Back to Journals » International Journal of Nanomedicine » Volume 17
Biological Effects, Applications and Design Strategies of Medical Polyurethanes Modified by Nanomaterials
Authors Wang J, Dai D, Xie H, Li D, Xiong G, Zhang C
Received 14 October 2022
Accepted for publication 20 December 2022
Published 29 December 2022 Volume 2022:17 Pages 6791—6819
DOI https://doi.org/10.2147/IJN.S393207
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Anderson Oliveira Lobo
Jianrong Wang,* Danni Dai,* Hanshu Xie, Dan Li, Gege Xiong, Chao Zhang
Stomatological Hospital, Southern Medical University, Guangzhou, 510280, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Chao Zhang, Email [email protected]
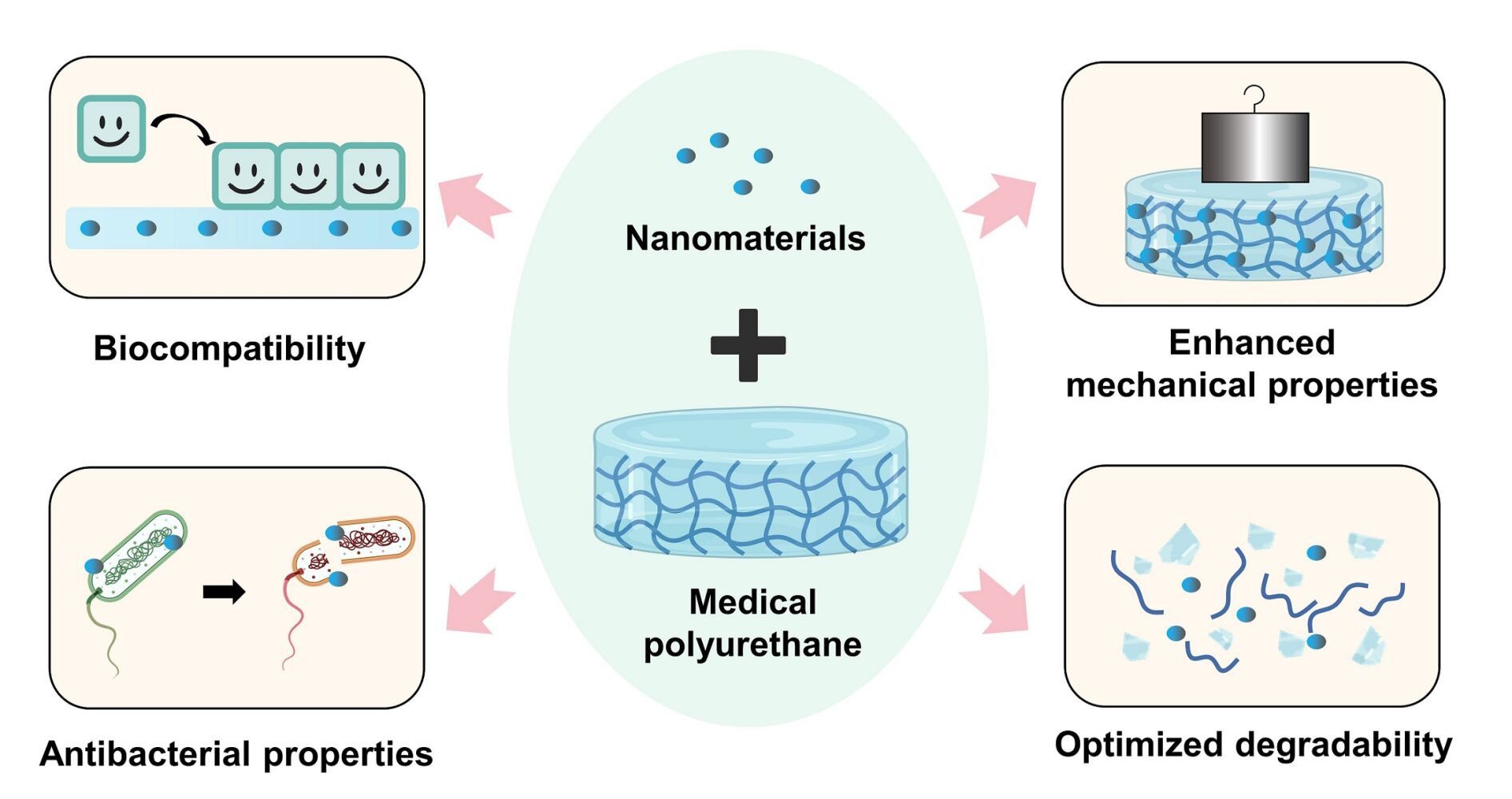
Abstract: Polyurethane (PU) has wide application and popularity as medical apparatus due to its unique structural properties relationship. However, there are still some problems with medical PUs, such as a lack of functionality, insufficient long-term implantation safety, undesired stability, etc. With the rapid development of nanotechnology, the nanomodification of medical PU provides new solutions to these clinical problems. The introduction of nanomaterials could optimize the biocompatibility, antibacterial effect, mechanical strength, and degradation of PUs via blending or surface modification, therefore expanding the application range of medical PUs. This review summarizes the current applications of nano-modified medical PUs in diverse fields. Furthermore, the underlying mechanisms in efficiency optimization are analyzed in terms of the enhanced biological and mechanical properties critical for medical use. We also conclude the preparation schemes and related parameters of nano-modified medical PUs, with discussions about the limitations and prospects. This review indicates the current status of nano-modified medical PUs and contributes to inspiring novel and appropriate designing of PUs for desired clinical requirements.
Graphical Abstarct:
Keywords: polyurethane, nanomaterial, modification, medical application
Graphical Abstract:
Introduction
Polyurethanes (PUs) are multiblock polymers composed of polyols, isocyanates, and chain extenders; polyols form the soft segment and the latter two components form the hard segment of these materials (Figure 1). By regulating the components’ types, proportion and distribution, it is flexible to produce PUs with desired properties to a certain degree. The soft segment endows PUs with flexibility and biodegradability, and the hard segment confers stable thermodynamic properties due to the link between urethane and hydrogen bonds.1 Such thermodynamic incompatibility between the soft and hard segments makes PUs’ unique microphase separation structure, which endows them with changeable physicochemical properties and structures. Up until now, PUs have been made into various medical apparatus (eg foams, coatings, fibers, hydrogels, and elastomers) and been used in biomedical fields for stents, catheters, drug-delivery vehicles, etc. (Figure 2).2,3 PUs have been used as stents since they could be easily made into a three-dimensional porous structure with high permeability and porosity, promoting uniform cell distribution and the spread of nutrients. PU-based catheters have excellent elasticity and hydrolysis resistance to be used as catheters in the urinary system, cardiovascular system, digestive system, and so on. Table 1 shows the representative list of the food and drug administration (FDA)-approved medical PUs. Moreover, biodegradable PUs are deemed efficient and safe drug-delivery vehicles. However, their clinical application is limited due to their vulnerability to microbial attack, lack of bioactivity, insufficient histocompatibility, unaccommodating long-time mechanical properties, and degradation trend.4,5 More importantly, owing to the particular microphase separation structure, PUs are expected to design as multifunctional medical apparatus and could have become promising therapeutic candidates for long-term implants, controllable drug delivery system, and tissue engineering. Thus, PUs must follow certain modifications to optimize their clinical effectiveness and expand the application fields.6–8
 |
Table 1 List of FDA-Approved Medical PUs |
 |
Figure 1 Synthesis (A) and structure (B) of a typical polyurethane, and microphase separation morphology (C). |
With the development of nanotechnology, nanomodification has provided new opportunities for optimizing the properties of PUs critical for medical use.9–12 On one hand, making use of the small size effect of NMs to combine with PUs could limit the flow of the soft and hard PU segments that regulate physicochemical properties.13 Since NMs themselves possess excellent thermal conductivity, electrical conductivity, mechanical strength, and considerable stability, their addition further enhances the physicochemical properties of medical PU.14 On the other hand, the inherent biological (eg antibacterial, osteogenic) effects of NMs confer PUs enhanced biological efficiency, which is favorable in biomedical fields.15,16 The nanostructures endowed by NMs were evidenced to promote cell adhesion, proliferation, and differentiation.17 The combination of medical PU and NM could optimize the drug loading capacity of PU and realized the slow drug release due to the high specific surface area of NM.18 Currently, the introduction of NMs has been found to markedly optimize the mechanical properties, biocompatibility, antibacterial effects, and biodegradation of PUs to improve application ranges and efficacy, which has attracted much attention in the past decade for the development of products such as tissue engineering scaffolds, wound dressings, and drug-delivery systems.1,13,19–22 Nonetheless, there needs to be a more comprehensive review to summarize the application scenarios and scope of these nano-modified medical PUs and explain the optimizing strategy and underlying mechanism from the perspective of particular effects.
This review summarizes the advantages of nano-modified medical PUs and research progress in diverse clinical areas, as well as the effects and mechanisms of action of different kinds of NM that improve PU performance. The preparation methods and parameters for nano-modified medical PUs are also described in detail. This review aims to understand how NM enhances the biological and mechanical properties of medical PUs, paying special attention to the underlying mechanism, which may provide guidance for the design, optimization, and clinical application of these materials.
