradiol 20 mcg and drospirenone 3 mg in an extended flexible regimen or a 24/4 cyclic regimen (24/4).11
The 11 Brazilian sites (Jundiaí School of Medicine) (RB Machado), ABC School of Medicine (LM Pompei), Federal University of Parana (R Andrade), Botucatu Medical School (Unesp) (E Nahas), Federal University of Sao Paulo (C Guazzelli), Federal University of Rio Grande do Sul (MC Wender), Centro de Pesquisa e Assistência em Reprodução Humana (CEPARH) (H Maia Filho), University of Sao Paulo – Ribeirao Preto (RA Ferriani), PUC Rio Grande do Sul (M Badalotti), Centro Paulista de Investigaçao Clinica (S Del Debio), Endocrinology Unit Federal University of Sao Paulo (M Lazaretti Castro) involved in the study obtained approval from their respective Research Ethics Committees. All women volunteered to participate in the study, after detailed information was provided and the informed consent form was signed, according to the Declaration of Helsinki.
The participants were women aged 18 to 39 years whose clinical histories included at least 3 consecutive regular menstrual cycles, defined as cycles at intervals between 25 and 35 days with bleeding for 3 to 7 days, immediately prior to the study. Women using OCP that did not contain drospirenone according to a regimen of 21 active pills followed by a 7-day HFI were also included so long as they experienced withdrawal bleeding corresponding to the HFI.
The exclusion criteria were the use of OCP containing drospirenone, any OCP regimen other than 21/7, or the use of progestogen-only pills, a levonorgestrel intrauterine system within 3 months before the start of the study, or medroxyprogesterone acetate administered as an intramuscular depot within 6 months before the start of the study or any condition in category 2, 3, or 4 of the Medical Eligibility Criteria for Contraceptive Use of the World Health Organization (WHO).12
Participants underwent a screening period corresponding to a full menstrual cycle (for non-users of OCP) or a 28-day treatment cycle (for OCP users). During this time, they used a diary to record their menstrual symptoms using the “Daily Symptoms Scale,” a version of the Penn Daily Symptom Report (DSR)13 (to fulfill the primary objective of this study protocol) and to complete the “Bleeding Pattern Registry.”
Treatment regimen assigned to each patient was determined according to a computer-generated central randomization list. The participants were then distributed among 2 groups using an OCP with a daily dose of ethinylestradiol 20 mcg in combination with drospirenone 3 mg: the extended regimen (also called extended scheduled regimen, EE/DRSPes) group, in which the participants used the combination for 168 consecutive days with the option of a 4-day HFI for management of persistent unexpected bleeding/spotting, defined in this protocol as presence of unexpected bleeding/spotting for 7 or more consecutive days, and the cyclic regimen (EE/DRSP24/4) group, in which the participants used active pills for 24 days with a 4-day HFI between the packs. The evaluation period was 168 consecutive days for both groups, corresponding to daily use of the combination for 168 consecutive days by the women in the EE/DRSPes group and six cycles of 28 days for the EE/DRSP24/4 group.
The bleeding pattern was evaluated from the notes in the participants’ diary according to the criteria suggested by the WHO.14 Bleeding was classified as the absence of bleeding or spotting, bleeding (vaginal bleeding requiring sanitary protection), and spotting (vaginal bleeding not requiring sanitary protection). The intensity of bleeding was graded on a scale of 0 to 4, where 0 was defined as the absence of bleeding or spotting, 1 as spotting, 2 as light bleeding (less than usual menstruation), 3 as moderate bleeding (same as usual menstruation), and 4 as heavy bleeding (heavier than usual menstruation).
Participants randomized to EE/DRSPes group who exhibited persistent unexpected bleeding/spotting were allowed a 4-day HFI provided that they had been taking the medication continuously for at least 28 days since the beginning of the study. The HFI for treatment of persistent unexpected bleeding/spotting was optional and was implemented when requested by the patient due to the inconvenience caused by the bleeding/spotting. Effectiveness of the HFI was defined as resolution of the bleeding/spotting, i.e., cessation of vaginal bleeding for at least 14 days after the initiation of the 4-day HFI.
