Back to Journals » International Journal of General Medicine » Volume 14
Biological Significance of 18F-FDG PET/CT Maximum Standard Uptake Value for Predicting EGFR Mutation Status in Non-Small Cell Lung Cancer Patients
Authors Wang Y, Han R, Wang Q, Zheng J , Lin C, Lu C , Li L, Chen H, Jin R , He Y
Received 22 October 2020
Accepted for publication 31 December 2020
Published 3 February 2021 Volume 2021:14 Pages 347—356
DOI https://doi.org/10.2147/IJGM.S287506
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Yubo Wang,1,* Rui Han,1,* Qiushi Wang,2 Jie Zheng,1 Caiyu Lin,1 Conghua Lu,1 Li Li,1 Hengyi Chen,1 Rongbing Jin,3 Yong He1
1Department of Respiratory Medicine, Daping Hospital, Army Medical University, Chongqing, People’s Republic of China; 2Department of Pathology, Daping Hospital, Army Medical University, Chongqing, People’s Republic of China; 3Department of Nuclear Medicine, Daping Hospital, Army Medical University, Chongqing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yong He
Department of Respiratory Medicine, Daping Hospital, Army Medical University, 10# Changjiangzhilu Daping, Yuzhong District, Chongqing, 400042, People’s Republic of China
Tel +86-2368757791
Fax +86-2368700970
Email [email protected]
Rongbing Jin
Department of Nuclear Medicine, Daping Hospital, Army Medical University, 10# Changjiangzhilu Daping, Yuzhong District, Chongqing, 400042, People’s Republic of China
Tel +86-2368757641
Email [email protected]
Purpose: To investigate the potential of maximum standardized uptake value (SUVmax) in predicting epidermal growth factor receptor (EGFR) mutation status in non-small cell lung cancer (NSCLC) patients.
Methods: Clinical data of 311 NSCLC patients who had undergone both EGFR mutation test and 18F-FDG PET/CT scans between January 2013 and December 2017 at our hospital were retrospectively analyzed. Patients were sub-grouped by their origin of SUVmax. Univariate and multivariate analyses were performed to investigate the association between clinical factors and EGFR mutations. Receiver operating characteristic curve (ROC) analysis was performed to confirm the predictive value of clinical factors. In vitro experiments were performed to confirm the correlation between EGFR mutations and glycolysis.
Results: EGFR-mutant patients had higher SUVmax than the wild-type patients in both primary tumors and metastases. In the multivariate analysis, SUVmax, gender and histopathologic type were determined as independent predictors of EGFR mutation status for patients whose SUVmax were obtained from the primary tumors; while for patients whose SUVmax were obtained from the metastases, SUVmax, smoking status and histopathologic type were regarded as independent predictors. ROC analysis showed that SUVmax of the primary tumors (cut off > 10.92), not of the metastases, has better predictive value than other clinical factors in predicting EGFR mutation status. The predict performance was improved after combined SUVmax with other independent predictors. In addition, our in vitro experiments demonstrated that lung cancer cells with EGFR mutations have higher aerobic glycolysis level than wild-type cells.
Conclusion: SUVmax of the primary tumors has the potential to serve as a biomarker to predict EGFR mutation status in NSCLC patients.
Keywords: non-small cell lung cancer, epidermal growth factor receptor, SUVmax, receiver operating characteristic curve
Introduction
Lung cancer is the leading cause of cancer-related death in the world.1 Non-small cell lung cancer (NSCLC), accounts for 80–85% of lung cancer cases, is the most common type of lung cancer. In recent years, epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs), represented by gefitinib, erlotinib and osimertinib, have revolutionized the treatment of NSCLC by prolonging the survival time and improving the quality of life of patients.2,3 Therefore, assessment of EGFR mutation status before treatment is vital for NSCLC patients. Although demographic characteristics such as gender, smoking status and histopathologic type are reported to correlate with the presence of EGFR mutation, they are insufficient to predict it. Current routine gene mutation test often involves invasive procedures such as tissue biopsy; however, it is not always feasible to obtain adequate amount of tissues to perform EGFR mutation test when the primary tumor is not resectable, particularly for advanced-stage NSCLC patients.