Advantages and Potential Application of Nano-Modified Medical PUs as Candidates in Clinic Areas
Novel nano-modified medical PUs are being considered qualified candidates in many clinical areas, as they make up the drawbacks of currently used PUs and endow them with new desired properties. Their biological and physicochemical properties are summarized in Tables 2 and 3.
 |
Table 2 Enhanced Biological Properties of PU Nanocomposites and Their Main Applications |
 |
Table 3 Enhanced Physicochemical Properties of PU Nanocomposites and Their Main Applications |
Bone Tissue Engineering
Flexible PU elastomers have been applied in the repair of cartilage defects in areas such as the maxillofacial region, throat, and meniscus.23–26 However, drawbacks such as unmatched stiffness and strength, insufficient osteoconduction, and the lack of antibacterial activity affect PU use in the treatment of load-bearing bone defects and chronic osteomyelitis.27–29 First, rigid nanoparticles (NPs) such as titanium dioxide (TiO2)-NPs are stronger and stiffer than PUs, and their addition in PUs could obtain higher tensile strength useful for load-bearing bone tissue.30 Second, nano-hydroxyapatite (nHA) and bioactive glass (BG)-NPs, as popular calcium-based NMs, are known for osteoinductive activity, whose introduction by forming covalent bonds with PUs enhances the mechanical strength as well as osteogenic activity, resulting in broadening the application of PU scaffolds.20,27,31 Moreover, nHA and BG-NPs have nanostructures similar to that of natural bone, which is attractive for osteoblasts to adhere to. Their nanoscale surface also provides a rough and hydrophilic surface for osteoblast growth and differentiation.32–34 Remarkedly, nano-fluorohydroxyapatite was found to increase the compressive strength of a polyester PU scaffold by 370 kPa and the compressive modulus by 500 kPa via enhanced hydrogen bonding.35 The introduction of fluorine in nHA also reduced the solubility of apatite and promoted the mineralization and crystallization of calcium phosphate compounds during bone regeneration, which facilitated the maintenance of long-term mechanical strength and an environment favoring bone regeneration during the repair of load-bearing bone defects. PU scaffolds modified with BG-NPs, which have osteogenic effects similar to that of nHA, induce osteogenic differentiation through the upregulation of the bone endostatin gene Runx2, promote the deposition of calcium ions, form hydroxyapatite layers in-vivo, and accelerate biomineralization in defective bone tissue.36
Recent research has indicated that the use of PU scaffolds modified with NMs loaded with biomolecules (such as osteogenic peptides) enables the achievement of a steady loading effect to induce osteoblast adhesion, proliferation, and differentiation and promote extracellular matrix (ECM) production.37,38 Carbon nanodots (CDs) can be used to load sequential peptides for cell adhesion, osteoblast differentiation, osteogenesis, and angiogenesis through the carboxylic group (–COOH), and they form carbamate bonds with urethane to create stable peptide-loading systems.24 Scaffolds with CDs have been shown to increase alkaline phosphatase activity by one-fold compared with pure PU scaffolds; to promote the reconstruction of defective bone tissue by regulating the recruitment of bone-marrow mesenchymal stem cells and preosteoblasts; and to stimulate osteoblast adhesion, proliferation, and differentiation in a targeted manner.24
As osteogenesis is not ideal in infected areas, the addition of antimicrobial components to regeneration scaffolds is desirable. PUs alone have low drug loading rates and limited capacity for the controlled release of antimicrobial drugs.39 The modification of PU scaffolds with osteogenic NPs that loads antimicrobial components such as antibiotics and silver ion (Ag+) could improve osteogenic effects and antimicrobial properties at the same time.23,40–42 An nHA/PU scaffold containing 3% Ag was proved suitable for the repair of chronic osteomyelitis since it achieved the slow release of Ag+ for up to 39 days, with significant increases in the number of new bone trabeculae (1.486 ± 0.129/mm) and bone thickness (0.140 ± 0.095 um at 12 weeks) compared with the use of pure PU scaffolds.23
Cardiovascular System
Given their excellent flexibility, wear resistance, and easy formability, PUs meet the tensile strength and elastic modulus requirements for the fluid environments of applications in the cardiovascular system.43,44 Thus, they are used to prepare prostheses such as artificial blood vessels and heart valves by electrostatic spinning and three-dimensional printing.45 However, PU exhibits hydrolytic degradation and thrombogenicity when in contact with blood components, such as platelets and cholesterol esterase.46–48 Surface endothelialization by endothelial cells to block coagulation cascade reactions and prevent platelet activation and aggregation is the key to preventing thrombogenesis and improving the hemocompatibility of implant materials in the cardiovascular system.45 The addition of graphene oxide (GO), carbon NPs, and Fe-NPs to PUs yields rough, hydrophilic surfaces that promote endothelial cell adhesion and proliferation.45,46,49,50 The hydrophilic hydroxyl and carboxyl groups in GO/PU nanocomposites prepared by plasma treatment reduce the water contact angle to 0°, thereby promoting the adhesion and proliferation of human umbilical-vein endothelial cells (HUVECs).45 In particular, PU modified by Fe-NPs could release Fe3+ to promote the adhesion of endothelial cells and enhance endothelial cell activity via attracting surface bovine serum albumin (BSA) and type I collagen, and form a micro-magnetic field, respectively, thus resulting in expected endothelialization and vascularization.50
The long-term stability of cardiovascular system implant PUs can also be fulfilled by constructing smooth hydrophobic surfaces to inhibit platelet and protein adhesion. The introduction of NMs to PU coating aids the steady release of nitric oxide (NO, a vasodilator) or heparin (an endogenous platelet inhibitor), whose application was severely limited due to poor long-term stability.51 PU doping with S-nitrosothiol (RSNO)-modified mesoporous silica nanoparticles (MSNs) increases NO loading and prolongs the duration of NO release.51,52 Given the large internal surface area of MSNs, RSNO groups stabilize in the pores through the “cage effect” and produce NO in situ at the polymer–blood interface, which plays a significant role in thromboprophylaxis. Besides preventing thrombogenesis, nano-modified PUs have advantages in avoiding hydrolytic degradation simultaneously. The silica-rich pendant groups of polyhedral oligomeric silsesquioxane (POSS) NPs fill the surface deformations of PU chains to create a smooth hydrophobic surface with a 9.45% increase in the water contact angle and 76.7% decrease in surface roughness, which inhibits platelet and protein adhesion, thereby preventing thrombogenesis.5 Furthermore, the addition of POSS NPs introduces strong, stable silicone–oxygen bonds into the hard segments of PU, increasing structural cross-linkage and limiting the flow of soft segments; this approach yields a substrate with a high degree of resistance to degradation (oxidation and hydrolysis) while maintaining the flexibility and elasticity of PU. In addition to solving those common problems of cardiovascular implant PUs, NMs also broaden their application range in this field. For example, NM-modified deformation memory PU with x-ray visibility was a promising therapeutic candidate for hemangioma occlusion. The incorporation of tungsten NMs into PU foams enabled control of the transition temperature and composite chain segment mobility, thereby enabling self-expansion for occlusion and blockage of the aneurysm blood supply in-vivo.53
Wound Dressing
PU wound dressings (foams, nanofiber pads, and films), which are economical and have excellent mechanical strength, promote hemostasis and aid exudate removal due to their ideal water absorption capacity (water vapor transmission rate >76 g/m2/day, porosity >90%).54–56 To compensate for the cytocompatibility and antibacterial shortcomings of PU, bioactive NMs such as silicon dioxide (SiO2)-, Ag-, and zinc oxide (ZnO)-NPs and GO have been introduced to promote cell proliferation and endow PU dressings with antibacterial properties, thereby promoting wound healing overall.54,57,58
On the one hand, the addition of NMs in PUs would stimulate the cellular pro-healing effects. GO plays a significant role in promoting wound healing by improving the hydrophilicity of PU composite scaffolds and providing an ideal electrophysiological environment for wound fibroblast adhesion and proliferation.59 The incorporation of SiO2-NPs enabled maintenance of the water absorption capacity and vapor permeability (>800 g/m2/day) of PU composite foam matrices, with the continuous provision of about 80 ppm water absorption, while increasing the stiffness by 1.5–2 times for adaptation to human skin (0.85 MPa under strain, 0.42 MPa under torsion).54 More importantly, these dressings provided about 80 ppm silicon ions persistently, promoting collagen regeneration and collagen fiber deposition, inducing the recruitment of inflammatory cells and secretion of growth factors [eg tumor necrosis factor-α (TNF-α), transforming growth factor (TGF)], and stimulating the expression of kinase insert domain receptor, the vascular endothelial growth factor receptor on HUVECs, resulting in the upregulation of NO expression and initiation of angiogenesis and thereby shortening the wound healing period.54
As for those infected wounds, PU foams loaded with ZnO-NPs reduced biofilm formation by common skin infection flora (eg Pseudomonas aeruginosa PAO1 and methicillin-resistant Staphylococcus aureus) by 95% through the activation of the oxidative stress response, thereby accelerating infected wound healing.58 PUs modified by Ag-NPs in combination with traditional wound-healing herbs (asiaticoside, badger oil, and curcumin) synergistically exerted anti-inflammatory and fibroblast proliferative effects, promoting the healing of infected wounds.55,56,60 Sodium alginate adsorbed with Ag-NPs and Centella asiatica glycosides enhanced the antibacterial activity of a PU nanocomposite foam in which they were compounded and induced type I collagen synthesis through phosphorylation of the Smad pathway, leading to 100% wound re-epithelialization in 12 days.60 Badger oil and Ag-NPs synergistically enhance the hydrophilic and antimicrobial properties of PU composites and promote fibroblast adhesion and proliferation during wound healing.55 Similarly, curcumin and reduced graphene oxide (rGO)/Ag-NPs encapsulated simultaneously in PU/cellulose acetate (CA) nanofiber mats to promote regeneration of the epidermal layer exerted antibacterial effects and synergistically enhanced tensile strength, hydrophilicity, and antibacterial ability, respectively.56
Drug-Delivery Scaffolds