The sample size was calculated from the primary efficacy parameter (mean percent decrease in the total symptom score assessed by the Penn Daily Symptom Report), which is the primary objective of the published study.11 Considering an expected improvement of 50% from baseline, a power of 80%, a significance level of 5%, and a non-inferiority margin of 15%, 276 patients were supposed to complete the study, assuming a standard deviation of 50%. Considering a 25% dropout rate, at least 368 women were supposed to be enrolled in the study. We would like to stress that, regarding sample size, if the study was outlined primarily for the analysis of the bleeding pattern, a smaller number of participants could be enrolled. Thus, when considering the 80% and 1.6% amenorrhea rates for extended use and cyclic use, respectively, as well as a dropout rate of 35–40%, a statistical power of 80%, and a significance level of 5%, 24 patients would be sufficient.
The Mann–Whitney test was used to compare the numbers of days with/without bleeding/spotting during the total treatment period and the reference periods between the groups. The bleeding pattern was analyzed for each 28-day treatment interval in each study group and compared using the chi-square test. The chi-square test was also used to compare the rates of amenorrhea (absence of bleeding or spotting) and absence of bleeding (with or without spotting) throughout the treatment between the groups. The analyses were performed using SAS® software, version 9.3, and the significance level was 0.05 (α = 5%) for every analysis performed.
Results
Flow through the study is presented in Figure 1 and the characteristics of the study participants are listed in Table 1.
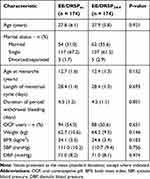 |
Table 1 Summary of Demographic and Baseline Characteristics of Participants Who Were Randomly Assigned (n = 348) |
|
Figure 1 Flow of participants through the trial. EE/DRSPes = ethinylestradiol 20 mcg + drospirenone 3 mg in an extended regimen. EE/DRSP24/4 = ethinylestradiol 20 mcg + drospirenone 3 mg in a 24/4 regimen. |
Among the analyzed variables describing the bleeding pattern, the number of days without bleeding (light, moderate, or heavy) throughout the entire evaluation period was significantly smaller in the EE/DRSPes group than in the EE/DRSP24/4 group. On the other hand, the number of days of spotting was similar between the groups (Figure 2). Women in the EE/DRSPes group had more days without bleeding or spotting and fewer days of bleeding or spotting during the entire treatment period (Table 2). Figure 3 shows the number of days of bleeding or spotting in both reference periods analyzed. The number of days of bleeding or spotting in reference period 1 was significantly lower for the EE/DRSPes regimen than for the cyclic regimen (P < 0.001). The result was similar for reference period 2. The percentage of women who did not experience any sort of bleeding, including spotting, for each 28-day treatment interval throughout the entire study period is shown in Figure 4. The rate of amenorrhea (no bleeding or spotting) associated with the extended flexible regimen varied by the treatment interval between 46.5% and 59.5%. This rate was lower among users of the 24/4 regimen, in whom it varied between 2.6% and 6.0%. The percentage of women without bleeding or spotting (i.e., with amenorrhea) was significantly larger in the EE/DRSPes group than in the EE/DRSP24/4 group for all 6 28-day treatment intervals (P < 0.001 for every interval). The same pattern was seen for the rate of bleeding with or without spotting (Figure 5). The optional 4-day HFI for management of persistent unexpected bleeding/spotting in women in the EE/DRSPes group was implemented by 34 women: 23 used it during 1 interval, 9 during 2 intervals, 1 during 3 intervals, and 1 during 4 treatment intervals. The percentage of women who are elected to use the HFI to manage persistent unexpected bleeding/spotting and the percentage in whom this measure is resolved the bleeding are shown in Figure 6.
