Back to Journals » OncoTargets and Therapy » Volume 10
Biosimilar Retacrit® (epoetin zeta) in the treatment of chemotherapy-induced symptomatic anemia in hematology and oncology in Germany (ORHEO) – non-interventional study
Authors Losem C, Koenigsmann M, Rudolph C
Received 15 September 2016
Accepted for publication 21 December 2016
Published 28 February 2017 Volume 2017:10 Pages 1295—1305
DOI https://doi.org/10.2147/OTT.S122427
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Carlos E Vigil
Christoph Losem,1 Michael Koenigsmann,2 Christine Rudolph3
1Praxis für Hämatologie und Onkologie, Neuss, 2Onkologisches Ambulanzzentrum, Marienstr, Hannover, 3Hospira Germany, A Pfizer Company, München, Germany
Background: Symptomatic anemia is a frequent and severe complication of chemotherapy that is commonly treated with erythropoiesis-stimulating agents. The primary objective of this study was to assess the change in hemoglobin levels in patients with chemotherapy-induced anemia (CIA) following treatment with biosimilar Retacrit® (epoetin zeta). Secondary objectives included changes in hematologic parameters and tolerability.
Methods: This was a non-interventional, multicenter, long-term observational study that is part of an ongoing surveillance program for epoetin zeta. Adult patients (N=291) with solid tumors, malignant lymphomas or multiple myeloma, and chemotherapy-induced symptomatic anemia, who were eligible for treatment with biosimilar epoetin zeta, were enrolled. Patients were evaluated at enrollment, 3 months, and 6 months.
Results: Evaluable patients had lymphoma or myeloma (n=30) or solid tumors (n=260). At 3 months, patients with lymphoma and myeloma showed the greatest increase in mean (SD) hemoglobin from 9.2 (0.9) to 11.0 (1.8) g/dL, whereas patients with breast cancer showed the smallest increase from 10.0 (1.0) to 11.1 (1.2) g/dL. At 6 months, the greatest mean increase occurred in patients with lymphoma or myeloma from 11.0 (1.8) to 11.7 (2.3) g/dL, and the smallest in patients with other solid tumors from 10.9 (1.4) to 11.1 (1.5) g/dL. Patient evaluation of epoetin zeta therapy was positive, as most patients expressed satisfaction with epoetin zeta treatment during the study, compliance with treatment was high, and most indicated their willingness to be retreated if necessary. Epoetin zeta was also well tolerated; overall, in 25 patients (8.6%), there were 31 adverse events.
Conclusion: Despite variability among different disease groups, epoetin zeta was effective and well tolerated in patients with different types of solid tumors and hematologic malignancies.
Keywords: epoetin, erythropoiesis-stimulating agents, anemia, chemotherapy, biosimilar epoetin zeta, safety, efficacy, real-world
Introduction
Symptomatic anemia is a frequent and severe complication of chemotherapy used to treat solid tumors and malignant diseases of the blood, leading to fatigue and other related symptoms, which can significantly compromise patient quality of life.1 Additionally, chemotherapy-induced anemia (CIA) may signal inferior clinical outcomes. Correction of anemia may be accomplished by transfusion with red blood cells (RBCs) or administration of erythropoiesis-stimulating agents (ESAs) with or without iron supplementation.2
RBC transfusions are efficient in rapidly increasing hemoglobin (Hb) levels and consequently reversing anemia quickly. However, their use implies potential high risks to patients such as procedural complications, iron overload, thrombosis, immunologic injury, exposure to viral and bacterial infections, and transfusion-related acute lung injury.2
ESAs are human recombinant forms of erythropoietin, which stimulate bone marrow to produce RBCs.3 The use of ESAs can alleviate symptomatic anemia and avoid potential complications associated with blood transfusions.4,5 Clinical trials show that ESAs reduce the number of RBC transfusions and decrease fatigue in CIA patients, which result in improved anemia and quality of life.6–12 However, trials using an off-label target Hb level of >12 mg/dL indicate possible safety issues associated with the use of ESA therapy-increased risk of thrombosis-related events and decreased overall survival. Weighing all the evidence, the European Medicines Agency’s Committee for Medicinal Products for Human Use suggests that the benefits of using ESAs in approved indications in CIA (Hb 10–12 g/dL) continue to outweigh the associated risks, including increased risk of tumor progression and venous thromboembolism and reduced survival.13
Since the patent protection of the first ESA ended (epoetin alfa), several new biosimilar agents have become available.14 Although the quality, safety, and clinical efficacy of biosimilar products has been established by preclinical, clinical, and post-approval assessments,15 continued post-approval safety studies of biosimilar products are recommended.16
The recent 6-month post-marketing ORHEO (BiOsimilaRs in the management of anemia secondary to chemotherapy in HaEmatology and Oncology) study assessed the Hb response in adult patients with chemotherapy-induced anemia (N=2,333) undergoing treatment for solid tumors, lymphoma, or myeloma following treatment with biosimilar epoetin zeta (Retacrit®; Hospira, a Pfizer Company).17 Overall mean standard deviation (SD) increase in Hb level was 1.5 (1.6) g/dL at 3 months and 1.7 (1.6) g/dL at 6 months.