The regulation of PU soft segment degradation and increased mobility of polymer chains allow for the control of scaffold swelling and prolongation of the diffusion paths of loaded antibiotics and anticancer drugs for slow-release delivery.7,61 Incorporating NMs with high specific surface areas or electrostatic action into PUs can increase drug loading and promote controllable degradation of PUs as well as sustained drug release, which could significantly increase bioavailability.62,63 First, using the great drug-loading ability with a large specific surface area of NM could enhance the utilization of PUs. PU-algin/chitosan (CS) nanomicrospheres constructed by electrostatic repulsion between hydroxyl groups of a sodium alginate–urethane blending system (nuclei) and positively charged CS (shell) NPs have high electrostatic stability, and the successive enzymatic digestion of the nano-chitosan (nCS) shell and drug-loaded sodium alginate nuclei in the intestinal environment increases the dissolution of PU composite drug-delivery scaffolds.62 The encapsulation rate for insulin protein drugs loaded with this system was >90%, and the degree of bioavailability increased by 10.36%. Second, structural changes of NM itself would endow PUs with sustained drug release capability. Nano-montmorillonite has a unique layered structure that enables the improvement of drug loading and sustained release via the expansion and contraction of interlayer spacing.64 The amine group of montmorillonite forms hydrogen bonding with the polar functional groups of PU fibers help change the microphase separation structure and accelerate the hydrolysis of ester bonds in PCL, with linear drug release over 371 days observed.64 Third, NM would help PU across a biological barrier. PU nanofibers modified with nCS and gold (Au)-NPs can be used in the treatment of glioblastoma. The Au-NPs enable the crossing of the blood–brain barrier to achieve targeted anticancer effects, and the construction of a drug-carrying gel system with positively charged nCS is easy and significantly improves the encapsulation rate of short half-life drugs such as temozolomide, with up to 30 days of slow release observed.65
Stimulus-responsive smart PU scaffolds constructed by the introduction of magnetic response, photosensitive, and pH-responsive NPs could enable the regulation of PU composite degradability in response to diseased tissue environments and light and magnetic stimulation in-vitro to achieve precise drug delivery (Figure 3). The magnetothermal effect that occurs under the action of an oscillating magnetic field enhances the mobility of PU molecular chains, accelerating degradation and thereby enhancing the drug release rate, which is suitable for hydrophobic anticancer drug-delivery systems.66–69 In a multi-responsive drug delivery system with a hollow hierarchical HA core and an aliphatic PU amine/Au-NP shell, the photo-thermal conversion of Au-NPs under near-infrared (NIR) laser irradiation is used to achieve rapid controlled drug release via PU chain disruption.70 nCS and biodegradable bifunctional PU are cross-linked by Schiff base bonds to form difunctional polyethylene glycol (PEG) as a drug carrier that is pH-responsive, injectable, and self-healing; the deformation and reorganization of dynamic Schiff base bonds under pH and aniline is used to achieve the control of hydrogel degradation.71
Muscle and Nerve Repair
Constructing aligned nanofibers using conductive polymers is favorable for the migratory growth of myocytes and neural stem cells (NSCs) in the repair of muscle and nerve, as these cells have limited regenerative capacity and are sensitive to electrical stimulation and surface morphology.72–75 Nanoscale PU fibers prepared by electrostatic spinning provide topographic guidance for such growth, and further doped with Au-NPs and carbon-based NPs with appropriate rigidity and electrical activity [eg graphene, carbon nanotubes (CNTs)] improves mechanical support and provides good electrical conductivity to promote muscle and nerve cell adhesion.76–78 The simultaneous use of physical cue sensor array of PU fibers and good electrical conduction of NMs could effectively control the migration of NSCs and myoblasts and has great potential in the treatment of muscle and tendon injuries and neurodegenerative diseases.72,79
After been added carbon-based NMs with excellent mechanical property and bioactivity, PUs’ elasticity would be improved to match the stress environment of the muscle tissue. A PU matrix modified with multi-walled carbon nanotubes (MWCNTs) showed unique advantages for tendon and ligament grafts, as the tensile stress was increased from 11.40 ± 0.9 to 51.25 ± 5.5 MPa due to the increased transfer stress interface area.80 GO has the similar effects of improving the elasticity, stress relaxation capacity, and hydrophilicity of PU nanofiber scaffolds.13,59 In contrast to those of CNTs, the surface functional (hydroxyl, carboxyl, carbonyl, and epoxy) groups of GO promote serum protein adsorption, upregulate myogenic mRNA levels and myosin heavy-chain expression in C2Cl2 skeletal muscle myogenic cells, accelerate spontaneous myogenic differentiation, fuse to form mature multinucleated myotubes, and aid tissue repair.13,59
Besides the promotion of nerve cell adhesion and growth, the prolongation of nerve processes is another substantial factor in nerve tissue repair. The introduction of 0.1% CNT rich in hydroxyl groups improved the hydrophilicity of PU scaffolds and promoted the adhesion of neuronal stem cells after 7 days of incubation; in addition, the electrostatic repulsion of CNTs led to the reduction of PU nanofiber diameters from 1000 ± 400 nm to 700 ± 300 nm, increasing the specific surface area and porosity and promoting neuronal cell growth.81 In particular, functionalized multi-walled carbon nanotubes (fMWCNTs) with amine, hydroxyl, sulfide, and carboxyl groups have inherent conductive activity to help maintain the ionic concentrations of Ca2+, K+, and Na+, thereby promoting neuronal cell growth and the reverse propagation of intercellular action potentials.78 Such CNTs used in modifying PU aid in guiding the spontaneous regeneration of nerve protrusions in neural injury repair.78 Self-healing PU hydrogels and regenerative scaffolds doped with polypyrrole NPs also show promise for use in neural repair. These NPs cross-link with aldehyde-terminated difunctional PU via dynamic Schiff base reaction, stimulating neuronal cell migration and proliferation while inhibiting M1 polarization and reducing immune scaffold rejection due to the abundant surface COO groups.82 Metal NPs, such as citrate-coated Au-NPs, also enhance the hydrophilicity and surface roughness of PU nanofibers, as their electrical activity dissipates the electrical field into cells and stimulates the elongation of nerve protrusions.76
Other Applications
Nano-modified PUs have also been used in disposable medical consumables, contact lenses, and dental materials, among others.64,83 Montmorillonite nanoclay particles increase the stiffness of PUs by 10 times to match that of the ocular soft tissue and are used in contact lenses.64 Researchers have attempted to incorporate NPs such as Ag, copper (Cu), ZnO, and TiO2 into PU medical consumables (eg catheters) to improve the materials’ antibacterial and anti-aging properties.84–86 In dentistry, PUs are used mainly as thermosetting sealers and filling materials. The inclusion of NPs such as SiO2 and nHA in PUs can improve antibacterial effects and adhesion to dental tissues.87–89 For example, nHA can be chemically bonded to the diisocyanate component of PU by grafting to realize direct bonding with dental structures.89 Nano-fluoroapatite (nFA) can be used to prevent caries through the slow release of fluoride ions at a pH equivalent to that in the oral cavity.88 In recent years, thermoplastic polyurethane (TPU)-based invisible aligners without brackets have been used widely in clinical practice. Researchers have attempted to incorporate Ag-NPs on these aligners and other orthodontic elastomeric modules, such as ligation rings, to inhibit the formation of caries-causing biofilm and prevent enamel demineralization.90,91
Nano-Modified PU Performance Optimization
Biocompatibility
NMs can enhance intercellular signaling, promoting cell adhesion, proliferation, differentiation, and migration via biophysical signals such as hydrophilic rough surfaces, micro-magnetic fields, electrical stimulation, and protein adsorption (Figure 4). Nanoscale SiO2 increases the spreading degree and speed of silicon ion, which are known to induce the expression of growth factors. Therefore, SiO2-NPs/PU composites containing 10% SiO2-NPs could induce the secretion of TNF-α and TGF, promote the proliferation of epidermal keratinocytes and skin fibroblasts, and increase new elastin fiber production to accelerate wound healing during the observed 14 days.54,99 The introduction of carbon-based NMs (eg MWCNTs, GO) with abundant hydrophilic surface functional (eg hydroxyl, carboxyl) groups increases PU nanocomposite surface roughness and hydrophilia, synergistically promoting protein and cell adhesion.13,78 The hydroxyl, carboxyl, carbonyl, and epoxy groups on the GO surface increase hydrophilicity and the negative surface charge, which improves serum protein adhesion.59 The preferential adsorption of these proteins promotes myoblast adhesion and differentiation.59 The loading of NMs such as Au- and platinum (Pt)-NPs onto TPU increases the hydrophilicity and negative surface charge, and only 0.1% addition of such NMs is sufficient for promoting binding to albumin and cytokines and the adhesion and proliferation of endothelial cells after 4 days of culturing.100 For neurons and myoblasts, which are highly dependent on electrical activity, conductive (eg Au-76 and carbon-based13,80) NMs can be introduced into PUs to promote intercellular signal recognition and electrical stimulation conduction, facilitating the propagation of intercellular action potentials in neurons and the elongation of neural protrusions.78 NMs appear to provide links between biocompatibility, conductivity, and catalytic activity to enhance signal transduction.101 The modification of PU nanopillar array surfaces with highly conductive silver nanowires (Ag-NWs) and rGO enhanced NSC differentiation by electrical stimulation and the growth of neural protrusions.72 Magnetic hematite NPs with good biocompatibility form a micro-magnetic field in scaffold pores to promote cell growth and can be metabolized by the spleen or kidney even when dispersed in the blood circulation.79
NMs can also form an ECM biomimetic structure by improving the PU pore size, roughness, and hydrophilic properties, promoting surface cell adhesion and growth.32,58,102,103 Properly proportioned and sized pores facilitate nutrient exchange and provide space for cell infiltration and migration.58,103 The introduction of viscosity-increasing NMs (eg tungsten) or conductive NPs (eg MWCNTs and GO) facilitates regulation of the pore size and porosity of PU foams and fibers formed by electrostatic spinning. Scaffolds prepared by the compounding of GO on tubular TPU have increased porosity and surface hydrophilicity, which enhances the attachment and proliferation of endothelial cells for the achievement of endothelialization. Loaded NMs enhanced the biophysical signaling between PU nanocomposites and cells, significantly improving biocompatibility, but research on the relationship between this signaling and cytological behavior is still at the level of in-vitro experiments; further in-vivo studies are needed.45,104 In addition, the targeted modulation of specific cell lines by PU nanocomposites remains difficult to achieve. NP-loaded peptides and other biomolecules should confer these materials’ specific cellular recognition ability and induction of cellular responses at the molecular level in the future.