In recent years, many studies had focused on the relationship between CT imaging characteristics and EGFR mutation.4–7 Fluorine-18 fluorodeoxyglucose (18F-FDG) positron emission tomography/computed tomography (PET/CT) is an imaging technology widely used in staging or restaging tumors and evaluating the therapeutic response of NSCLC patients.8 The maximum standardized uptake value (SUVmax), an important metabolic parameter used in 18F-FDG PET/CT, can reflect the metabolic activity of tumors.9 Given that the EGFR signaling pathway plays important roles in cell survival and proliferation and the glucose metabolism has close relationship with disease aggressiveness and cell proliferation, researchers assumed that SUVmax may be used to predict the EGFR mutation status.10 In the past few years, several studies have investigated the correlation between SUVmax and EGFR mutation status; however, the value of SUVmax in predicting EGFR mutation status remains controversial. Some reports showed that there was no difference between EGFR-mutants and wild-type lung cancers regarding SUVmax.11,12 In contrast, other evidences indicated that patients with lower13–19 or higher20–22 SUVmax were more likely to have EGFR mutations.
In this retrospective study, we try to explore the potential of SUVmax in predicting EGFR mutation status in NSCLC patients. In addition to the analysis of clinical data, in vitro experiments were also performed to confirm the correlation between EGFR mutations and glycolysis.
Patients and Methods
Patient Selection
Medical records from 311 patients with histopathologically confirmed NSCLC who had undergone EGFR mutation test and 18F-FDG PET/CT scanning prior to receiving treatment between January 2013 and December 2017 at our institute were retrospectively analyzed. Clinical characteristics, including age, gender, smoking history, histopathologic type, clinical stage, EGFR mutation status and SUVmax of the biopsy site, either from the primary tumors or from the metastases, were obtained from each patient. Clinical staging was based on the American Joint Committee on Cancer 7th edition TNM staging system of NSCLC.
EGFR Mutation Test
EGFR mutation test was performed on tissue biopsies of all patients using an amplification refractory mutation system (ARMS) with the ADx-ARMS EGFR Mutation Test Kit (Amoy Diagnostics Co., Ltd., Xiamen, China) as previously described.23
18 F-FDG PET/CT Scanning
18 F-FDG PET/CT scanning was performed in all patients using the Biograph TruePoint 16 PET/CT (Siemens Medical Systems/CTI, Knoxville, TN, USA). All patients were required to fast for 6 h before examination. Serum glucose levels were measured and confirmed to be less than 6.6 mmol/L prior to 18F-FDG injection. One hour before image acquisition, approximately 5.5 MBq/kg of 18F-FDG, provided by the PET Center of the Daping Hospital, was intravenously administered to each patient. After an initial low-dose non-contrast CT scan, standard PET imaging was performed with an acquisition time of 2.5 min/bed at three-dimensional mode. Images were then reconstructed using an iterative reconstruction algorithm. All PET/CT images were reviewed by an experienced nuclear physician on a GE AW 4.0 workstation (GE Healthcare, Milwaukee, WI, USA). Volume of interest (VOI) was drawn on the biopsies to derive SUVmax as previously described.24 18 F-FDG PET/CT scanning was performed in accordance with the Declaration of Helsinki. Written informed consent was obtained from all patients. Data collection was retrospectively performed and approved by the Ethical Committee of the Army Medical University (ethics approval number: 2,020,101).
Cell Culture
Human NSCLC cell lines PC-9 (del19), H1299 (wild-type) and H460 (wild-type) were cultured in RPMI-1640 (Hyclone) with Earle’s salts at 37°C, with 5% CO2 and 90% humidity. RPMI-1640 medium was supplemented with 10% FBS (Gibco), 2 mmol/L L-glutamine (Gibco), 100U/mL penicillin (HyClone) and 100µg/mL streptomycin (Hyclone). All cell lines were obtained from ATCC (Manassas, VA, USA) except for PC-9 which was generously provided by Prof. J. Xu and Dr. M. Liu (Guangzhou Medical University, Guangzhou, China). PC-9 cell line authentication was performed by Shanghai OrigiMed Clinical Laboratory Co., Ltd (Shanghai, China). The use of PC-9 cell line was recorded (not approved due to lack of authority) by the Ethical Committee of the Army Medical University.