The primary objective of this subanalysis of the OHREO Study (OHREO Trial registration: NCT01626547) was to assess the change in Hb levels in solid tumor and lymphoma or multiple myeloma subgroups. Secondary objectives were to evaluate the profiles of treated patients, evaluate the treatment plans for each prescriber, observe changes in blood values in each subgroup, evaluate the correlation between the therapy plans of prescribing oncologists and patient characteristics, and assess the tolerability profile of epoetin zeta.
Methods
This study was approved by Germany Federal Institute for Drugs and Medical Devices, which decided that patients were not required to provide written informed consent because this study was observational and non-interventional. The guideline Quality-Assurance Measures in Non-Interventional Studies was used in this study.18
Study participants
Patients from oncology and hematology practices (N=291) were aged ≥18 years with solid tumors, malignant lymphomas or multiple myeloma, and chemotherapy-induced symptomatic anemia, regardless of chemotherapy treatment cycle and who were eligible for treatment with epoetin zeta, were enrolled. Patients not undergoing chemotherapy, had previously participated in a biosimilar epoetin zeta study, had known contraindications or were hypersensitive to the biosimilar epoetin zeta product, had erythroblastopenia or “pure red cell aplasia”, had uncontrolled hypertension, or for whom adequate prophylaxis against thrombosis was not possible were excluded from the study.
Study design
This was a non-interventional, multicenter, long-term observational study. Enrolled patients were evaluated at an enrollment visit, a 3-month follow-up visit, and a final 6-month visit. Since the dose per injection and the administration sequence and dosage form of the epoetin zeta products were not uniform, the weekly dose was calculated for each patient to ensure comparability. Demographic data were collected using patient and physician questionnaires.
Using the WHO definition, chemotherapy-induced anemia was defined as a decrease in Hb level to <11 g/dL following the start of treatment. The widely accepted guidelines for the application of epoetin are described in the reports of the American Society of Clinical Oncology/American Society of Hematology (ASCO/ASH)19 and European Organization for Research and Treatment of Cancer (EORTC) Working Groups.20 ASCO/ASH recommends patients with chemotherapy-induced symptomatic anemia whose Hb values are ≤10 g/dL should be treated with ESAs, and if Hb is between 10 and 12 g/dL, consider ESAs according to clinical condition.19,21 The EORTC recommends that ESAs should be initiated at an Hb level of 9–11 g/dL based on anemia-related symptoms and be considered in selected asymptomatic patients with an Hb level of 11–11.9 g/dL if this would prevent a further decline in Hb.22
Statistical analysis
Analyzed data from a physician questionnaire included demographics, area of specialization, treatment of symptomatic anemia, use of epoetin zeta, including reasons for and against its use, and the use of other ESAs. Clinical data obtained for each patient included type of malignancy, presence of symptomatic anemia, vital signs, hematologic parameters, treatment with epoetin zeta, concomitant therapeutic interventions, clinical course, changes in Hb levels, and adverse events (AEs). All reported AEs were documented regardless of causality; however, only AEs of special interest are reported here.