Nano-modified medical PUs are also able to regulate mitochondria-mediated oxidative stress. The increase of reactive oxygen species (ROS) generation and the resulting oxidative stress lead to the material aging of PU and promote the release of pro-inflammatory mediators to damage normal tissues and cells.105 NM with superoxide dismutase (SOD)-like and catalase-like activities can consume ROS produced by mitochondria to prevent PU from aging and degradation due to oxidation. The trivalent and tetravalent oxidation state of cerium oxide NM can simulate SOD to remove the ROS generated after the PU implantation as artificial vessels, thereby avoiding the resulting endothelial cell apoptosis and failure of vascular endothelialization.106 Using the large specific surface area of NM to load antioxidants to modify PU can not only improve the loading rate of antioxidants but also avoid their rapid invalidation due to the encapsulation effect of NM. The biological PU scaffold composed of SiO2-NP loaded with antioxidative phenolic acid can reach a slow release for 2 weeks, which protects cells from oxidative stress and promotes wound healing.107 There are risks in some NMs to cause mitochondrial toxicity, aggravating oxidative stress reactions. About 25 μg/mL ZnO-NPs can target mitochondria to damage the mitochondrial respiratory chain, increase the level of ROS, and cause cell damage.108 Excess TiO2-NPs in medical PU can activate the Caspase-8/Bid pathway in a concentration-dependent manner and induce mitochondria-mediated apoptosis.109 Since mitochondria are the control scenter of cellular activities and the main target organelle exposed to many NMs, their functional changes have gradually become one of the main evaluating standards of the biocompatibility of nano-modified medical PUs.
Antibacterial Properties
PUs are susceptible to microbial attack because the urethane bonds in their main chains are highly similar to peptide bonds in biological macromolecules.110,111 Doping or coating with hydrophobic or antimicrobial NMs can prevent bacterial adhesion and biofilm formation on PU surfaces (Figure 5).93,112,113 The preparation of superhydrophobic surfaces on PUs with hydrophobic silica-based NPs, such as POSS, can inhibit bacterial adhesion.54,87 PU modification with NPs such as Ag-NPs, graphene family NMs, and nCS improves antibacterial efficacy and avoids the development of resistance with long-term antibiotic use.21 Ag-NP–modified PUs have been studied most widely.92,114–116 In a recent study, Ag+ was released continuously from waterborne PU/Ag-NP complexes for more than 21 days, exerting a long-lasting antibacterial effect.116 rGO/Ag-NPs loaded in PU/CA nanofiber dressings inhibit bacterial growth by Ag+ diffusion and exert a synergistic antibacterial effect via the oxidative stress sterilization of rGO sheets to destroy bacterial membranes.56 The positively charged amine group of nCS not only has inherent antibacterial activity but also adsorbs and slowly releases anionic antibacterial drugs to improve the antibacterial activity of PUs.117 TPUs modified by nCS combined with quaternary ammonium salt are usually used as antibacterial tracheal intubation materials; they maintain excellent mechanical properties and long-term antibacterial performance in hydrodynamic environments.118
In recent years, the intelligent antibacterial effects of PU nanocomposites modified with photosensitive NPs (Au-, TiO2-, and ZnO-NPs) have been examined. PUs modified with gold nanorods (Au-NRs) and PEG have antifouling and photothermal bactericidal properties; Au-NRs exhibit efficient photothermal bactericide under 808 nm NIR irradiation, and hydrophilic PEG layers remove dead surface bacteria, inhibiting long-term biofilm formation.119 TiO2- and ZnO-NPs have similar photocatalytic antibacterial properties; they also inactivate microorganisms through electrostatic interactions, the generation of ROS, and the destruction of cell membranes. The addition of photosensitive bactericidal Crystal violet–enhanced ZnO-NPs resulted in the significant inhibition of S. aureus and Escherichia coli activity on PU surfaces under white light.120 However, bactericidal NMs may kill normal cells as well as bacteria, and most recent studies of their effects on cells have been in-vitro proliferation experiments. In-vivo experiments are needed to verify the safety and effectiveness of these NMs before they are applied clinically. Their antimicrobial properties and biocompatibility should be coordinated via the modulation of their shapes and ratios, the surface modification of nanocomposites, and the introduction of new targeted antimicrobial agents (eg nanozymes) to advance the clinical application of NM-modified PU.
Enhanced Mechanical Properties
Mechanical properties such as compressive strength, tensile strength, and shape memory performance are required for prostheses and wound dressings applied to bones, muscles, and cardiovascular components, which are continuously subjected to loading, shear stress, and abrasion.1 Measures undertaken previously to improve the mechanical properties of PUs, such as the adjustment of the composition and ratio of the materials’ soft and hard segments, reduced PU flexibility and produced toxic products (eg aromatic isocyanates) during synthesis and degradation.19,60,121 The introduction of rigid NMs can disperse the stress of a PU matrix, and regulate porosity and retain flexibility through a dissipation mechanism.30,122 NMs can also be embedded into or bonded with the hard segment components of PU to improve rigidity and tensile strength.32,54 PU nanocomposites containing TiO2-NPs, nHA, SiO2-NPs, and carbon-based NPs can adapt to different tissue systems via functional group interactions and chemical bonding. BG-NPs and nHA form covalent bonds with PUs through –OH groups to obtain tensile properties and compressive strength matching those of cancellous bone.30,32,102,123 SiO2-NPs enhance hard segment properties due to the generation of dipole–dipole intermolecular forces between silicone groups and the hydrophilic functional groups of PU, resulting in mechanical strength matching that of skin.56 The reactive polar functional groups in fMWCNTs form intra- and intermolecular covalent, hydrogen, and peptide bonds with –COOH, C=O, –OH, and –NCOO– in PU/serine, which enhances Young’s modulus and tensile fracture stress and provides a stable environment for the migratory growth of neuronal cells.78,81 The amine group in silylated GO and the NCO in PU chains form chemical urea bonds (–NH–C=O–NH–), which improve the tensile and compressive strength of PU nanocomposites, enabling adaptation to the mechanical requirements of arteries and veins.45,124
Shape memory property is required by implantable PUs including self-expanding scaffolds, smart sutures, and stents for controlled drug release. The addition of stimulus-responsive NMs or augmentation of NM bonding energy storage can be used to regulate the degree of phase separation between hard and soft PU segments, thus conferring shape memory to these PUs.96 Given the excellent NIR photothermal properties of PU/black phosphorus (BP) nanoplates, the glass transition temperature of the PU in these plates can be increased rapidly for shape restoration and cardiovascular integration.96 The addition of GO improves the rapid shape recovery of shape-memory PU and halves the actuation time of neat PU under NIR irradiation (Figure 6).125–127 Given their polar oxygen and Ag–oxygen bonding abilities, hybrid CD/Ag NMs can be covalently bonded to hyperbranched PU hard segment matrices to increase energy storage, conferring strain release and enabling rapid self-expansion to fill tissue defect gaps.128
Notably, the enhancement of NM dispersion in PU composites is key for the improvement of PU mechanical properties. Currently, NMs are modified mainly by surface functionalization to establish chemical bonding with PU chains, which improves interfacial interaction and thus the microphase separation and soft-segment orderliness of PU nanocomposites, ultimately conferring mechanical properties that are suitable for the human body.129 However, changes in the pore structure of the PU matrix during NM modification can affect the matrix’s mechanical properties.27 Mechanical property enhancement must be undertaken in specific application environments with the regulation of NM amounts; for example, the ratio of nHA added to modified guided bone regeneration films needs to be considered to meet the high individual flexibility and tensile property requirements.32
Optimized Degradability
The improvement and stabilization of the PU degradation rate in body-temperature environments is key for the improvement of medical PU biostability, as PU degradation is accompanied by the loss of excellent original physicochemical properties, such as the elastic modulus. In the past, the degradation rate could be controlled to a certain extent via adjustment of the chemical structure of the soft segment or the soft/hard segment ratio and porosity.20 NMs can be used to optimize the PU degradation performance in two main ways: 1) for tissue engineering, bioactive NMs (eg nHA, MWCNTs) can be used to construct biodegradable PU scaffolds with degradation rates comparable to tissue repair rates; and 2) for drug delivery, stimulus-responsive NMs (eg superparamagnetic NPs and BP) can be used to synthesize intelligent PU scaffolds with targeting and tunability. NMs can influence matrix contact with water, enzymes, and other components by regulating the scaffold pores and hydrophilic surface; furthermore, their polar groups can change the molecular structure of PU and the degree of chain segments’ microphase separation, thereby regulating scaffold degradation.79 For example, the formation of hydrogen bonds between the hydroxyl group of nHA and the amide group of PU promotes microphase separation between the soft and hard segments to regulate thermal stability;48 in addition, nHA acts as a mass transport barrier, lengthening the escape path for volatile products generated during decomposition and further improving thermal stability.32,130 Nanofillers with hydroxyl-rich surfaces also regulate the thermal stability and degradability of nanocomposites by competing with –NH groups for carbonyl groups in carbamate bonds.58 For example, the covalent bonding of the large number of active hydroxyl groups in PU/thiacalix[4]arenes–functionalized ferroferric oxide (Fe3O4)-NPs to the PU matrix reduces PU chain mobility and improves thermal stability.129 Nanomontane amine groups interfere with the formation of hydrogen bonds with PU polar groups (eg carbonyl groups in ester bonds, urethane or urea bonds) and accelerate PU microphase separation and the hydrolysis of PCL ester bonds in the soft segment, thereby promoting PU nanocomposite degradation and drug release.33,64,79
The synergistic promotion of slow drug release through the introduction of stimulus-responsive NMs [eg superparamagnetic iron oxide (SPIO)-NPs131 and BP96 to PU scaffolds to modulate degradation has received much research interest in recent years. The difficulty of regulating PU nanocomposite scaffold degradation lies in the achievement of adaptation to the tissue regeneration rate. In addition, a means of in-vivo monitoring is lacking and relevant degradation indices are difficult to obtain. The development and optimization of the in-vitro visualization of NMs used to modify PU composites, such as magnetic NMs, would aid the comprehensive in-vivo monitoring of degradation. In addition, the degradation products of PU nanocomposite scaffolds may cause tissue immune reactions and related metabolic pathways and biosafety need to be investigated further and verified.