Lactate Production and Glucose Consumption
After 48h culture, lactate and glucose levels of H460 and PC-9 cells were quantified using the K627-100 Lactate Assay kit (BioVision, Mountain View, CA, USA) and K686-100 Glucose Assay kit (BioVision, Mountain View, CA, USA) according to the manufacturer’s instructions. All experiments were performed in triplicate.
FLAG-Tagged EGFR Expression
EGFR wild-type H460 and H1299 cells were transiently transfected with empty vector or FLAG-tagged plasmids containing either wild-type EGFR or mutant EGFR (del19 or L858R) using Lipofectamine 2000 (Life Technologies, CA, USA) according to the manufacturer’s instructions. Western blot was performed to detect the exogenous expression of EGFR. Glucose consumption and lactate production experiments were performed 48h after transfection. All experiments were performed in triplicate.
Statistical Analysis
Continuous variables and categorical variables were analyzed using Student’s t-test and Pearson’s chi-square test, respectively. Significant parameters were selected for further multivariate logistic regression analysis to investigate the association between clinical features and EGFR mutation status. Receiver operating characteristic (ROC) curve analysis was performed using the MedCalc version 12.3 Software (Mariakerke, Belgium). All analyses except for ROC curve analysis were performed using SPSS 19.0 (SPSS Incorporated, Chicago, IL, USA). A p value less than 0.05 was considered statistically significant.
Results
Patient Characteristics
Of the 311 patients, 60.8% (189/311) were males and 39.2% (122/311) were females. The majority of patients were diagnosed with adenocarcinoma (74.9%, 233/311) and squamous carcinoma (14.1%, 44/311). There were 12.9% (40/311) with stage I–IIIa, 15.8% (49/311) with stage IIIb and 71.4% (222/311) with stage IV. 41.2% (128/311) of patients were EGFR-mutant and 58.8% (183/311) were EGFR wild-type. Among the 128 EGFR-mutant patients, majority had mutation either on exon 19 (49.2%, 63/128) or exon 21 (43.0%, 55/128). In addition, 1 patient (0.78%) had mutation on exon 18, 1 patient (0.78%) had mutation on exon 20 and 8 patients (6.25%) had multiple EGFR mutations. The clinical features of participants are listed in Table 1.
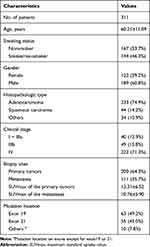 |
Table 1 Baseline Clinical Features of the Included Patients |
Correlation Between SUVmax and EGFR Mutation Status
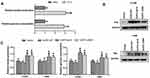
Interestingly, our results showed that the mean SUVmax of the primary tumors was significantly higher than that of the metastases (12.31±6.52 vs 10.76±5.90, P=0.038). To explore the correlation between SUVmax and EGFR mutation status in NSCLC patients, we divided patients into two groups according to origin of SUVmax. Our results showed that EGFR-mutant patients had higher SUVmax than the wild-type patients in both primary tumors (Figure 1A) and metastases (Figure 1C). Similar results also observed in the analyses based on EGFR mutation spectra, in which patients with exon 19 or exon 21 mutation or other mutations had higher SUVmax than the wild-type patients in both primary tumors (Figure 1B) and metastases (Figure 1D).