The response to treatment was evaluated for patients receiving treatment with epoetin zeta. Responders were defined as patients satisfying any of the following criteria: a 1 g/dL rise in Hb after enrollment, Hb ≥10 g/dL at 6 months, or achieving a patient-specific Hb goal defined at enrollment visit, without blood transfusion during the last 3 weeks. Reasons for interrupted or discontinued treatment were recorded.
The tolerability analysis was based on the Safety Analysis Set (SAF), consisting of patients with at least one dose of epoetin zeta. The Efficacy Analysis Set (PPS) included SAF patients fulfilling all inclusion and exclusion criteria, who had an evaluable Hb value at baseline and during or shortly after receiving treatment.
Results
Study patients
Among the 291 enrolled patients, 290 (99.7%) were included in the safety analysis and 278 (95.5%) were included in the efficacy analysis. Among patients stratified by disease, 30 (10.3%) had a lymphoma/myeloma and 260 (89.7%) had a solid tumor (two patients with both types of malignancy were assigned to the solid tumor group). The 3-month follow-up visit was completed by 251 patients and the final 6-month visit was completed by 190 patients. There was a mean of 81.4 days between enrollment and follow-up and 148.0 days between enrollment and the final visit. Except for gender differences among patients with breast cancer, patient demographics and baseline clinical characteristics were similar across groups (Table 1). Patients with breast cancer were non-metastatic (n=65), metastatic (n=21), or undetermined (n=9). There were no differences in baseline ECOG/WHO status stratified by disease across groups (Table 2).
  | Table 1 Demographics and baseline clinical characteristics |
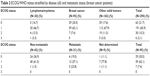  | Table 2 ECOG/WHO status stratified by disease (all) and metastatic status (breast cancer patients) |
Among patients with solid tumors (N=260), the mean (SD) disease duration was 3.9 (3.3) years. Tumors were localized to the breast (n=95), ovaries (n=32), lungs (n=27), colon or rectum (n=22), cervix (n=15), urological system (n=12), liver (n=6), and neck or head (n=5). The current stage of disease in most patients was T-3 (22.1%), N-0 (25.2%), and M-0 (40.7%).
Among patients with hematological malignancies (N=32), most (68.8%) had lymphoma. The mean disease duration was 5.0 (4.6) years and the mean number of prior chemotherapy cycles was 5.4 (8.5). There was one autologous stem cell transplant and no allogeneic stem cell transplants. Overall, 89.3% of patients were treated with chemotherapy alone, with little difference across disease groups. Radiochemotherapy was used to treat two patients with lymphoma/myeloma (6.7%), 13 patients with breast cancer (13.7%), and 16 patients with other solid tumors (9.7%).
Hemoglobin
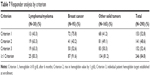
For 45% of the patients, the Hb level was between 9 and 10 g/dL, and in 27% of patients, levels ranged from 10 to 11 g/dL. For patients with breast cancer, 36.2% were in the range of 9–10 g/dL, which was the lowest proportion of patients in this category, and 50% of patients with lymphoma/myeloma, which was the highest proportion of patients. In the range from 10 to 11 g/dL, it is the opposite. Here, patients with breast cancer with 37.2% were the largest proportion and patients with lymphoma/myeloma with 10% were the smallest proportion (Table 3).
  | Table 3 Hemoglobin stratified by disease at study entry |
The mean hematocrit was 29.9%, with little difference across groups. Unfortunately, only a minority of all patients had a complete examination of iron storage including transferrin saturation thus precluding a detailed analysis. Since the reticulocyte count results were only available for 89 patients and showed an extremely large variation among patients with solid tumors, median values were determined with an overall median value of 34.6 mm3 (Table 4). The median values were 19.0 and 41.3 mm3 for patients with lymphoma/myeloma and breast cancer, respectively. The overall mean serum iron value was 71.5 μg/dL and as high as 75.8 μg/dL in patients with lymphoma/myeloma and as low as 65.5 μg/dL in patients with breast cancer. The total serum iron level was only available for 89 patients.
  | Table 4 Most recently performed blood tests at enrollment |
Treatment of symptomatic anemia with epoetin zeta
Epoetin zeta was administered subcutaneously to all patients. The median weekly dose was 40,000 IU for patients with solid tumors and 30,000 IU for patients with lymphoma/myeloma. The majority of patients received epoetin zeta weekly with little difference between the underlying diseases.