Preparation Schemes for the Nanomodification of Medical PUs According to NM Characteristics
During the design and fabrication of nano-modified medical PUs, the choice of raw materials and methods directly affects the final performance. The chemical compositions of raw materials are linked closely to their properties, including chemical bonding ability, thermostability, electroconductivity, and appearance, and must be considered to properly retain and utilize the desired properties. The most thoroughly studied NMs used in this process and mature fabrication methods are described below.
Characteristics of NMs
NMs classification is based largely on the materials’ biological performance, chemical reactivity, optical properties, magnetism, and superconductivity. NMs used to modify medical PUs have excellent bioactivity and mechanical strength, which enhance the physical and chemical performance parameters (eg degradability and antibacterial action) of the final materials.56,132,133 Most of these NMs can be classified as metal-based, inorganic non-metallic, and organic materials (Figure 7). Recently discovered NMs are expected to bond to PU; they include BP and nano-tungsten, which respond well to stimuli, and CDs, which can load biopeptides and endow them with smart targeting properties. These materials and properties may represent future directions for tissue repair and drug-carrying scaffold production.
Metal-Based NMs
Metal NMs have antimicrobial properties due to their release of metal ions with strong oxidizing ability, and they improve biocompatibility and help to maintain the normal electrophysiological activities of cells, especially neurons on PU scaffolds.97,134,135 Ag- and Au-NPs are the most thoroughly studied pure metal NPs used to modify PUs. The urethane groups of PUs bind with Ag ions, stabilizing Ag-NPs for the promotion of antimicrobial activity. The addition of Ag-NPs also enhanced the mechanical strength and biological stability of composites.98,115 Ag-NPs transfer stress from PU nanofibers to themselves through a dissipative mechanism and limit the migration of waterborne PU chains through eCOO-Ag, which in turn improves the tensile strength and Young’s modulus.115,122 Ag-NPs also increase the microphase separation of PU matrices to increase the pyrolysis temperature, and the enhance biological stability.136 In addition, Ag-NWs, with strong electrical conductivity, can impart tunable electrostriction to PU elastomers, improving the sensitivity of nanocomposite sensor systems.137 As for Au-NPs, they have high electrical conductivity, photosensitive activity, versatile surface modification capacity due to their flexibility, and excellent biocompatibility, making them powerful materials for bacterial control and drug loading and transmission. Although Au-NPs themselves have poor bacteria killing ability, NIR laser irradiation activates their surface plasmon resonance, thereby producing heat suitable for the killing of bacteria.138 In an Ag-Au–NP/toluidine blue O (TBO)/PU system, the Au-NPs catalyzed the photochemical reaction of TBO molecules under white light to accelerate the release of ROS and reactive singlet oxygen. The combination of Au-NPs with Ag-NPs and other inherently germicidal active NPs yields a more effective and intelligent germicidal effect.139 Taking advantage of the potential of Au-NPs to cross the blood–brain barrier and kill tumor cells, drug-loaded Au-NP/CS/PU scaffolds can be applied in the treatment of brain tumors such as glioblastomas.97
Metal oxide NMs exert antimicrobial activity by releasing ROS to stimulate oxidative stress reactions.140 Fe3O4-, TiO2-, and ZnO-NPs have different degrees of photosensitivity and magnetic effects, and their combination with PUs enables the intelligent regulation of composites’ antibacterial properties and deformation memory. Magnetic hematite NPs have electromagnetic activity and activate cellular signal mechanotransduction pathways through in-vitro magnetic fields to enhance cellular activity.141 They also release Fe3+, which promotes erythropoiesis and increases the absorption of BSA and type I collagen, thereby promoting cell adhesion and proliferation after covalent bonding with PU.50,79,142 These NMs also impart a magneto-thermal effect to PU, modulate the degradation of the PU matrix under an external magnetic field, and control the slow release of loaded drugs for drug-delivery systems and cancer therapy.69,131 For example, SPIO-NP/PU composites have high cancer-cell recognition efficiency and magnetic heating properties, and they can be used to accelerate the release of encapsulated hydrophobic anticancer drugs (MAMA and VK3) and their retention in target organs under external magnetic field heating, which has great potential in the treatment of liver cancer.66,69,143 TiO2-NPs has great photocatalytic activity, and TiO2-modified PUs exhibit intelligent and controllable photocatalytic antibacterial activity due to the creation of oxidative stress under photocatalysis.1,97,144 TiO2-NPs also interfere with hydrogen bonding to accelerate the decomposition of PU hard segments, thereby enhancing stability, and improve the absorption of ultraviolet light and reflectivity of visible light, enhancing the aging resistance of PU.144,145 The introduction of ZnO-NPs into PU through amine, hydroxyl, and hydrogen bonding or direct reaction with isocyanates can enhance the tensile strength of PU composites and confer antimicrobial properties similar to those of TiO2-NPs.120,146,147
Inorganic Nonmetallic NMs
Inorganic nonmetallic NMs are less toxic than metal NMs since they do not release metal ions. They are characterized by good phase mixing with PUs, and they improve mechanical properties such as tensile and compressive resistance. They have excellent osteogenic and anti-inflammatory properties and promote cell adhesion and proliferation, thereby enhancing the biological activity of PU. nHA, graphene, CNTs, and silica NPs are the most thoroughly studied inorganic non-metallic NMs in modifying PU. nHA has excellent mechanical strength and osteogenic properties, which can improve the tensile strength, elastic modulus, and compressive capacity of PUs and promote osteogenesis.6,13,32,34,148 Besides their affinity with bone, nHA/PU composites can be used on dentin as restorative or adhesive materials. The NCO group at the phenyl end of the isocyanate component of the PU hard segment reacts with the –OH group of nHA to form a carbamate bond, thereby improving the compressive properties of restorative materials. The urethane functional group of PU bonds strongly to the dentin collagen matrix to enhance the adhesion of nHA/PU nanocomposites.89 When the nHA in the nanocomposites changes into nFA, the obtained modified PU is able to promote enamel mineralization and prevent caries through controlled fluoride release.88
Carbon-based NMs, such as graphene and CNTs, have good rigidity and enhance the mechanical properties of PU.140,148 The induction of the abundant amine groups on the surfaces of carbon-based NMs to link with PU chains greatly enhances the thermal stability and tensile properties of PU nanocomposites. Polar functional groups on fMWCNT can form strong hydrogen bonds with the carbonyl and amino groups of PUs, which improves compression resistance.22 CNTs and GO also have hydrophilic and conductive activities that promote the adhesion and proliferation of fibroblasts and endothelial cells.45 PU/silk/fMWCNT scaffolds enhance cellular signal recognition through conductive stimulation and contribute to neural tissue regeneration.78 Silica-based (eg SiO2- and POSS-) NPs are rigid, hydrophobic, and hemocompatible, and have outstanding advantages for cardiovascular system and wound dressings.47 They provide superhydrophobic interfaces that inhibit microbial and platelet adhesion to nanocomposite surfaces, and the Si4+ released from such systems promotes the regeneration of elastin fibers and accelerates wound healing.55 Silica NPs and POSS could be used in intravascular catheter polymer coatings to inhibit thrombus formation, can be used in polymer coatings for intravascular catheters to inhibit thrombus formation; while layered silicate crystals, such as montmorillonite nanoclay, are used as hydrophobic cationic calcium drug carriers in TPU nanofibers for local drug delivery and the long-term management of chronic wound healing via the formation of intercalated composites with PU.5,52,64,149
Organic NMs
Organic NMs include synthetic and natural polymeric NMs. Synthetic polymer NMs, such as double-bonded chitosan-modified polypyrrole, are characterized by excellent degrees of phase mixing with PU matrices and conductive activity to stimulate neuronal cell migration and proliferation.60,71,82 Natural polymers such as CS have hydrophilic, hemostatic, and anti-inflammatory properties that enable the achievement of a balance between biocompatibility and mechanical strength when used in combination with PU for wound dressings and drug-carrying scaffolds.1,60,117,150 nCS uses the hydroxyl group to react with water through hydrogen bonding, which enhances the hydrophilicity of the PU matrix. In addition, the –NH2 in its molecules combines with anions dissociated from silicic acid and phosphate contained in microorganisms’ cell walls to exert antibacterial effects in the treatment of infected wounds.118 The addition of temperature-sensitive materials to compounds of the drug carrier nCS and PU helps to control the degradation of PU nanocomposite hydrogels and achieve the slow release of antibacterial drugs.62,71,117 Cellulose nanocrystals have good thermostability due to their highly crystalline structure, and their introduction into composites improves the thermal properties of PUs.151 In addition, their nanoscale effect aids dispersion in the third functional phrase in PU nanocomposites.152 The inherent effect of cellulose also facilitates the adhesion of PU-based nanocoatings.152
Preparation Schemes
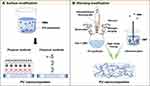
In the production of medical PU nanocomposites (fibers, elastomers, gels, and foams), the blending of NMs into molten PUs by direct mixing and in-situ polymerization promotes sufficient contact and chemical bonding with the PU matrix.153 For materials such as medical catheters, the attachment of NMs to the PU matrix surface is more suitable because the excellent flexibility and other mechanical properties of the matrix are retained.154,155 PU nanocomposites with antibacterial and antithrombotic surfaces are produced by coating, layer-by-layer (LBL) self-assembly, and chemical graft polymerization (Figure 8).
