Back to Journals » OncoTargets and Therapy » Volume 13
Biomarkers of Insulin and the Insulin-Like Growth Factor Axis in Relation to Breast Cancer Risk in Chinese Women
Authors Zhu Y, Wang T, Wu J, Huang O, Zhu L, He J, Li Y, Chen W, Chen X , Shen K
Received 15 April 2020
Accepted for publication 10 July 2020
Published 11 August 2020 Volume 2020:13 Pages 8027—8036
DOI https://doi.org/10.2147/OTT.S258357
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Leo Jen-Liang Su
Yifei Zhu,1,* Tiange Wang,2,* Jiayi Wu,1 Ou Huang,1 Li Zhu,1 Jianrong He,1 Yafen Li,1 Weiguo Chen,1 Xiaosong Chen,1 Kunwei Shen1
1Comprehensive Breast Health Center, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, People’s Republic of China; 2Shanghai Institute of Endocrine and Metabolic Diseases, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xiaosong Chen; Kunwei Shen
Comprehensive Breast Health Center, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, People’s Republic of China
Email [email protected] [email protected]
Background: The interplay between biomarkers of insulin and the insulin-like growth factor (IGF) axis in the context of breast cancer risk is unclear.
Methods: We measured the concentrations of insulin, C-peptide, IGF1, and IGF binding protein 3 (IGFBP3) and calculated the homeostasis model assessment of insulin resistance (HOMA-IR) index and the IGF1/IGFBP3 ratio among 2536 patients with breast cancer and 2528 patients with benign breast disease recruited from Ruijin Hospital, Shanghai, China, between 2012 and 2017.
Results: Multivariable-adjusted odds ratios (ORs) for breast cancer associated with the highest quartiles versus the lowest quartiles of insulin and IGF factors were 1.45 (95% CI, 1.20– 1.75) for insulin, 1.32 (1.08– 1.60) for C-peptide, 1.53 (1.26– 1.85) for HOMA-IR, and 1.27 (1.05– 1.53) for IGF1; these associations did not differ substantially across stratifications of age, body mass index, age at menarche, or menopausal status (all P for interaction > 0.05). In the joint analysis, the highest quartile of IGF1 was associated with the greatest risk of breast cancer in the highest quartiles of insulin (OR, 1.77; 95% CI, 1.29– 2.44), C-peptide (1.60; 1.17– 2.20), and HOMA-IR (1.90; 1.38– 2.62), compared with the risks associated with the combination of the lowest quartiles of IGF1 and each insulin factor. In stratification analysis, the positive association between IGF1 and breast cancer was stronger in the highest quartiles of insulin (P[interaction] = 0.29), C-peptide (P[interaction] = 0.020), and HOMA-IR (P[interaction] = 0.075).
Conclusion: Our findings indicate effect modifications of insulin, C-peptide, and insulin resistance on the relationship between IGF1 and breast cancer risk in Chinese women.
Keywords: insulin, insulin-like growth factor 1, C-peptide, insulin resistance, breast cancer
Introduction
Breast cancer is the most frequently diagnosed cancer and the leading cause of cancer-related death in women worldwide.1 In China, the incidence of breast cancer has increased more than twice as fast as the total rates worldwide over the past three decades.2 Current cases of breast cancer in China account for 12.2% of all newly diagnosed breast cancers and 9.6% of all deaths from breast cancer globally.2
Breast cancer is an endocrine-related cancer, and endocrine biomarkers involved in the etiology of breast cancer may serve as predictors for prevention and early detection.3 Insulin and insulin-like growth factors (IGFs) are key regulators of energy metabolism and cellular growth. Preclinical evidence from experimental investigations has revealed the important role of these hormones, mainly insulin, C-peptide, IGF1, and IGF binding protein 3 (IGFBP3), in the development of multiple cancers, including breast cancer.4–7 In contrast, clinical and epidemiological studies have revealed mixed findings with regard to the relationships of the biomarkers of insulin and the IGF axis with breast cancer.8–13
Most epidemiological studies have supported a positive association between circulating IGF1 and breast cancer risk but have been inconsistent as to whether this association is affected by confounders such as menopausal status.8–10 Regarding insulin biomarkers, current findings on their relationships with breast cancer have been inconclusive.11–13 There is experimental evidence indicating the occurrence of crosstalk among biomarkers between insulin signaling pathways and the IGF axis.4–7 However, to date, few studies have extensively assessed the joint association and interaction patterns of biomarkers of insulin and the IGF axis with breast cancer. In addition, previous studies have mainly focused on European and American women, while evidence from Chinese women is limited.8–13
Therefore, we examined the individual and joint associations of biomarkers of insulin and the IGF axis, as well as insulin resistance, with breast cancer and specifically tested the interaction patterns of these markers with breast cancer in this clinical case-control study conducted in China.