Univariate and Multivariate Analyses of Clinical Features
Considering the primary tumors had significant higher SUVmax than the metastases, to avoid the influence of heterogeneity, following univariate and multivariate analyses were performed on different patients stratified by the origin of SUVmax. As shown in Tables 2 and 3, in the univariate analyses, SUVmax, gender, smoking status and histopathologic type were significantly associated with EGFR mutation status; while, in the multivariate analyses, SUVmax, gender and histopathologic type were determined as independent predictors of EGFR mutation status in patients whose SUVmax were obtained from the primary tumors, and SUVmax, smoking status and histopathologic type were independent predictors of EGFR mutation status in patients whose SUVmax were obtained from the metastases.
 |
Table 2 Univariate and Multivariate Analyses for NSCLC Patients with SUVmax Obtained from Primary Tumors |
 |
Table 3 Univariate and Multivariate Analyses for NSCLC Patients with SUVmax Obtained from Metastases |
ROC Curve Analysis
Based on the multivariate analyses results, ROC curve analyses were performed to determine the discrimination values of these clinical features to predict EGFR mutation status. Our results showed that, in patients whose SUVmax was obtained from the primary tumors, SUVmax (cut off >10.92; AUC=0.773; sensitivity=78.2%; specificity=66.4%; 95% CI=0.709 to 0.829) has better EGFR mutation predictive value than gender (cut off ≤0; AUC=0.640, sensitivity=56.3%; specificity=71.7% and 95% CI=0.569 to 0.707) and histopathologic type (cut-off of >0; AUC=0.602; sensitivity =85.1%; specificity=35.4%; 95% CI=0.531 to 0.761). A combined variable, derived by including all three factors into a logistic regression analysis, can better predict EGFR mutation status (cut off >0.3003, AUC=0.825, sensitivity=87.4%, specificity =63.7% and 95% CI=0.766 to 0.875) (Figure 2A). In patients whose SUVmax were obtained from the metastases, SUVmax (cut off >10.6; AUC=0.728; sensitivity=70.7%; specificity=70.0%; 95% CI=0.636 to 0.808) has comparable EGFR mutation predictive value with smoking status (cut off ≤0; AUC=0.682, sensitivity=70.7%; specificity=65.7% and 95% CI=0.587 to 0.767) and histopathologic type (cut-off of >0; AUC=0.659; sensitivity =73.2%; specificity=34.3%; 95% CI=0.563 to 0.747). The predictive value was significantly improved after combined SUVmax with smoking status and histopathologic type (cut off >0.4852, AUC=0.859, sensitivity=73.2%, specificity =85.7% and 95% CI=0.780 to 0.918) (Figure 2B).
In vitro Investigation of Association Between EGFR Mutation and Glycolysis
In vitro, we further investigated the association between EGFR mutation and glycolysis. Our results revealed that EGFR-mutant PC-9 cells had significantly higher glucose consumption and lactate production than EGFR wild-type H460 cells (P<0.05, Figure 3A). To further elucidate the role of EGFR mutation on glycolysis, we transfected EGFR wild-type NSCLC cell lines H1299 and H460 with plasmids carrying wild-type EGFR or mutant EGFR including del19 or L858R. Our results revealed that the exogenous expression of EGFR was increased after plasmids transfection (Figure 3B); besides, the levels of glucose consumption and lactate production were significantly higher in both H1299 and H460 cell lines transiently expressing EGFR exon 19 deletion or L858R mutation than those transfected with wild-type EGFR or vector (P<0.05, Figure 3C). These results demonstrated that the NSCLC cells harboring common EGFR sensitizing mutations have higher glycolysis phenotype than the wild-type NSCLC cells.
Discussion
In the present study, our results demonstrated that EGFR-mutant patients have higher SUVmax than the wild-type patients in both primary tumors and metastases. Interestingly, SUVmax of the primary tumors was significantly higher than that of the metastases. For patients whose SUVmax was obtained from the primary tumors, SUVmax has better EGFR mutation predictive value than gender and histopathologic type; while for patients whose SUVmax was obtained from the metastases, the predictive value of SUVmax was comparable with that of the smoking status and histopathologic type. The predict performance was significantly improved after combined SUVmax with other independent predictors. Our in vitro studies also demonstrated that EGFR-mutant lung cancer cells have higher aerobic glycolysis level than EGFR wild-type cells.