Blending Modification
Blending modification techniques used to prepare nanocomposites include in-situ blending, in-situ polymerization, and the sol–gel method. Blending involves the direct addition of NMs with different morphologies to the polymer matrix for mixing and can be performed mechanically or ultrasonically, in solution or emulsion, and by melting. This method is mature and simple and is commonly used for PU modification with GO,156 Ag-NPs,136 ZnO-NPs,146 nBG,36 and nHA.130 However, in-situ blending can result in the agglomeration of NMs in the polymer matrix; NM pretreatment with surface modifiers or ultrasonic oscillation to assist dispersion is required.
In in-situ polymerization, NMs are mixed with PU monomers, followed by polymerization, which stabilizes the NM size and disperses them uniformly in the PU matrix. PU nanocomposites prepared by this method (eg those containing Fe3O4-NPs) have shown greater interfacial adhesion.6,67,68 A disadvantage of this method is that it can easily result in the alteration of the molecular weight of PU and increase the viscosity of the system; it is suitable for use only with solutions containing metals, sulfides, or hydroxides.
The sol–gel method involves the dissolution of NM precursors, such as siloxane or metal salts, in water or organic solvents, followed by the addition of PU monomers to trigger their polymerization and finally the removal of the solvent to obtain PU nanocomposites.68 With this method, the NMs are bound covalently in the polymer matrix to achieve uniform dispersion and precise composition control.146 However, the volatilization of solvents, water, and small molecules often causes shrinkage stress and brittle cracking inside the material. In addition, as most NM precursors are expensive and toxic alkyl silicate esters that volatilize easily, the application of this method is limited. It is used most often to introduce SiO2-NPs into PU, as the pH can affect the size and shape of silica NPs and the final polymer structure and properties.54,149
Prepolymerization, intercalation polymerization and radiation synthesis are other feasible methods for NM/PU composite production.64,136 For example, many inorganic NMs with layered structures (eg nanomontane) can be mixed or embedded in PU matrices and fully combined by prepolymerization and intercalation polymerization. Radiation synthesis is generally applicable for the mixing of PU monomers with metal salts (eg silver nitrate) at the molecular level and in-situ reduction to nanoscale metals.115
Surface Modification
The surface nano-modification of PUs is used to produce biocompatible coatings while retaining the excellent inherent properties of the PU matrix.157–159 Physical coating methods (eg dip coating, ultrasonic deposition, electrodeposition) do not require complex chemical treatment; examples are HA deposition on PU by dipping in a supersaturated calcification solution and GO deposition on PU foam.2,59,98,160,161 However, this method depends on the non-specific interaction of NMs with the PU substrate surface, and adhesion to the substrate may be poor.162
In LBL assembly, electrostatic interactions are used to drive the sequential adsorption of oppositely charged materials onto a PU substrate, resulting in a multilayer nanoscale coating.27,163 These coatings have controllable thickness, predictable physicochemical properties, and bioefficacy (eg antibacterial and anticoagulative properties) and are used primarily for cardiovascular system which multiple properties are needed.164–166
In chemical grafting, NMs such as nHA89 and Au-NPs119 are introduced by physically or chemically activating the PU surface to produce reactive groups, which triggers a grafting polymerization reaction.67,68,116 Depending on the NM properties of composite material applications, other methods used to compound NMs on PU surfaces include plasma ink-jet printing, ion beam-assisted treatment, and multi-target plasma sputtering.4,72,150
Key Parameters of PU Nanomodification
NM Morphology
Nanofillers with different morphologies, such as NPs, NWs, NTs, NRs, and nanosheets, bring different physical and chemical properties to PU.167–169 For example, Ag-NWs improve the conductivity of PU more efficiently than do Ag-NPs due to their high aspect ratio.72 The inherent characteristics (eg crystallographic form) of NMs are important parameters to adjust the properties of PU nanocomposites. For instance, anatase TiO2 has a surface structure that is more easily combined with waterborne PU, and it endows better antibacterial ability to PU than do rutile and plate TiO2.170 NM size also greatly influences the performance of modified PUs. The micro-addition of NMs <50 nm, which are easily dispersed fully in PU systems, significantly improves the biological activity of PU nanocomposites.170–172 The addition of nBGs with medium and small particle sizes to PU matrices significantly improves the osteogenic activity and mechanical properties of nanocomposites, whereas larger nBGs tend to aggregate in PU matrices, forming stress concentration points and resulting in increased brittleness.161 Thus, NMs with appropriate morphologies need to be selected according to the application requirements, and the synthesis parameters of NMs need to be adjusted to retain the particles’ size and microstructure for improved nano-modified PU efficiency.
NM Components and Ratios
Single NMs have limited performance and cannot simultaneously confer multiple biological properties. Joint PU modification with multiple NM types has attracted much recent research attention.173 For example, combined modification with GO and Au- and Ag-NPs enables the stabilization and dispersal of Ag to improve antibacterial properties, and improves the biocompatibility of PU composites due to the hydrophilicity of Ag and the electrophysiological environment provided by GO and Au-NPs.2,23,53,56,139,143 Natural polymer NPs (eg nCS) are often combined with metal NPs (eg TiO2-NPs and Ag-NPs) for PU modification.1,150 nCS synergistically improve metal NM dispersal in PU matrices and enhance antibacterial activity, and rigid metal NMs compensate for the decrease in tensile and breaking strength caused by the introduction of CS, thereby enhancing the mechanical strength of nanocomposite.