Methods
Study Setting, Patients and Recruitment
Between November 2012 and June 2017, patients aged 14 years or older who had undergone surgical procedures were enrolled from the Comprehensive Breast Health Center, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine. Stored hematoxylin and eosin-stained sections from all patients were evaluated by experienced pathologists in the Department of Pathology, Ruijin Hospital. Breast cancer and benign breast disease were diagnosed by histopathologic examination according to the 4th edition of the World Health Organization Classification of Tumors of the Breast.14 Patients who met the following criteria were included in this study: (1) absence of pregnancy, (2) breast cancer or benign breast disease proven by core needle biopsy or open excision biopsy, (3) no preoperative therapy, and (4) complete clinical data. A total of 2536 cases of breast cancer and 2528 age-matched cases with benign breast disease were included in the analysis. The study protocol was approved by the Medical Ethics Committee of Ruijin Hospital, Shanghai Jiao Tong University. All study participants provided written informed consent. A parent or legal guardian signed informed consent for any participant under the age of 18 years. This study was conducted in accordance with the Declaration of Helsinki.
Data Collection
Standardized clinical data of patients with breast cancer were obtained from the Shanghai Jiao Tong University Breast Cancer Database. For patients with benign breast disease, standardized clinical data were derived from the Electronic Medical Records of Ruijin Hospital. Clinical information included demographic characteristics, reproductive factors (including age at menarche, number of full-term pregnancies, breastfeeding history, menopausal status, and age at menopause), hormone replacement therapy, and family history of breast cancer.
Body weight and height were measured with patients wearing light indoor clothing and no shoes to the nearest 0.1 kg and 0.1 cm, respectively. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared.
Before the surgical procedure, blood samples were obtained after an overnight fast of at least 8 hours. Fasting glucose was measured by a Beckman Coulter-AU 5800 (Beckman Coulter, Inc., Atlanta, GA, USA). Serum insulin (catalog number: 12,017,547 122; lower detection limit: 0.200 uIU/mL) and C-peptide (catalog number: 03184897 190; lower detection limit: 0.010 ug/L) were measured by electrochemiluminescence immunoassay on Cobas E601 analyzers (Hoffman-La Roche Ltd, Basel, Switzerland). Plasma IGF1 (catalog number: L2KGF2; lower detection limit: 20 ng/mL) and IGFBP3 (catalog number: L2KGB2; lower detection limit: 0.1 ug/mL) were tested by chemiluminescent immunoassay using the IMMULITE 2000 system (Siemens AG, Munich, Germany). Each assay had high specificity, for the cross-reactivity with recombinant analogs or relative substances was not detectable or with no clinical significance. The homeostasis model assessment of insulin resistance (HOMA-IR) index was applied to evaluate insulin resistance and was calculated as fasting insulin (µIU/mL) × fasting glucose (mmol/L)/22.5.15 The IGF1/IGFBP3 ratio was calculated.
Statistical Analysis
Patients with benign breast disease were age-matched (plus or minus 1 year) with patients with breast cancer. Characteristics of study participants with breast cancer or benign breast disease were described as the mean (SD) for continuous variables with normal distribution, median (interquartile range) for continuous variables with skewed distribution, or number (proportion) for categorical variables. Differences in distributions of continuous variables between the breast cancer and benign breast disease groups were examined using analysis of variance (ANOVA). Differences in proportions of categorical variables between the breast cancer and benign breast disease groups were compared using the chi-square test. Correlation coefficients between biomarkers of insulin and the IGF axis as well as HOMA-IR were analyzed by Pearson r correlation. Multivariable logistic regression analysis was used to estimate odds ratios (ORs) and 95% confidence intervals (CIs) to represent the relative risk of breast cancer associated with quartiles of the biomarkers of insulin and the IGF1 axis including insulin, C-peptide, IGF1, IGFBP3, and the IGF1/IGFBP3 ratio as well as HOMA-IR. ORs (95% CIs) were adjusted for age (< 45, 45-< 65, ≥ 65 years), BMI (< 18.5, 18.5-< 23, 23-< 27.5, ≥ 27.5 kg/m2), age at menarche (< 14, ≥ 14 years), breastfeeding (yes, no), number of full-term pregnancies (0, 1 or 2, ≥ 3), postmenopausal status (yes, no), hormone replacement therapy (yes, no), and family history of breast cancer (yes, no). Stratification analyses were performed to evaluate whether these associations varied across subgroups of age (< 50 or ≥ 50 years), BMI (< 23 or ≥ 23 kg/m2), age at menarche (< 14 or ≥ 14 years), or menopausal status (premenopausal or postmenopausal status).