It is well known that EGFR mutation status of lung cancer is very important because it indicates induction of EGFR-TKIs treatment. Although evidences have suggested that EGFR mutation was related with clinical features such as adenocarcinoma, female, never-smoker and Asians,25 more specific and accurate factors are needed to help predict EGFR mutation. The potential of SUVmax as a predictor for EGFR mutation status has attracted more attention in recent years. Putora et al11 and Lee et al12 found no statistical difference in the SUVmax between the EGFR-mutant and EGFR wild-type lung cancers, concluding that SUVmax cannot predict EGFR mutation status. In contrast, more studies had suggested that SUVmax was significantly correlated with EGFR mutation status but with opposite findings. Several studies have reported that patients with low SUVmax, with cut-off values from ≤2.69 to <11.5, were more likely to have EGFR mutations than those with high SUVmax.13–19 In contrary, some studies have reported that patients with higher SUVmax, with cut-offs from ≥6 to >13.65, were more likely to have EGFR mutations.20–22 Our results are in favor that patients with higher SUVmax were more likely to have EGFR mutations. The discrepancy of these clinical studies maybe related to many factors such as different evaluation protocol and ethnicity/histopathologic type of included patients. Detailed information about the above-mentioned studies is summarized in Table 4.
 |
Table 4 Summary of the Clinical Studies Evaluated the Relationship Between SUVmax and EGFR Mutation Status |
One of the highlights of the present study is that we divided patients into two groups and separately analyzed the potential of SUVmax in predicting EGFR mutation status because the metabolic phenotype of the primary tumors differs from that of metastases, which was consistent with the observations of Lee et al;26 however, they drew different conclusion from ours and suggested that SUVmax ≤7.2 in metastasis could predict EGFR mutations with high specificity in stage IV lung adenocarcinoma patients. In the present study, we found EGFR-mutant patients had higher SUVmax than the wild-type patients in both primary tumors and the metastases. Notably, SUVmax obtained from the primary tumors, not from the metastases, has better predictive value than other clinical features in predicting EGFR mutation status. When combined SUVmax with other clinical factors such as gender, smoking status and histopathologic type, the prediction ability was profoundly improved.
SUVmax obtained from PET/CT is a semi-quantitative metabolic parameter that indicates the degree of aerobic glycolysis in tumor.27 Considering the controversial correlation between SUVmax and EGFR mutation status reported in previous studies, we further used human lung cancer cell lines that harbored EGFR mutation or transiently expressing FLAG-tagged EGFR mutations such as del19 and L858R to confirm the relationship between EGFR mutation and aerobic glycolysis. Our in vitro experiments showed that EGFR-mutant NSCLC cells have higher aerobic glycolysis level as compared with the wild-type cells. This was consistent with our clinical observations that EGFR-mutant patients had higher SUVmax value than the wild-type patients. In a study performed by Kim et al, authors found that EGFR-mutant NSCLCs had significant higher glucose uptake and lactate production compared with wild-type NSCLCs. They concluded that EGFR mutation-regulated glycolysis enhancement was required for fueling the tricarboxylic acid cycle which was essential for survival of EGFR mutant NSCLCs.28 In addition, other studies also demonstrated that EGFR mutation can increase glycolysis and promotes glucose consumption via activation of the Akt pathway.29,30 Taken together, these evidences indicated that EGFR mutant NSCLCs probably had higher aerobic glycolysis level, in other words, higher SUVmax value, than the wild-type NSCLCs.
Although SUVmax seems as a promising factor for predicting EGFR mutation status, our findings must be interpreted in the context of the retrospective nature of the study, which may introduce biases. In the future, large-scale, prospective studies are warranted to further validate the value of SUVmax in predicting EGFR mutations.
Conclusions
SUVmax of the primary tumors has the potential to serve as a biomarker to predict EGFR mutation status in NSCLC patients.