 |
Table 2 Median Number of Days During the Entire Treatment Period (ITT, n = 309) Without Bleeding/Spotting, with Bleeding/Spotting, and with Bleeding of Each Intensity Level |
|
Figure 2 Number of days in the total treatment period (ITT, n = 309) with spotting only or bleeding only. |
|
Figure 3 Numbers of days with bleeding or spotting, spotting only, and bleeding only in the 2 90-day reference periods in both groups. (A) Reference Period 1 (RP 1); (B) Reference Period 2 (RP 2). |
|
Figure 4 Percentage of women exhibiting amenorrhea (neither spotting nor bleeding) during each of the 6 28-day treatment intervals. Between-groups difference, P < 0.001. |
|
Figure 5 Percentage of women without bleeding (with or without spotting) during each of the 6 28-day treatment intervals. Between-groups difference, P < 0.001. |
|
Figure 6 Percentage of women in the EE/DRSPes group who implemented the 4-day HFI for management unexpected bleeding/spotting and the rate of effectiveness thereof during each of the 6 28-day treatment intervals. |
It is noteworthy that the HFI was effective in every case in which it was used during treatment intervals 1, 2, 3, and 6, and in 83.3% and 81.8% of cases in intervals 4 and 5, respectively.
Discussion
Findings and Interpretation
In the present study, we have evaluated an OCP containing a lower dose of estrogen (20 mcg ethinylestradiol) in combination with drospirenone. The use of this formulation in an extended regimen significantly reduced the number of days of bleeding during a 6-month (168-day) period relative to the use of a cyclic regimen of the same combination.
Differences and Similarities in Relation to Other Studies
The results shown here agree in part with the findings of Jensen15 and Klipping,16 who have also evaluated the same contraceptive composition. While the cyclic group (24/4 regimen) in our study experienced a median of 15 days of bleeding, both prior studies showed mean values of 33 and 43 days during 1 and 2 years, respectively.15,16 Although the different study lengths complicate the comparison, the mean frequency of total days of bleeding during the extended regimen was in fact higher in these 2 publications than in our present report. Notably, our present study assessed the use of the extended regimen for 168 consecutive days without a programmed pause but allowed the user to elect a 4-day HFI to manage persistent unexpected bleeding/spotting; we have termed this modified treatment schedule the extended flexible regimen. However, the studies by Jensen et al15 and Klipping et al16 allowed users of their extended regimens to include a 4-day HFI in case of bleeding for 3 consecutive days after the first 24 days of use and a scheduled 4-day HFI after the 120th day, making their regimens flexible extended regimens of 124 days with management of intracyclic bleeding (flexible MIB regimen). These studies also included extended use in which the 4-day HFI was allowed regardless of bleeding, with a programmed pause after the 120th day, defined as a flexible extended regimen with active period control (flexible APC regimen) and a fixed extended regimen in which the 4-day HFI was allowed only after every 120 days of use. The mandatory contraceptive pause every 4 months and the greater likelihood that the user would experience an HFI during the flexible MIB and APC regimens may explain the greater mean frequencies of bleeding days relative to the study length than observed in our study. The extended regimen that we evaluated also allows flexibility, particularly regarding the possibility of an HFI, but does not include a mandatory HFI after 120 days of use.
As seen by other authors,15,16 the extended regimen decreased the rate of bleeding/spotting relative to that observed under the 24/4 cyclic regimen, resulting in significantly more days free of bleeding/spotting and fewer days of bleeding in reference periods 1 and 2.