The main association between IGF1 and breast cancer was additionally adjusted for insulin, C-peptide, and HOMA-IR to examine whether the IGF1-breast cancer relationship is independent of insulin, C-peptide, and insulin resistance. Joint associations and interactions of IGF1 with insulin, C-peptide, and HOMA-IR on breast cancer were further analyzed to evaluate the effect modifications of insulin, C-peptide, and HOMA-IR on the association between IGF1 and breast cancer. Multiplicative interactions were tested by including respective product terms (for instance, IGF1 × insulin) as well as the main associations in the models.
All statistical analyses were performed by using SAS software, version 9.2 (SAS Institute). All reported P values are nominal and 2-sided, and a P value of < 0.05 was considered statistically significant.
Results
Patient Characteristics
The current analyses included 2536 patients with breast cancer and 2528 age-matched controls with benign breast disease. The basic characteristics of patients with breast cancer or benign breast disease are shown in Table 1. Compared with women with benign breast disease, women with breast cancer had a higher BMI (mean value of 23.1 kg/m2 versus 22.8 kg/m2, P = 0.002) and were more likely to have 3 full-term pregnancies or more (24.6% versus 20.7%, P < 0.001) and to be postmenopausal (45.7% versus 42.9%, P = 0.045). In addition, women with breast cancer had higher concentrations of insulin (mean of 7.67 µIU/mL versus 7.13 µIU/mL, P < 0.001), C-peptide (mean of 1.92 µg/L versus 1.84 µg/L, P < 0.001), IGF1 (mean of 175.3 ng/mL versus 171.5 ng/mL, P = 0.032) and IGFBP3 (mean of 4.07 µg/mL versus 4.01 µg/mL, P = 0.049), as well as higher HOMA-IR (mean of 1.75 versus 1.61, P < 0.001) and fasting glucose (mean of 5.3 mmol/L versus 5.2 mmol/L, P = 0.001), than women with benign breast disease. Overall, there were statistically significant positive correlations among insulin, C-peptide, HOMA-IR, IGF1 and IGFBP3 (Supplemental Table 1 in additional file).
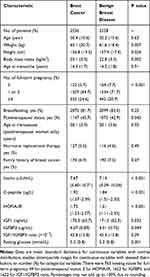 |
Table 1 Basic Characteristics of Study Patients |
Associations of Insulin and IGF Factors with Breast Cancer
In general, increasing quartiles of insulin, C-peptide, HOMA-IR, and IGF1 were gradually associated with a higher risk of breast cancer (Table 2). Multivariable-adjusted ORs (95% CIs) for breast cancer associated with the highest quartile versus the lowest quartile of the markers were as follows: 1.41 (1.17–1.71) for insulin, 1.28 (1.05–1.56) for C-peptide, 1.49 (1.23–1.80) for HOMA-IR, and 1.27 (1.05–1.53) for IGF1. The corresponding ORs (95% CIs) of breast cancer were 1.10 (1.04–1.17), 1.07 (1.01–1.14), 1.12 (1.05–1.19), and 1.07 (1.01–1.14) for each 1-quartile increase in insulin, C-peptide, HOMA-IR, and IGF1, respectively. There were no statistically significant associations of IGFBP3 (OR, 1.04; 95% CI, 0.98–1.11 per 1-quartile increase) or the IGF1/IGFBP3 ratio (OR, 1.05; 95% CI, 0.98–1.13 per 1-quartile increase) with breast cancer. Medians and ranges of insulin, C-peptide, HOMA-IR, IGF1, IGFBP3, and the IGF1/IGFBP3 ratio across respective quartiles are shown in Supplemental Table 2 in the additional file.