Acknowledgments
We thank Dr. Analyn Lizaso of Burning Rock Biotech for editorial assistance and valuable discussion.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
This work was supported by grants from National Nature Science Foundation of China (grant numbers 81672284, 81702291).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Ujiie H, Yasufuku K. The importance of novel molecular biomarker of early stage lung adenocarcinoma. Transl Cancer Res. 2017;6:S964–S968. doi:10.21037/tcr.2017.06.12
2. Cadranel J, Ruppert AM, Beau-Faller M, Wislez M. Therapeutic strategy for advanced EGFR mutant non-small-cell lung carcinoma. Crit Rev Oncol Hematol. 2013;88(3):477–493. doi:10.1016/j.critrevonc.2013.06.009
3. Patel N, Wu P, Zhang H. Comparison of gefitinib as first- and second-line therapy for advanced lung adenocarcinoma patients with positive exon 21 or 19 del epidermal growth factor receptor mutation. Cancer Manag Res. 2017;9:243–248. doi:10.2147/CMAR.S138643
4. Liu Y, Kim J, Qu F, et al. CT features associated with epidermal growth factor receptor mutation status in patients with lung adenocarcinoma. Radiol. 2016;280(1):271–280. doi:10.1148/radiol.2016151455
5. Hasegawa M, Sakai F, Ishikawa R, Kimura F, Ishida H, Kobayashi K. CT features of epidermal growth factor receptor–mutated adenocarcinoma of the lung: comparison with nonmutated adenocarcinoma. J Thorac Oncol. 2016;11(6):819–826. doi:10.1016/j.jtho.2016.02.010
6. Dai J, Shi J, Soodeen-Lalloo AK, et al. Air bronchogram: a potential indicator of epidermal growth factor receptor mutation in pulmonary subsolid nodules. Lung Cancer. 2016;98:22–28. doi:10.1016/j.lungcan.2016.05.009
7. Cheng Z, Shan F, Yang Y, Shi Y, Zhang Z. CT characteristics of non-small cell lung cancer with epidermal growth factor receptor mutation: a systematic review and meta-analysis. BMC Med Imaging. 2017;17(1):5. doi:10.1186/s12880-016-0175-3
8. Pauwels EK, Coumou AW, Kostkiewicz M, Kairemo K. [(1)(8)F]fluoro-2-deoxy-d-glucose positron emission tomography/computed tomography imaging in oncology: initial staging and evaluation of cancer therapy. Med Princ Pract. 2013;22(5):427–437. doi:10.1159/000346303
9. Wang H, Li J, Chen F, et al. Morphological, functional and metabolic imaging biomarkers: assessment of vascular-disrupting effect on rodent liver tumours. Eur Radiol. 2010;20(8):2013–2026. doi:10.1007/s00330-010-1743-5
10. Gandhi J, Zhang J, Xie Y, et al. Alterations in genes of the EGFR signaling pathway and their relationship to EGFR tyrosine kinase inhibitor sensitivity in lung cancer cell lines. PLoS One. 2009;4(2):e4576. doi:10.1371/journal.pone.0004576
11. Putora PM, Fruh M, Muller J. FDG-PET SUV-max values do not correlate with epidermal growth factor receptor mutation status in lung adenocarcinoma. Respirology. 2013;18(4):734–735. doi:10.1111/resp.12083
12. Lee SM, Bae SK, Jung SJ, Kim CK. FDG uptake in non-small cell lung cancer is not an independent predictor of EGFR or KRAS mutation status: a retrospective analysis of 206 patients. Clin Nucl Med. 2015;40(12):950–958. doi:10.1097/RLU.0000000000000975
13. Na II, Byun BH, Kim KM, et al. 18F-FDG uptake and EGFR mutations in patients with non-small cell lung cancer: a single-institution retrospective analysis. Lung Cancer. 2010;67(1):76–80. doi:10.1016/j.lungcan.2009.03.010
14. Guan J, Xiao NJ, Chen M, et al. 18F-FDG uptake for prediction EGFR mutation status in non-small cell lung cancer. Medicine. 2016;95(30):e4421. doi:10.1097/MD.0000000000004421
15. Cho A, Hur J, Moon YW, et al. Correlation between EGFR gene mutation, cytologic tumor markers, 18F-FDG uptake in non-small cell lung cancer. BMC Cancer. 2016;16:224. doi:10.1186/s12885-016-2251-z