The extended regimen produced significantly higher rates of absence of bleeding and/or spotting and absence of bleeding (with or without spotting) than did the cyclic regimen. During the 6th treatment interval, the rate of absence of bleeding/spotting (amenorrhea) was 57.6% and the rate of absence of bleeding (with or without spotting) was 73.9%. These findings were compatible with the usual rates of amenorrhea reported among users of continuous or extended regimens of OCPs. The rate of absence of bleeding (with or without spotting) after the 6th month of uninterrupted use of the combination of ethinylestradiol 30 mcg/gestodene 75 mcg is 81%, almost the same as that observed with continuous use of ethinylestradiol 20 mcg/levonorgestrel 90 mcg, whereas the combination of EE 30 mcg/DRSP 3 mg produces a rate of absence of bleeding (with or without spotting) after the 6th month of around 78%.8–10
Users of extended or continuous regimens of OCPs are more susceptible to treatment interruption due to unpredictable bleeding than are women who use contraceptives in the conventional cyclic regimen.17 For this reason, appropriate pre-treatment counseling, and instruction in proper management of bleeding during the use of oral contraceptives according to non-standard schedules of administration are fundamental to improving the success, adherence to, and continuity of the use of such contraceptive regimens. In this study, we evaluated the effectiveness of an HFI in cases of persistent unexpected bleeding or spotting. Unlike women in previous studies,15,16 the participants in our study were allowed to choose whether to implement an HFI to manage unexpected bleeding or spotting. We observed a small number of women who instituted the 4-day HFI as a consequence of persistent unexpected bleeding/spotting. This finding suggests that persistent unexpected bleeding/spotting during extended use was uncommon; we can also assume that each woman’s individual interpretation of unexpected bleeding/spotting was different, making it difficult to establish quantitatively the best interval for a scheduled HFI. Furthermore, these findings lead us to question the advantages of flexible regimens with a predetermined HFI (for example, after 120 days) because although this interval model is associated with lower rates of unpredictable bleeding, the withdrawal bleeding produced may not be necessary for motivated women who have been counseled regarding bleeding during extended use of OCPs.
For the management of persistent unexpected bleeding/spotting, we used the HFI described by Sulak et al18 in a study involving the extended use of a combination of ethinylestradiol and drospirenone. In the present study, we observed that the 4-day HFI resolved persistent unexpected bleeding/spotting in 94% of the cases in which it was implemented over the 168 days of the extended use regimen, proving the effectiveness of this simple measure for control of unexpected bleeding/spotting.
Strengths and Weaknesses of the Study
We consider the strong points of this study to be the large sample population and the choice of an extended regimen allowing an optional HFI (also called extended scheduled regimen) at the woman’s discretion, making the clinical evaluation closer to a “real-life” scenario. The main limitation of our study is the follow-up time, which is shorter than in other studies that assessed the use of the same formulation according to an extended regimen.15,16
Conclusion
In conclusion, the bleeding pattern in women using an extended regimen of ethinylestradiol 20 mcg and drospirenone 3 mg showed no bleeding rates comparable to other contraceptive formulations, including those with higher doses of the estrogen component. Elective use of a 4-day HFI was an efficient way to manage unexpected bleeding/spotting, allowing control of the unpredictable bleeding associated with the use of an extended flexible regimen.
Data Sharing Statement
All data from the current study, including individual data from unidentified patients, are filed by the author and may be made available at any time, also highlighting that all data from the full clinical report are available in Portuguese.
Disclosure
Libbs Farmacêutica Ltda (Brazil) provided funding and material support for this research (protocol number LB0901). Dr. Achilles Machado Cruz is a medical consultant with Libbs Farmacêutica Ltda and reports study funding from Libbs Farmacêutica during the conduct of the study. Rogerio Bonassi Machado reports grants from Libbs Farmaceutica Ltda, Bayer, Exeltis, Grunenthal, MSD and Pfizer outside the submitted work. Luciano Melo Pompei reports grants from Libbs Farmacêutica, MSD Brasil, Grünenthal do Brasil, and Bayer outside the submitted work. The other authors have no other potential conflicts of interest to disclose.
References
1. Nelson AL. Extended-cycle oral contraception: a new option for routine use. Treat Endocrinol. 2005;4(3):139–145. doi:10.2165/00024677-200504030-00002
2. den Tonkelaar I, Oddens BJ. Preferred frequency and characteristics of menstrual bleeding in relation to reproductive status, oral contraceptive use, and hormone replacement therapy use. Contraception. 1999;59(6):357–362. doi:10.1016/S0010-7824(99)00043-8
3. Anderson FD, Hait H. A multicenter, randomized study of an extended cycle oral contraceptive. Contraception. 2003;68(2):89–96. doi:10.1016/S0010-7824(03)00141-0
4. Edelman A, Gallo MF, Jensen JT, Nichols MD, Grimes DA. Continuous or extended cycle vs. cyclic use of combined hormonal contraceptives for contraception. Cochrane Database Syst Rev. 2011;8:CD004695.