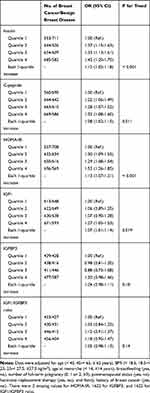 |
Table 2 Multivariable Adjusted Odds Ratio (95% CI) of Breast Cancer According to Quartiles of Insulin, C-Peptide, HOMA-IR, IGF1, IGFBP3, and IGF1/IGFBP3 Ratio |
Moreover, the positive associations of each 1-quartile increase in insulin, C-peptide, HOMA-IR, and IGF1 with breast cancer were consistent across stratifications of age, BMI, age at menarche, and menopausal status (all P for interaction ≥ 0.08), indicating no evidence of effect modifications of these conventional risk factors on these associations (Figure 1). No statistically significant associations of IGFBP3 or the IGF1/IGFBP3 ratio with breast cancer were observed across these stratifications (Supplemental Table 3 in additional file).
Independent and Joint Associations of IGF1, Insulin, C-Peptide, and HOMA-IR with Breast Cancer
As shown in Figure 2, when additionally adjusted for insulin, the OR (95% CI) of breast cancer associated with the highest quartile compared with the lowest quartile of IGF1 decreased from 1.27 (1.05–1.53) to 1.17 (0.97–1.42); in contrast, the significant positive association between insulin and breast cancer persisted with adjustment for IGF1 (OR, 1.41; 95% CI, 1.17–1.71 without adjustment for IGF1; OR, 1.36; 95% CI, 1.12–1.66 with adjustment for IGF1). Similarly, the association between IGF1 and breast cancer was largely attenuated after additional adjustment for C-peptide or HOMA-IR, but the associations of C-peptide and HOMA-IR with breast cancer were not substantially changed after adjusting for IGF1.
When analyzed jointly, compared with the combined category of the lowest quartile of IGF1 and the lowest quartile of insulin, the highest quartile of IGF1 was associated with an OR (95% CI) of breast cancer of 1.02 (0.68–1.54), 1.51 (1.09–2.10), 1.55 (1.14–2.10), and 1.74 (1.26–2.40) across increasing quartiles of insulin; the significant association pattern between increasing quartiles of IGF1 and elevated risk of breast cancer was more prominent in the highest quartile of insulin (Figure 3). Compared with the corresponding reference category, the highest quartile of IGF1 was associated with the greatest risk of breast cancer in the highest quartiles of C-peptide (OR, 1.58; 95% CI, 1.15–2.16) and HOMA-IR (OR, 1.87; 95% CI, 1.35–2.58). Similar patterns of joint associations of IGF1 with C-peptide and HOMA-IR on breast cancer were observed.
Interactions of IGF1, Insulin, C-Peptide, and HOMA-IR with Breast Cancer
Consistently, the association between each 1-quartile increase in IGF1 and breast cancer was strongest in the highest quartiles of insulin (OR, 1.18; 95% CI, 1.04–1.33), C-peptide (OR, 1.23; 95% CI, 1.09–1.39), and HOMA-IR (OR, 1.19; 95% CI, 1.06–1.35; Figure 4). A statistically significant interaction between IGF1 and C-peptide (P for interaction = 0.016) and a borderline significant interaction between IGF1 and HOMA-IR (P for interaction = 0.061) were detected in the context of breast cancer.
Discussion
In this large, clinic-based case-control study, we found that concentrations of insulin, C-peptide, and IGF1, as well as HOMA-IR levels, were positively and synergistically associated with breast cancer in Chinese women, and these associations did not differ markedly according to stratification by age, BMI, or reproductive factors such as age at menarche and menopausal status. Specifically, the significant positive association between IGF1 and breast cancer was substantially dependent on, and strengthened by, high levels of insulin and C-peptide as well as insulin resistance estimated by HOMA-IR, indicating effect modifications of insulin, C-peptide, and insulin resistance status on the relationship between IGF1 and breast cancer.