The key to the balancing of PU mechanical properties and NM biological activity is the adjustment of NM proportions in nanocomposites. Usually, the incorporation of <10 wt% NMs significantly improves the biochemical performance of medical PUs.174–176 Due to their high surface energy, the addition of large proportions of NMs to PU matrices tends to result in uneven dispersion, which affects the biochemical performance of composites. When two or more types of NM are added, their proportions should be adjusted according to the target tissue requirements. For example, the proportions of rigid NMs (eg nHA, nBGs) and bacteriostatic NMs (eg Ag-NPs, nCS) should be regulated in PU nano-scaffolds used for the repair of load-bearing bone defects to achieve a balance between mechanical and antibacterial properties.23,114
PU Nanocomposite Forms and Preparation
To meet diverse biomedical requirements, different forms of PU nanocomposite (eg foams, fibers, gels, and elastomers) need to be produced using appropriate methods (Figure 9). PU nanocomposite fibers used in wound dressings and tissue engineering scaffolds are generally prepared by electrostatic spinning, electrospraying, or thermally induced phase separation to provide bionic environments for cell attachment, migration and proliferation.150,177–179 PU nanofibers prepared by electrostatic spinning mimic the reticular structure of the ECM; the addition of conductive NMs (eg MWCNTs, GO, and Au-NPs) increases the jet charge density of the preparation, reducing the PU nanofiber diameter and increasing the porosity, and provides an electrophysiological environment for directional cell attachment, migration, and proliferation.78,180–183 Drug-loaded scaffolds and injectable PU gels for minimally invasive tissue defect filling are generally prepared by hydrogel polymerization and loaded with Au-, TiO2-, and other stimulus-responsive NMs, for targeted and controlled drug release, and the effective balancing of viscosity and elasticity.3,184 PU foams and elastomers for tissue engineering scaffolds, artificial blood vessels, cardiac patches, barrier membranes, and many other implants can be prepared by gas foaming, solvent leaching, and other processes; the addition of NMs such as nHA and nano-tungsten enables the regulation of the pore diameter and morphology. The further addition of NMs such as BP to increase the deformation memory performance of these materials for aneurysm embolization therapy has received much recent research attention.47,95,145
 |
Figure 9 Main clinical applications of biomedical PU nanocomposites. Abbreviation: PU, polyurethane. |
Balancing the Biological Effects and Potential Toxicity of NMs
The sustained release of NMs from modified PUs confers a variety of physicochemical and biological effects, but also toxicological risk.30,59 First of all, NMs with oxidative properties such as Ag-NPs, GO, and TiO2-NPs enter cells through direct osmosis or endocytosis, induce ROS synthesis, and cause DNA damage and toxicity.30,140,185 Among them, the ROS level is the most sensitive to external stimuli, which is also an initiator of damage in the cell membrane, mitochondria, lysosomes, and other organelles.186 Surface modifications of NMs allow for functional diversity and enhanced dispersion, and the biological action is also accompanied by changes in its surface properties. Functionalizing NMs or modifying them with small molecules, such as PEG, facilitate to reduce the possibilities of nano-modified medical PUs to generate ROS, which may induce oxidative damage and autolysis of cells.106,187 Ion shedding is an important property for some metal-based NM and has more complex toxicity since the released ions could sharpen the cellular injury. For example, Zn2+ released by ZnO-NPs has been proven to alkalize lysosomes and interfere with physiological autophagy;188 Ag+ dissolved from Ag-NPs may bind to protein filaments and damage the cytoskeleton.189 The cytotoxicity of NMs has been found to increase with decreasing particle size and the internalization rates of spherical NMs are usually much greater than those of flat ones.140,190 For example, NMs with particle sizes <2 nm are more likely to induce oxidative stress through phagocytosis or endocytosis.138 As for shape-dependent toxicological effects, studies indicated that two-dimensional materials, like graphene and black phosphorus, are worthy of special attention. They could adhere and interfere with cell membranes in a plane-to-plane type and also directly insert into cells when a certain angle exists to cause cell cytoplasm leakage.191,192 The regulation of NM toxicity in PU nanocomposites is related closely to the improvement of antibacterial performance.59,140,193 PU gels loaded with Ag-NPs release Ag+ and increased local Ag+ concentrations around normal tissue are potentially toxic. The risk of Ag+ poisoning can be reduced by adding biocompatible surface nHA, phospholipids, peptides, or polymer barrier layers (eg polyaniline, CS).23,39,140,150,193 The time period and dose selection should also be taken into consideration since NMs exposed for extended periods or at high doses would result in severe global cytotoxicity. For different NMs, the standards of safe dose and effective dose are also different. So far, adding NMs no more than 10% is a relatively safe dose for PUs’ modification.58,85,93 Though PUs with a content of ZnO-NPs nanofiller less than 5% showed a concentration-dependent growing cell proliferation, a decreased cell viability would occur when the ZnO-NPs content exceeded 10%.58 As for the observing time period, 7 days are needed to identify whether cytotoxicity exists in nano-modified PU, while the histocompatibility requires a cultured time of more than 1 month to approve. Before clinical application, adequate biocompatibility testing and even oxidative stress testing of modified PU nanocomposites is crucial to strike a balance between biopotency and toxicity (Figure 10).
Future Perspective
Although the medical polyurethane modified by NMs has made great progress in recent years, there are still many performances that need to be improved. Firstly, the improvement and application of nano-modified medical PUs depend on the development of novel kinds and/or effects of NMs. The NMs with enhanced bioactivity are developed and yet to be used in the modification of medical PUs. For instance, nano-tantalum oxide has a similar elasticity modulus to cartilage and is a facilitator of stimulating chondrocytes to secrete extracellular matrices like glycosaminoglycan and type II collagen. Nano-tantalum oxide loading via elastic PU enables the rapid repair of cartilage defect.194,195 Nano-niobium oxide is deemed a promoter of fast vascular remodeling, whose addition into porous PU is beneficial to accelerate osteogenesis, muscular repair, and wound healing since tissue repair is dependent on blood supply.196 The application of these newly developed NMs in PU may significantly improve the biological performance of modified PU in the future.
Secondly, the application of intelligent and targeted NM in modifying medical PU is one of the most potential directions. The intelligent response to the diverse in-vivo environments endows medical PU with a different reaction to stimuli. Nano-modified PU response to pH, temperature, magnetic field, and light has been reported, but those able to respond to more endogenous or exogenous stimuli, such as pressure, friction, fluid force, and ultrasound, still need to be developed.50,71,96 Moreover, encapsulation of PU drug carriers by cell membrane is suitable for recognizing target cells and immune escape. Applications of NMs coated by erythrocyte membrane, platelet membrane, leukocyte membrane, and macrophage membrane can be the permeation enhancers of medical PUs to pass biological barriers such as blood–brain barrier, intestinal barrier, and pulmonary barrier to improve the drug delivery efficiency.197–199
Thirdly, a comprehensive understanding of the in-depth studies of biological mechanisms between cells and nano-modified medical PUs is worth attention. Existing studies mainly evaluate the biological performance evaluation and mechanism of NM itself, but studies on the cellular and molecular mechanisms of the composite materials after the nanomodification of PU lag far behind that of NM alone. In addition, the creation of suitable in-vivo treatment and regeneration environments requires the comprehensive, simultaneous enhancement of properties such as biocompatibility, antibacterial activity, and degradation. Efforts should be made to establish a balance between cell proliferation ability and antibacterial activity in PU nanocomposites. The potential toxicity of self-degrading PU nanocomposite products poses potential risks for the human body and natural environment. At present, methods for the clear characterization of degradation products and their toxicity are lacking; in-vivo studies and clinical trials are urgently needed to address this issue.
Conclusions
Given its characteristic microphase separation structure, PU is degradable and easily processed into various bioengineering materials; thus, it has great potential for numerous biomedical applications. PU modification with NMs through blending and surface alteration enhances the strength and degradability of composites while creating bionic environments that are suitable for cells and conferring properties such as electrical conductivity and antibacterial activity. Nanotechnology can fabricate a new generation of medical PUs with high efficiency, providing desired biological responses and mechanical properties beneficial to a wide range of clinical fields. The type and concentration of NMs used for modification and the specific modification scheme should be designed according to the application scenario, so as to achieve the best clinical effect. Different directions and applications of studies have been reported, but the internal rules of modification are still worth summarizing and analyzing. This review will serve as a significant step for the popularization and industrialization of nano-modified PUs. Despite multiple promising studies applying NMs for optimizing the medical PUs, nanotechnology is still costly and technically challenging. The future development and application of nano-modified medical PUs are always based on reasonable design, complete biological effect analysis, and careful preclinical biosafety detection.
Abbreviations