5. Seidman DS, Yeshaya A, Ber A, et al. A prospective follow-up of two 21/7 cycles followed by two extended regimen 84/7 cycles with contraceptive pills containing ethinyl estradiol and drospirenone. Isr Med Assoc J. 2010;12(7):400–405.
6. Foidart JM, Sulak PJ, Schellschmidt I, Zimmermann D; Yasmin Extended Regimen Study Group. The use of an oral contraceptive containing ethinylestradiol and drospirenone in an extended regimen over 126 days. Contraception. 2006;73(1):34–40. doi:10.1016/j.contraception.2005.06.068
7. Kwiecien M, Edelman A, Nichols MD, Jensen JT. Bleeding patterns and patient acceptability of standard or continuous dosing regimens of a low-dose oral contraceptive: a randomized trial. Contraception. 2003;67(1):9–13. doi:10.1016/S0010-7824(02)00445-6
8. Archer DF, Jensen JT, Johnson JV, et al. Evaluation of a continuous regimen of levonorgestrel/ethinyl estradiol: phase 3 study results. Contraception. 2006;74(6):439–445. doi:10.1016/j.contraception.2006.07.005
9. Machado RB, Fabrini P, Cruz AM, Maia E, da Cunha Bastos A. Clinical and metabolic aspects of the continuous use of a contraceptive association of ethinyl estradiol (30 microg) and gestodene (75 microg). Contraception. 2004;70(5):365–370. doi:10.1016/j.contraception.2004.06.001
10. Machado RB, de Melo NR, Maia H
11. Machado RB, Pompei LM, Badalotti M, et al. Effects of an extended flexible regimen of an oral contraceptive pill containing 20 μg ethinylestradiol and 3 mg drospirenone on menstrual-related symptoms: a randomised controlled trial. Eur J Contracept Reprod Health Care. 2017;22(1):11–16. doi:10.1080/13625187.2016.1239077
12. World Health Organization. Medical Eligibility Criteria for Contraceptive Use [Internet].
13. Freeman E, DeRubeis RJ, Rickelsa K. Reliability and validity of a daily diary for premenstrual syndrome. Psyc Res. 1996;65(2):97–106. doi:10.1016/S0165-1781(96)02929-0
14. Belsey EM, Machin D, d’Arcangues C. The analysis of vaginal bleeding patterns induced by fertility regulating methods. World Health Organization Special Programme of Research, Development and Research Training in Human Reproduction. Contraception. 1986;34(3):253–260. doi:10.1016/0010-7824(86)90006-5
15. Jensen JT, Garie SG, Trummer D, Elliesen J. Bleeding profile of a flexible extended regimen of ethinylestradiol/drospirenone in US women: an open-label, three-arm, active-controlled, multicenter study. Contraception. 2012;86(2):110–118. doi:10.1016/j.contraception.2011.12.009
16. Klipping C, Duijkers I, Fortier MP, Marr J, Trummer D, Elliesen J. Contraceptive efficacy and tolerability of ethinylestradiol 20 μg/drospirenone 3 mg in a flexible extended regimen: an open-label, multicentre, randomised, controlled study. J Fam Plann Reprod Health Care. 2012;38(2):73–83. doi:10.1136/jfprhc-2011-100213
17. Hitchcock CL, Prior JC. Evidence about extending the duration of oral contraceptive use to suppress menstruation. Womens Health Issues. 2004;14(6):201–211. doi:10.1016/j.whi.2004.08.005
18. Sulak PJ, Kuehl TJ, Coffee A, Willis S. Prospective analysis of occurrence and management of breakthrough bleeding during an extended oral contraceptive regimen. Am J Obstet Gynecol. 2006;195(4):935–941. doi:10.1016/j.ajog.2006.02.048
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