Previous clinical and epidemiological studies assessing the relationship between biomarkers of insulin and the IGF axis and breast cancer have mainly concentrated on individual insulin and IGF factors.8–13 The most mainstream previous findings documented a positive association between IGF1 and breast cancer, although such an association could vary according to menopausal status.8–10 A positive but weak association between IGFBP3 and breast cancer has been reported among postmenopausal women, and such an association was more likely attributed to a positive correlation between IGFBP3 and IGF1.9 Epidemiological studies on associations of insulin and C-peptide with breast cancer have yielded contradictory findings, suggesting a positive association,11 an inverse association,12 or no clear association at all.13 These divergent results might be partly due to the heterogeneity of the study populations, the bias introduced by the self-reported diagnosis of breast cancer, and the influence of potential modifiers or confounders.11–13 Our study extends the current evidence by providing novel findings that IGF1, the primary biomarker of the IGF axis, together with insulin, C-peptide, and insulin resistance, were synergistically associated with breast cancer. Interestingly, the significant association between IGF1 and breast cancer was substantially diminished after additionally adjusting for insulin, C-peptide, or HOMA-IR, suggesting that the positive association between IGF1 and breast cancer was largely dependent on the correlations of IGF1 with these insulin markers.
Importantly, in this study, the positive association between IGF1 and breast cancer was mostly seen in the highest quartiles of insulin, C-peptide, and HOMA-IR. The interaction of IGF1 with C-peptide was most significant, followed by a borderline significant interaction with HOMA-IR. C-peptide and HOMA-IR are markers for pancreatic insulin secretion and insulin resistance, respectively. Laboratory studies have reported that increased insulin can upregulate the production and biological activity of IGF1, which in turn overstimulates cellular proliferation and leads to conditions favorable to tumor growth.16–18 Our findings further emphasize the effect modifications of elevated insulin secretion and insulin resistance on the relationship of IGF1 with breast cancer, in keeping with the biological function of insulin and the IGF signaling systems.
Furthermore, several confounders, such as age, BMI, and reproductive factors, including age at menarche and menopausal status, may influence breast cancer risk, partially through their relationships with insulin and IGF1.2 As previously detected, the associations of insulin and IGF1 with breast cancer were more prominent in premenopausal women than in postmenopausal women.9,19-21 IGF1 concentration decreased with increasing age, with no obvious additional decline after approximately 50 years of age.22–24 Thus, it has been speculated that the inverse association between IGF1 and age might be the key determinant of the varied association patterns by menopausal status.9 Age at menarche is an indicator of puberty development and has also been associated with higher concentrations of IGF1.25,26 In addition, a U-shaped relationship between IGF1 and BMI has been described, such that circulating IGF1 was lower in thin and obese individuals than in individuals with normal weight.27–29 In this study, the significant associations of insulin and IGF factors with breast cancer persisted after adjustment for age, BMI, age at menarche, and menopausal status, and these associations did not differ markedly across stratifications of these confounders. Our findings indicate that the positive associations of insulin and IGF markers with breast cancer appear to be independent of these conventional confounders. Currently, anticancer therapeutic strategies, including lifestyle and pharmacological interventions, have aimed to reduce circulating insulin or reduce signaling downstream of insulin and the IGF1 receptors.30–32 Our results further highlight the necessity of considering the interplay between insulin and IGF factors in the prediction and treatment of breast cancer.
The strengths of this study include the relatively large sample size, the pathologically proven breast cancer cases, and the comprehensive measurements of biomarkers of insulin and the IGF axis. Our study also has evident limitations. First, due to a cross-sectional study design, reverse causality may exist. Second, in this study, biomarkers of insulin and the IGF axis as well as HOMA-IR were based on a single measurement for each patient. Thus, random errors associated with assay variation and fluctuations in these measurements within each individual patient may influence the stability of the findings. Third, although we carefully controlled for multiple confounders, residual and unmeasured confounding may still exist; therefore, these findings should be interpreted with caution.
Conclusions
In this large, case-control study, circulating insulin, C-peptide, and IGF1 as well as insulin resistance were positively and synergistically associated with breast cancer in Chinese women. In particular, the association between IGF1 and breast cancer was mainly attributed to effect modifications of insulin, C-peptide, and insulin resistance. Our findings underline the importance of taking into account the synergistic and interactive relations of insulin and IGF factors when developing innovative strategies for targeted prevention and treatment of breast cancer.
Data Sharing Statement
The data are available in coded form at the Comprehensive Breast Health Center, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine.