Concomitant therapies
Patients with solid tumors were treated with concomitant B-vitamins (4.1%) and other vitamins (4.8%), folate (4.5%), and intravenous (13.8%) or oral iron (3.1%). Only one patient with lymphoma/myeloma (3.3%) was treated with intravenous iron. Sixty-five patients (22.4%) received a blood transfusion during the year prior to enrollment, including patients with lymphoma/myeloma (n=10, 33.3%) and other solid tumors (n=10, 10.5%). Among patients with breast cancer, 45 (27.3%) received a blood transfusion during the year prior to enrollment.
Primary study objective: changes in Hb
Follow-up visit
Among the enrolled patients (N=290), the follow-up and final visits were completed by 251 (86.6%) and 190 (65.5%), respectively. Efficacy was analyzed in both the Safety and Efficacy Analysis sets, but the differences between them were small. At enrollment, there were 289 patients in the SAF versus 278 patients in the PPS. At the follow-up and final visits, the difference between the populations was only one patient (251 vs 250 and 183 vs 182).
Overall, mean (SD) Hb level increased from 9.7 (1.0) g/dL at enrollment to 11.0 (1.4) and 11.4 (1.5) g/dL at the follow-up and final visits, respectively (Table 5). Patients with breast cancer had the highest mean Hb values at the follow-up and final visits at 11.1 (1.2) and 11.7 (1.2) g/dL, respectively; however, these patients also had the highest mean Hb value of 10.0 (1.0) g/dL at enrollment. Patients with lymphoma and myeloma showed the greatest increase in mean Hb from 9.2 (0.9) to 11.0 (1.8) g/dL to 11.7 (2.3) g/dL (Table 5). Changes in Hb for all patients with solid tumors are shown in Table 5.
  | Table 5 Changes in mean hemoglobin values |
The overall Hb values for most patients at enrollment were ≤9–10 g/dL; however, they rose to 10–12 g/dL for most patients at the follow-up visit. Especially notable were increases to >12 g/dL, which were uncommon at enrollment (Table 6). Hb values for most patients were 10–12 g/dL at the follow-up visit and 11–12 g/dL at the final visit. Hb levels ≥13 g/dL, which were observed in 4.8% of patients at the follow-up visit, increased to 10.9% at the final visit.
  | Table 6 Hemoglobin values by malignancy type |
Responder analysis
Patients meeting one or more of the following criteria were defined as responders: Criterion 1, Hb ≥10 g/dL after 6 months; Criterion 2, rise in Hb value by 1 g/dL; or Criterion 3, reaching the individual patient Hb target established at enrollment (without blood transfusion during the prior 3 weeks). Overall, 84.8% of patients being treated with epoetin zeta responded with little difference across groups (Table 7). Overall, the mean Hb level in responders increased from 9.8 (0.9) g/dL at enrollment to 11.2 (1.2) g/dL at follow-up. Patients with lymphoma/myeloma had the highest mean Hb of 11.4 (1.2) g/dL. The greatest mean increase was from 9.3 (0.9) to 11.4 (1.2) g/dL in patients with lymphoma/myeloma, while the smallest increase was in patients with breast cancer from 10.0 (1.0) to 11.2 (1.2) g/dL.
Changes in therapy
At the follow-up visit, three patients with breast cancer (3.2%) and three patients with other solid tumors (1.8) had a change in epoetin zeta dosage. Among patients with breast cancer, reasons for the change were Hb above target level (n=1, 1.1%), other reasons (n=1, 1.1%), and one reason not recorded. Among patients with other solid tumors, reasons for changing therapy were Hb below target level (n=1, 0.6%), Hb above target level (n=1, 0.6%), and other reasons (n=1, 0.6%). At the final visit, three patients with breast cancer (3.2%) had a change in epoetin zeta therapy due to other reasons. Among four patients with other solid tumors (2.4%), three had changes due to Hb value still below the target range. These included one patient with and one patient without metastases and one patient with undetermined metastatic status. Overall, 92 patients (31.7%) had treatment with epoetin zeta interrupted or disrupted by the final visit, including 3 patients with lymphoma/myeloma (10%) and 51 with breast cancer (53.7%).