16. Takamochi K, Mogushi K, Kawaji H, et al. Correlation of EGFR or KRAS mutation status with 18F-FDG uptake on PET-CT scan in lung adenocarcinoma. PLoS One. 2017;12(4):e0175622. doi:10.1371/journal.pone.0175622
17. Lv Z, Fan J, Xu J, et al. Value of (18)F-FDG PET/CT for predicting EGFR mutations and positive ALK expression in patients with non-small cell lung cancer: a retrospective analysis of 849 Chinese patients. Eur J Nucl Med Mol I. 2018;45(5):735–750. doi:10.1007/s00259-017-3885-z
18. Gao X-C, Wei C-H, Zhang R-G, et al. 18 F-FDG PET/CT SUV max and serum CEA levels as predictors for EGFR mutation state in Chinese patients with non-small cell lung cancer. Oncol Lett. 2020;20(4):61. doi:10.3892/ol.2020.11922
19. Zhu L, Yin G, Chen W, et al. Correlation between EGFR mutation status and F18-fluorodeoxyglucose positron emission tomography-computed tomography image features in lung adenocarcinoma. Thoracic Cancer. 2019;10(4):659–664. doi:10.1111/1759-7714.12981
20. Huang CT, Yen RF, Cheng MF, et al. Correlation of F-18 fluorodeoxyglucose-positron emission tomography maximal standardized uptake value and EGFR mutations in advanced lung adenocarcinoma. Med Oncol. 2010;27(1):9–15. doi:10.1007/s12032-008-9160-1
21. Ko KH, Hsu HH, Huang TW, et al. Value of (1)(8)F-FDG uptake on PET/CT and CEA level to predict epidermal growth factor receptor mutations in pulmonary adenocarcinoma. Eur J Nucl Med Mol I. 2014;41(10):1889–1897. doi:10.1007/s00259-014-2802-y
22. Kanmaz ZD, Aras G, Tuncay E, et al. Contribution of (1)(8)Fluorodeoxyglucose positron emission tomography uptake and TTF-1 expression in the evaluation of the EGFR mutation in patients with lung adenocarcinoma. Cancer Biomark. 2016;16(3):489–498. doi:10.3233/CBM-160588
23. Wang Q, Mou J, Yang X, et al. EGFR mutations in patients with lung adenocarcinoma in southwest China: are G719S/A and L861Q more likely detected in tumors derived from smokers? Lung Cancer. 2013;4:27–33.
24. Chong GO, Lee YH, Hong DG, Cho YL, Lee YS. Unabsorbed polylactide adhesion barrier mimicking recurrence of gynecologic malignant diseases with increased 18F-FDG uptake on PET/CT. Arch Gynecol Obst. 2015;292(1):191–195. doi:10.1007/s00404-014-3587-8
25. Usuda K, Sagawa M, Motono N, et al. Relationships between EGFR mutation status of lung cancer and preoperative factors - are they predictive? Asian Pac J Cancer Prev. 2014;15(2):657–662. doi:10.7314/APJCP.2014.15.2.657
26. Lee EYP, Khong P-L, Lee VHF, Qian W, Yu X, Wong MP. Metabolic phenotype of stage IV lung adenocarcinoma: relationship with epidermal growth factor receptor mutation. Clin Nucl Med. 2015;40(3):e190–e195. doi:10.1097/RLU.0000000000000684
27. Yu C, Xia X, Qin C, Sun X, Zhang Y, Lan X. Is SUVmax helpful in the differential diagnosis of enlarged mediastinal lymph nodes? A pilot study. Contrast Media Mol I. 2018;2018:3417190. doi:10.1155/2018/3417190
28. Kim JH, Nam B, Choi YJ, et al. Enhanced glycolysis supports cell survival in EGFR-mutant lung adenocarcinoma by inhibiting autophagy-mediated EGFR degradation. Cancer Res. 2018;78(16):4482. doi:10.1158/0008-5472.CAN-18-0117
29. Xu W, Yin G, Zhang Y. Predictive power of a radiomic signature based on 18F-FDG PET/CT images for EGFR mutational status in NSCLC. Front Oncol. 2019;9:1062. doi:10.3389/fonc.2019.01062
30. Yip SS, Kim J, Coroller TP, et al. Associations between somatic mutations and metabolic imaging phenotypes in non–small cell lung cancer. J Nucl Med. 2017;58(4):569. doi:10.2967/jnumed.116.181826
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.