Ethical Approval and Consent
All patients included in the study (recruited at the Comprehensive Breast Health Center, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China) signed informed consent forms. The study protocol was approved by the Medical Ethics Committee of Ruijin Hospital, Shanghai Jiao Tong University. All participants provided expressed consent for publication of their details. All personal information has been made anonymous.
Acknowledgment
The authors would like to thank Ms. Yidong Du for her assistance in the operation and management of the Shanghai Jiao Tong University Breast Cancer Database.
Author Contributions
All authors made substantial contributions to the study conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
None of the authors has any conflicts of interest to declare in regard to this work.
References
1. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
2. Fan L, Strasser-Weippl K, Li JJ, et al. Breast cancer in China. Lancet Oncol. 2014;15(7):e279289. doi:10.1016/S1470-2045(13)70567-9
3. Kang C, LeRoith D, Gallagher EJ. Diabetes, obesity, and breast cancer. Endocrinology. 2018;159(11):3801–3812. doi:10.1210/en.2018-00574
4. Pollak M. Insulin and insulin-like growth factor signaling in neoplasia. Nat Rev Cancer. 2008;8(12):915–928. doi:10.1038/nrc2536
5. Gallagher EJ, LeRoith D. The proliferating role of insulin and insulin-like growth factors in cancer. Trends Endocrinol Metab. 2010;21(10):610–618. doi:10.1016/j.tem.2010.06.007
6. D’Esposito V, Passaretti F, Hammarstedt A, et al. Adipocyte-released insulin-like growth factor-1 is regulated by glucose and fatty acids and controls breast cancer cell growth in vitro. Diabetologia. 2012;55(10):2811–2822. doi:10.1007/s00125-012-2629-7
7. Christopoulos PF, Msaouel P, Koutsilieris M. The role of the insulin-like growth factor-1 system in breast cancer. Mol Cancer. 2015;14(1):43. doi:10.1186/s12943-015-0291-7
8. Schernhammer ES, Holly JM, Hunter DJ, et al. Insulin-like growth factor-I, its binding proteins (IGFBP-1 and IGFBP-3), and growth hormone and breast cancer risk in the nurses health study II. Endocr Relat Cancer. 2006;13(2):583–592. doi:10.1677/erc.1.01149
9. Key TJ, Appleby PN, et al. Endogenous Hormones and Breast Cancer Collaborative Group. Insulin-like growth factor 1 (IGF1), IGF binding protein 3 (IGFBP3), and breast cancer risk: pooled individual data analysis of 17 prospective studies. Lancet Oncol. 2010;11(6):530–542.
10. Kaaks R, Johnson T, Tikk K, et al. Insulin-like growth factor I and risk of breast cancer by age and hormone receptor status-A prospective study within the EPIC cohort. Int J Cancer. 2014;134(11):2683–2690. doi:10.1002/ijc.28589
11. Verheus M, Peeters PH, Rinaldi S, et al. Serum C-peptide levels and breast cancer risk: results from the European Prospective Investigation into Cancer and Nutrition (EPIC). Int J Cancer. 2006;119(3):659–667. doi:10.1002/ijc.21861
12. Eliassen AH, Tworoger SS, Mantzoros CS, et al. Circulating insulin and c-peptide levels and risk of breast cancer among predominately premenopausal women. Cancer Epidemiol Biomarkers Prev. 2007;16(1):161–164. doi:10.1158/1055-9965.EPI-06-0693
13. Autier P, Koechlin A, Boniol M, et al. Serum insulin and C-peptide concentration and breast cancer: a meta-analysis. Cancer Causes Control. 2013;24(5):873–883. doi:10.1007/s10552-013-0164-6