Hematologic changes
Hematocrit
Overall, the median hematocrit increased by 5.3% during the course of the study from 29.9% to 35.2% (Table 8). There was little difference between the malignancy types.
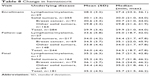  | Table 8 Change in hematocrit |
Reticulocytes
The overall median reticulocyte count decreased during the study period from 34.6 to 31.4 mm3 at the follow-up visit and 30.0 mm3 at the final visit. The median reticulocyte count during the study increased in patients with lymphoma and myeloma from 19.0 mm3 at enrollment to 21.0 mm3 at the follow-up visit and 68.0 mm3 at the final visit; however, this last value was from only one patient. Among patients with other solid tumors, the median reticulocyte count increased from 33.0 mm3 at enrollment to 36.7 mm3 at follow-up, decreasing to 31.2 mm3 at the final visit.
Serum iron
Overall median values for serum iron increased from 50.9 μg/dL at enrollment to 51.5 μg/dL and 59.6 μg/dL at the follow-up and final visits, respectively; however, median serum iron increased in patients with lymphoma/myeloma from 54.3 μg/dL at enrollment to 64.3 and 109.4 μg/dL at the follow-up and final visits, respectively. Among patients with other solid tumors, the median serum iron increased from 46.6 μg/dL at enrollment to 47.0 and 49.0 μg/dL at the follow-up and final visits, respectively.
Ferritin
Overall median ferritin levels initially increased from 396.5 μg/L at enrollment to 488.0 μg/L at the follow-up visit, declining to 271.9 μg/L at the final visit. Among patients with lymphoma/myeloma, the median ferritin level increased from 390.3 μg/L at enrollment to 417.6 μg/L at the follow-up visit, decreasing to 414.3 μg/L at the final visit. Patients with other solid tumors showed the greatest variation, with median ferritin levels increasing from 364.0 μg/L at enrollment to 622.0 μg/L at the follow-up visit and decreasing to 258.0 μg/L at the final visit.
Transferrin
Overall median transferrin saturation decreased from 21.1% at enrollment to 20.2% and 17.5% at the follow-up and final visits, respectively; however, median transferrin saturation increased in patients with lymphoma/myeloma increased from 17.1% at enrollment to 22.4% and 31.1% at the follow-up and final visits, respectively. Among breast cancer patients, the median transferrin saturation varied from 18.5% at enrollment to 17.4% at the follow-up visit and 25.0% at the final visit.
The overall number of patients with ferritin levels ≥100 ng/mL and transferrin saturation >20% declined during the study from 20 patients at enrollment (40.8%) to 16 patients (42.1%) at the follow-up visit and 6 patients (37.5%) at the final visit. It should be noted that the transferrin saturation values were only available for 49, 38, and 16 patients at each respective visit.
Treatment of symptomatic anemia with epoetin zeta
Overall, the mean weekly dose of epoetin zeta increased at the follow-up visit and decreased at the final visit. Among lymphoma/myeloma patients, the weekly dose also increased at follow-up visit, but decreased to a mean dose significantly below baseline at the final visit (31,000 IU vs 34,333 IU). The dose in patients with breast cancer and other solid tumors remained relatively stable. Among patients with breast cancer, the mean weekly dose decreased from 557.6 IU/kg at enrollment to 541.3 IU/kg at the final visit.
Concomitant therapy
Twenty-two patients (8.8%) received a blood transfusion at the follow-up visit and eight (4.2%) had a blood transfusion at the final visit. They were primarily patients with breast cancer.
Patient evaluation of biosimilar epoetin zeta therapy
Among patients providing a response at the follow-up visit, overall treatment satisfaction ranged from 57.7% to 62.8% across malignancy types and dissatisfaction ranged from 0% to 4.3% and approximately half (42.3%–50.6%) were willing to undergo re-treatment. Patient compliance was Very Good or Good for 69.3%–80.5% of patients and patient independence improved for 19.2%–26.6%.
Among patients providing a response at the final visit, overall treatment satisfaction ranged from 33.3% to 66.7% across malignancy types and dissatisfaction ranged from 0% to 1.2%. A somewhat smaller percentage of patients (30.0%–60.2%) were willing to undergo re-treatment. Patient compliance was Very Good or Good for 51.5%–76.3% of patients, and patient independence improved for 14.5%–30.1%. Patient satisfaction was similar regardless of metastatic status (62.5%–71.4%).
Adverse events
All AEs that were reported during the study were recorded; however, only AEs of special interest are reported. Overall, 25 patients (8.6%) reported 31 AEs (Table 9). None of the patients with lymphoma/myeloma reported an AE. Eight patients with solid tumors (2.8%) reported eight serious AEs (Table 10).
  | Table 9 Treatment-emergent adverse events |
  | Table 10 Treatment-emergent serious adverse events |
Discussion
Anemia is an expected complication for patients who are receiving chemotherapy treatment. Although patients with CIA experience reductions in transfusion requirements and fatigue17 with ESAs, they come with a high cost, which may limit access in some countries.23 Biosimilar medicines approved in the EU can offer physicians the reassurance of rigorous comparability studies and extensive clinical and post-marketing surveillance programs, in addition to cost savings due to lower development costs.17 Post-approval surveillance, real-world studies are an important component of post-approval efficacy and safety assessment of biosimilar medicines. Following the original ORHEO study, this study forms part of an ongoing surveillance program for epoetin zeta, a biosimilar of epoetin alfa.
The original OHREO study conducted in France demonstrated that biosimilar epoetin zeta was effective and well tolerated in the management of chemotherapy-induced anemia in patients with solid tumors, lymphoma, and myeloma.17 Mean baseline Hb was 9.6 g/dL, with 35.6% of patients having moderate anemia (8–9.5 g/dL). Hb response was achieved in 81.6% and 86.5% of patients at 3 and 6 months, respectively. Overall mean (SD) change in Hb level was 1.5 (1.6) and 1.72 (1.6) g/dL at 3 and 6 months, respectively. Transfusion and thromboembolic event rates were 9.4% and 2.4% at 3 months, and 5.8% and 1.5% at 6 months, respectively.17 Patients with solid tumors other than breast cancer exhibited a much better response than those patients with breast cancer. There are several possible explanations including the nature of the disease, the duration of the disease, and the intensity of chemotherapy. Furthermore, the results of this study question the role of epoetin in breast cancer patients, given the modest response. Further study is therefore warranted. The results from this ORHEO study conducted in Germany showed similar results to the original French study, although no statistical analysis was conducted. Additionally, these results mimic and reinforce the efficacy observed in the pre-approval studies of Retacrit.
In this study, numerous improvements in other laboratory parameters were also observed among different patient populations. The median total reticulocyte count increased in patients with lymphoma/myeloma. Among patients with lymphoma and myeloma, the mean serum iron level initially decreased at the follow-up visit but then rose sharply at the final visit. The overall mean ferritin levels initially increased at the follow-up visit and decreased at the final visit but remained above baseline. A similar pattern was observed in patients with solid tumors. In contrast, mean ferritin levels decreased in patients with lymphoma and myeloma, but then increased to levels above baseline at the final visit. Mean transferrin saturation for these patients also increased. In patients with breast cancer, mean transferrin saturation remained relatively unchanged. However, given that the majority of patients did not have ferritin and transferrin levels available, these data should be interpreted with caution.
Secondary objectives were to evaluate treated patient profiles, observe changes in blood values, assess the safety profile of epoetin zeta, and describe prescriber treatment plans; however, little meaningful information was available regarding prescribing practices, as only nine prescribers completed the Physician Questionnaire. The overall median hematocrit increased with little difference across malignancy types. Median serum iron, ferritin levels, and transferrin saturation increased in patients with lymphoma/myeloma, median serum iron increased in solid tumors, and median transferrin saturation increased among breast cancer patients.