14. Lakhani S, Ellis I, Schnitt S, et al. WHO Classification of Tumors of the Breast.
15. Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. doi:10.1007/BF00280883
16. Erdem C, Nagle AM, Casa AJ, et al. Proteomic screening and lasso regression reveal differential signaling in insulin and Insulin-like Growth Factor I (IGF1) pathways. Mol Cell Proteomics. 2016;15(9):3045–3057. doi:10.1074/mcp.M115.057729
17. Maki RG. Small is beautiful: insulin-like growth factors and their role in growth, development, and cancer. J Clin Oncol. 2010;28(33):4985–4995. doi:10.1200/JCO.2009.27.5040
18. Massoner P, Ladurner-Rennau M, Eder IE, et al. Insulin-like growth factors and insulin control a multifunctional signalling network of significant importance in cancer. Br J Cancer. 2010;103(10):1479–1484. doi:10.1038/sj.bjc.6605932
19. Schairer C, McCarty CA, Isaacs C, et al. Circulating insulin-like growth factor (IGF)-I and IGF binding protein (IGFBP)-3 levels and postmenopausal breast cancer risk in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial (PLCO) cohort. Hormones Cancer. 2010;1(2):100–111. doi:10.1007/s12672-010-0013-y
20. Gunter MJ, Hoover DR, Yu H, et al. Insulin, insulin-like growth factor-I, and risk of breast cancer in postmenopausal women. J Natl Cancer Inst. 2009;101(1):48–60. doi:10.1093/jnci/djn415
21. Vatten LJ, Holly JM, Gunnell D, et al. Nested case-control study of the association of circulating levels of serum insulin-like growth factor I and insulin-like growth factor binding protein 3 with breast cancer in young women in Norway. Cancer Epidemiol Biomarkers Prev. 2008;17(8):2097–2100. doi:10.1158/1055-9965.EPI-08-0212
22. Brabant G, von Zur Mühlen A, Wüster C, et al. Serum insulin-like growth factor I reference values for an automated chemiluminescence immunoassay system: results from a multicenter study. Horm Res. 2003;60(2):53–60. doi:10.1159/000071871
23. Landin-Wilhelmsen K, Lundberg PA, Lappas G, et al. Insulin-like growth factor I levels in healthy adults. Horm Res. 2004;62(Suppl 1):8–16. doi:10.1159/000080753
24. Zhu H, Xu Y, Gong F, et al. Reference ranges for serum insulin-like growth factor I (IGF-I) in healthy Chinese adults. PLoS One. 2017;12(10):e0185561. doi:10.1371/journal.pone.0185561
25. Probst-Hensch NM, Wang H, Goh VH, et al. Determinants of circulating insulin-like growth factor I and insulin-like growth factor binding protein 3 concentrations in a cohort of Singapore men and women. Cancer Epidemiol Biomarkers Prev. 2003;12(8):739–746.
26. Johansson H, Baglietto L, Guerrieri-Gonzaga A, et al. Factors associated with circulating levels of insulin-like growth factor-I and insulin-like growth factor binding protein-3 in 740 women at risk for breast cancer. Breast Cancer Res Treat. 2004;88(1):63–73. doi:10.1007/s10549-004-0746-9
27. Lukanova A, Söderberg S, Stattin P, et al. Nonlinear relationship of insulin-like growth factor (IGF)-I and IGF-I/IGF-binding protein-3 ratio with indices of adiposity and plasma insulin concentrations (Sweden). Cancer Causes Control. 2002;13(6):509–516. doi:10.1023/A:1016392129279
28. Schneider HJ, Saller B, Klotsche J, et al. Opposite associations of age-dependent insulin-like growth factor-I standard deviation scores with nutritional state in normal weight and obese subjects. Eur J Endocrinol. 2006;154(5):699–706. doi:10.1530/eje.1.02131
29. Völzke H, Nauck M, Rettig R, et al. Association between hepatic steatosis and serum IGF1 and IGFBP-3 levels in a population-based sample. Eur J Endocrinol. 2009;161(5):705–713. doi:10.1530/EJE-09-0374
30. Dowling RJ, Zakikhani M, Fantus IG, et al. Metformin inhibits mammalian target of rapamycin-dependent translation initiation in breast cancer cells. Cancer Res. 2007;67(22):10804–10812. doi:10.1158/0008-5472.CAN-07-2310
31. Venkateswaran V, Haddad AQ, Fleshner NE, et al. Association of diet-induced hyperinsulinemia with accelerated growth of prostate cancer (LNCaP) xenografts. J Natl Cancer Inst. 2007;99(23):1793–1800. doi:10.1093/jnci/djm231
32. Algire C, Zakikhani M, Blouin MJ, et al. Metformin attenuates the stimulatory effect of a high-energy diet on in vivo LLC1 carcinoma growth. Endocr Relat Cancer. 2007;15(3):833–839. doi:10.1677/ERC-08-0038
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