In general, epoetin zeta was administered once weekly with little difference between the disease groups or metastatic status. The weekly dose of epoetin zeta was decreased in patients with lymphoma and myeloma, while the dose in patients with breast cancer and other solid tumors remained stable.
Patient evaluation of epoetin zeta therapy was positive; as most patients expressed satisfaction with epoetin zeta treatment during the study, compliance with treatment was high and most indicated their willingness to be re-treated with epoetin zeta if necessary. Epoetin zeta was also well tolerated with relatively few AEs of interest occurring in patients with breast cancer (n=11, 11.6%) and other solid tumors (n=14, 8.5%) and none in patients with lymphoma and myeloma. The absence of AEs in patients with lymphoma and myeloma may be due to the small number of patients evaluated. There were few thromboembolic complications in patients with breast cancer (n=3, 3.2%) and other solid tumors (n=4, 2.4%).
The results of this observational study contribute to the growing body of evidence regarding the efficacy and safety of epoetin biosimilars in oncology. The efficacy results achieved with epoetin zeta in this study relate closely with previous research. A phase III study of epoetin zeta in CIA over a 12-week period in 216 patients reported a similar overall mean Hb increase of 1.8 g/dL, with 81.5% of patients achieving ≥1 g/dL Hb increase.24 A recent review summarized the post-marketing patient exposure and clinical observational studies regarding the clinical efficacy and safety of epoetin zeta. Between December 18, 2007, and November 30, 2013, the total estimated population exposure (in the oncology and nephrology indication), based on post-marketing sales, was 54,554,947 patient-days (WHO daily defined dose of 1,000 IU) with >35,000,000 patient-days’ experience in the oncology indication. During this timeframe, the observed post-marketing safety experience with epoetin zeta was consistent with the known profile of epoetin alfa with no cases of pure red cell aplasia reported.25
Conclusion
Overall, epoetin zeta was effective and well tolerated in patients with different types of solid and hematologic malignancies despite variability among different disease groups. Patient acceptance of epoetin zeta therapy was high, as most patients were compliant, expressed high satisfaction with treatment, and indicated their willingness to be re-treated if necessary.
Acknowledgments
Medical writing and editorial assistance were provided by Anne Gentry of Gentry Medical Communications, LLC, and funded by Hospira, which was acquired by Pfizer in September 2015.
Disclosure
CR was an employee of Hospira Germany, a Pfizer company, when the manuscript was developed. CL and MK report no conflicts of interest in this work.
References
Groopman JE, Itri LM. Chemotherapy-induced anemia in adults: incidence and treatment. J Natl Cancer Inst. 1999;91(19):1616–1634. | ||
Mountzios G, Aravantinos G, Alexopoulou Z, et al. Lessons from the past: Long-term safety and survival outcomes of a prematurely terminated randomized controlled trial on prophylactic vs. hemoglobin-based administration of erythropoiesis-stimulating agents in patients with chemotherapy-induced anemia. Mol Clin Oncol. 2016;4(2):211–220. | ||
Macdougall IC. Novel erythropoiesis-stimulating agents: a new era in anemia management. Clin J Am Soc Nephrol. 2008;3(1):200–207. | ||
Stramer SL. Current risks of transfusion-transmitted agents: a review. Arch Pat Lab Med. 2007;131(5):702–707. | ||
Bernard AC, Davenport DL, Chang PK, Vaughan TB, Zwischenberger JB. Intraoperative transfusion of 1 U to 2 U packed red blood cells is associated with increased 30-day mortality, surgical-site infection, pneumonia, and sepsis in general surgery patients. J Am Coll Surg. 2009;208(5):931–937. | ||
Galli L, Ricci C, Egan CG. Epoetin beta for the treatment of chemotherapy-induced anemia: an update. Onco Targets Ther. 2015;8:583–591. | ||
Maenpaa J, Puistola U, Riska H, et al. Impact of epoetin-beta on anemia and health-related quality of life in cancer patients: a prospective observational study using the generic 15D instrument. Anticancer Res. 2014;34(5):2325–2329. | ||
Crathorne L, Huxley N, Haasova M, et al. The effectiveness and cost-effectiveness of erythropoiesis-stimulating agents (epoetin and darbepoetin) for treating cancer treatment-induced anaemia: a systematic review and economic model. Health Technol Assess. 2016;20(13):1–632. | ||
Littlewood TJ, Bajetta E, Nortier JW, Vercammen E, Rapoport B; Epoetin Alfa Study Group. Effects of epoetin alfa on hematologic parameters and quality of life in cancer patients receiving nonplatinum chemotherapy: results of a randomized, double-blind, placebo-controlled trial. J Clin Oncol. 2001;19(11):2865–2874. | ||
Osterborg A, Brandberg Y, Molostova V, et al. Randomized, double-blind, placebo-controlled trial of recombinant human erythropoietin, epoetin beta, in hematologic malignancies. J Clin Oncol. 2002;20(10):2486–2494. | ||
Boogaerts M, Coiffier B, Kainz C; Epoetin Beta QOL Working Group. Impact of epoetin beta on quality of life in patients with malignant disease. Br J Cancer. 2003;88(7):988–995. | ||
Aapro MS. Anemia management with erythropoiesis-stimulating agents: a risk-benefit update. Oncologist. 2008;13(Suppl 3):1–3. | ||
European Medicines Agency. Neorecormon. Available from: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000116/human_med_000921.jsp&mid=WC0b01ac058001d124. Accessed November 30, 2016. | ||
Brinks V, Hawe A, Basmeleh AH, et al. Quality of original and biosimilar epoetin products. Pharm Res. 2011;28(2):386–393. | ||
European Generic Medicines Association: Biosimilars Handbook. 2nd ed. London: Sage Publications Ltd; 2011. | ||
Committee for Medicinal Products for Human Use: Guideline on similar biological medicinal products containing biotechnology-derived proteins as active substance: non-clinical and clinical issues. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003920.pdf. Accessed April 29, 2016. | ||
Michallet M, Luporsi E, Soubeyran P, et al. BiOsimilaRs in the management of anaemia secondary to chemotherapy in HaEmatology and Oncology: results of the ORHEO observational study. BMC Cancer. 2014;14:503. | ||
Theobald K, Capan M, Herbold M, Schinzel S, Hundt F. Quality-assurance measures in non-interventional studies. Ger Med Sci. 2009;7:Doc29. Available from: http://www.egms.de/static/en/journals/gms/2009-7/000088.shtml. Accessed December 8, 2016. | ||
Rizzo JD, Brouwers M, Hurly P, et al. ASCO-ASH clinical practice guideline update on the use of epoetin and darbepoetin in adult patients with cancer. J Clin Oncol. 2010;28(33):4996–5010. | ||
Aapro MS, Link H. September 2007 update on EORTC guidelines and anemia management with erythropoiesis-stimulating agents. Oncologist. 2008;13(Suppl 3):33–36. | ||
Theobald K, Capan M, Herbold M, Schinzel S, Hundt F. Quality assurance in non-interventional studies. Ger Med Sci. 2009;7:29. | ||
Bokemeyer C, Aapro MS, Courdi A, et al. EORTC guidelines for the use of erythropoietic proteins in anaemic patients with cancer: 2006 update. Eur J Cancer. 2007;43(2):258–270. | ||
Aapro M, Jelkmann W, Constantinescu SN, Leyland-Jones B. Effects of erythropoietin receptors and erythropoiesis-stimulating agents on disease progression in cancer. Brit J Cancer. 2012;106(7):1249–1258. | ||
Tzekova V, Mihaylov G, Elezovic I, Koytchev R. Therapeutic effects of epoetin zeta in the treatment of chemotherapy-induced anaemia. Curr Med Res Opin. 2009;25(7):1689–1697. | ||
Michallet M, Losem C. Biosimilar epoetin zeta in oncology and haematology: development and experience following 6 years of use. Acta Haematol. 2016;135(1):44–52. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.