γ-Fe2O3, γ-iron oxide; Ag, silver; Ag+, silver ion; Ag-NWs, silver nanowires; ALG, algin; Au, gold; Au-NRs, gold nanorods; BG, bioactive glass; BP, black phosphorus; BSA, bovine serum albumin; CA, cellulose acetate; CDs, carbon nanodots; CNTs, carbon nanotubes; CS, chitosan; Cu, copper; ECM, extracellular matrix; FDA, food and drug administration; Fe, iron; Fe2O3, iron oxide; Fe3O4, ferroferric oxide; fMWCNTs, functionalized multi-walled carbon nanotubes; GNP, gold nanoparticle; GO, graphene oxide; HUVECs, human umbilical-vein endothelial cells; LBL, layer-by-layer; MSNs, mesoporous silica nanoparticles; MWCNTs, multi-walled carbon nanotubes; nBG, nano-bioactive glass; nCS, nano-chitosan; nFA, nano-fluoroapatite; nHA, nano-hydroxyapatite; NIR, near-infrared; NM, nanomaterial; NO, nitric oxide; NPs, nanoparticles; NSCs, neural stem cells; PEG, polyethylene glycol; PLLA, poly-l-lactic acid; POSS, polyhedral oligomeric silsesquioxane; Pt, platinum; PU, polyurethane; rGO, reduced graphene oxide; ROS, reactive oxygen species; RSNO, S-nitrosothiol; SMPU, shape memory polyurethane; SiO2, silicon dioxide; SOD, superoxide dismutase; SPIO, superparamagnetic iron oxide; TBO, toluidine blue O; TPU, thermoplastic polyurethane; TGF, transforming growth factor; TiO2, titanium dioxide; TNF-α, tumor necrosis factor-α; ZnO, zinc oxide.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81801007); the Science and Cultivation Foundation of Stomatological Hospital of Southern Medical University (PY2021016); the Natural Science Foundation of Guangdong Province (2018A030310442); and the Major of Basic and Applied Basic Research Project of Guangzhou City (202201011601).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Jafari A, Hassanajili S, Karimi MB, et al. Effect of organic/inorganic nanoparticles on performance of polyurethane nanocomposites for potential wound dressing applications. J Mech Behav Biomed Mater. 2018;88:395–405. doi:10.1016/j.jmbbm.2018.09.001
2. Balasundaram G, Storey DM, Webster TJ. Novel nano-rough polymers for cartilage tissue engineering. Int J Nanomedicine. 2014;9:1845–1853. doi:10.2147/ijn.S55865
3. Gajbhiye KR, Chaudhari BP, Pokharkar VB, Pawar A, Gajbhiye V. Stimuli-responsive biodegradable polyurethane nano-constructs as a potential triggered drug delivery vehicle for cancer therapy. Int J Pharm. 2020;588. doi:10.1016/j.ijpharm.2020.119781
4. Paladini F, Pollini M, Tala A, Alifano P, Sannino A. Efficacy of silver treated catheters for haemodialysis in preventing bacterial adhesion. J Mater Sci Mater Med. 2012;23(8):1983–1990. doi:10.1007/s10856-012-4674-7
5. Zaredar Z, Askari F, Shokrolahi P. Polyurethane synthesis for vascular application. Prog Biomater. 2018;7(4):269–278. doi:10.1007/s40204-018-0101-6
6. Selvakumar M, Jaganathan SK, Nando GB, Chattopadhyay S. Synthesis and characterization of novel polycarbonate based polyurethane/polymer wrapped hydroxyapatite nanocomposites: mechanical properties, osteoconductivity and biocompatibility. J Biomed Nanotechnol. 2015;11(2):291–305. doi:10.1166/jbn.2015.1975
7. Rusu L-C, Ardelean LC, Jitariu -A-A, Miu CA, Streian CG. An insight into the structural diversity and clinical applicability of polyurethanes in biomedicine. Polymers. 2020;12(5). doi:10.3390/polym12051197
8. Andriani Y, Morrow IC, Taran E, et al. In vitro biostability of poly(dimethyl siloxane/hexamethylene oxide)-based polyurethane/layered silicate nanocomposites. Acta Biomater. 2013;9(9):8308–8317. doi:10.1016/j.actbio.2013.05.021
9. Dai D, He L, Chen Y, Zhang C. Astrocyte responses to nanomaterials: functional changes, pathological changes and potential applications. Acta Biomater. 2021;122:66–81. doi:10.1016/j.actbio.2020.12.013
10. Sutthavas P, Tahmasebi Birgani Z, Habibovic P, van Rijt S. Calcium phosphate-coated and strontium-incorporated mesoporous silica nanoparticles can effectively induce osteogenic stem cell differentiation. Adv Healthc Mater. 2022;11(4). doi:10.1002/adhm.202101588
11. Wu T, Li B, Wang W, et al. Strontium-substituted hydroxyapatite grown on graphene oxide nanosheet-reinforced chitosan scaffold to promote bone regeneration. Biomater Sci. 2020;8(16):4603–4615. doi:10.1039/d0bm00523a
12. Lv Y, Zhang T, Zhang X, et al. The synergistic effect of Ag and ZnO on the microstructure, corrosion resistance and in vitro biological performance of titania coating. Surf Coat Technol. 2021;426. doi:10.1016/j.surfcoat.2021.127798
13. Jo SB, Erdenebileg U, Dashnyam K, et al. Nano-graphene oxide/polyurethane nanofibers: mechanically flexible and myogenic stimulating matrix for skeletal tissue engineering. J Tissue Eng. 2020;11. doi:10.1177/2041731419900424
14. Mamidi N, Delgadillo RMV, Barrera EV, Ramakrishna S, Annabi N. Carbonaceous nanomaterials incorporated biomaterials: the present and future of the flourishing field. Compos B Eng. 2022;243. doi:10.1016/j.compositesb.2022.110150
15. Chen J, Wang Q, Luan M, et al. Polydopamine as reinforcement in the coating of nano-silver on polyurethane surface: performance and mechanisms. Prog Org Coat. 2019;137. doi:10.1016/j.porgcoat.2019.105288
16. Kuang Z, Dai G, Wan R, et al. Osteogenic and antibacterial dual functions of a novel levofloxacin loaded mesoporous silica microspheres/nano-hydroxyapatite/polyurethane composite scaffold. Genes Dis. 2021;8(2):193–202. doi:10.1016/j.gendis.2019.09.014
17. Mamidi N, Gutierrez Garcia R, Hernandez Martinez JD, et al. Recent advances in designing fibrous biomaterials for the domain of biomedical, clinical, and environmental applications. ACS Biomater Sci Eng. 2022;8(9):3690–3716. doi:10.1021/acsbiomaterials.2c00786
18. Mamidi N, Velasco delgadillo RM. Design, fabrication and drug release potential of dual stimuli-responsive composite hydrogel nanoparticle interfaces. Colloids Surf B Biointerface. 2021;204. doi:10.1016/j.colsurfb.2021.111819
19. Wendels S, Averous L. Biobased polyurethanes for biomedical applications. Bioact MaterBioact Mater. 2021;6(4):1083–1106. doi:10.1016/j.bioactmat.2020.10.002
20. Naureen B, Haseeb ASMA, Basirun WJ, Muhamad F. Recent advances in tissue engineering scaffolds based on polyurethane and modified polyurethane. Mater Sci Eng C Mater Biol Appl. 2021;118. doi:10.1016/j.msec.2020.111228
21. Farrokhi Z, Ayati A, Kanvisi M, Sillanpaa M. Recent advance in antibacterial activity of nanoparticles contained polyurethane. J Appl Polym Sci. 2019;136(4). doi:10.1002/app.46997
22. Bharadwaz A, Jayasuriya AC. Recent trends in the application of widely used natural and synthetic polymer nanocomposites in bone tissue regeneration. Mater Sci Eng C Mater Biol Appl. 2020;110. doi:10.1016/j.msec.2020.110698
23. Zhang D, Liu W, Wu X-D, et al. Efficacy of novel nano-hydroxyapatite/polyurethane composite scaffolds with silver phosphate particles in chronic osteomyelitis. J Mater Sci Mater Med. 2019;30(6). doi:10.1007/s10856-019-6261-7
24. Gogoi S, Maji S, Mishra D, et al. Nano-bio engineered carbon dot-peptide functionalized water dispersible hyperbranched polyurethane for bone tissue regeneration. Macromol Biosci. 2017;17(3). doi:10.1002/mabi.201600271
25. Castro NJ, Tan WN, Shen C, Zhang LG. Simulated body fluid nucleation of three-dimensional printed elastomeric scaffolds for enhanced osteogenesis. Tissue Eng Part A. 2016;22(13–14):940–948. doi:10.1089/ten.tea.2016.0161
26. Kon E, Filardo G, Zaffagnini S, et al. Biodegradable polyurethane meniscal scaffold for isolated partial lesions or as combined procedure for knees with multiple comorbidities: clinical results at 2 years. Knee Surg Sports Traumatol Arthrosc. 2014;22(1):128–134. doi:10.1007/s00167-012-2328-4
27. Ziminska M, Chalanqui MJ, Chambers P, et al. Nanocomposite-coated porous templates for engineered bone scaffolds: a parametric study of layer-by-layer assembly conditions. Biomed Mater. 2019;14(6). doi:10.1088/1748-605X/ab3b7b
28. Li LM, Li JD, Zou Q, et al. Enhanced bone tissue regeneration of a biomimetic cellular scaffold with co-cultured MSCs-derived osteogenic and angiogenic cells. Cell Prolif. 2019;52(5). doi:10.1111/cpr.12658
29. John L. Selected developments and medical applications of organic-inorganic hybrid biomaterials based on functionalized spherosilicates. Mater Sci Eng C Mater Biol Appl. 2018;88:172–181. doi:10.1016/j.msec.2018.02.007
30. Zhu Q, Li X, Fan Z, et al. Biomimetic polyurethane/TiO2 nanocomposite scaffolds capable of promoting biomineralization and mesenchymal stem cell proliferation. Mater Sci Eng C Mater Biol Appl. 2018;85:79–87. doi:10.1016/j.msec.2017.12.008
31. Shoaib M, Rahman MSU, Saeed A, Naseer MM. Mesoporous bioactive glass-polyurethane nanocomposites as reservoirs for sustained drug delivery. Colloids Surf B Biointerface. 2018;172:806–811. doi:10.1016/j.colsurfb.2018.10.030
32. Liu H, Zhang L, Li J, et al. Physicochemical and biological properties of nano-hydroxyapatite-reinforced aliphatic polyurethanes membranes. J Biomater Sci Polym Ed. 2010;21(12):1619–1636. doi:10.1163/092050609x12524778957011
33. Jiang J, Li L, Li K, et al. Antibacterial nanohydroxyapatite/polyurethane composite scaffolds with silver phosphate particles for bone regeneration. J Biomater Sci Polym Ed. 2016;27(16):1584–1598. doi:10.1080/09205063.2016.1221699
34. Hou Y, Song Y, Sun X, et al. Multifunctional composite hydrogel bolus with combined self-healing, antibacterial and adhesive functions for radiotherapy. J Mater Chem B. 2020;8(13):2627–2635. doi:10.1039/c9tb02967b
35. Asefnejad A, Behnamghader A, Khorasani MT, Farsadzadeh B. Polyurethane/fluor-hydroxyapatite nanocomposite scaffolds for bone tissue engineering. Part I: morphological, physical, and mechanical characterization. Int J Nanomedicine. 2011;6:93–100. doi:10.2147/ijn.S13385
36. Aguilar-Perez FJ, Vargas-Coronado RF, Cervantes-Uc JM, et al. Preparation and bioactive properties of nano bioactive glass and segmented polyurethane composites. J Biomater Appl. 2016;30(9):1362–1372. doi:10.1177/0885328215626361
37. Tahlawi A, Klontzas ME, Allenby MC, et al. RGD-functionalized polyurethane scaffolds promote umbilical cord blood mesenchymal stem cell expansion and osteogenic differentiation. J Tissue Eng Regen Med. 2019;13(2):232–243. doi:10.1002/term.2784
38. Shaabani A, Sedghi R, Motasadizadeh H, Dinarvand R. Self-healable conductive polyurethane with the body temperature-responsive shape memory for bone tissue engineering. Chem Eng J. 2021;411. doi:10.1016/j.cej.2021.128449
39. Liu H, Zhang L, Shi P, et al. Hydroxyapatite/polyurethane scaffold incorporated with drug-loaded ethyl cellulose microspheres for bone regeneration. J Biomed Mater Res B Appl Biomater. 2010;95B(1):36–46. doi:10.1002/jbm.b.31680
40. Tian X, Yuan X, Feng D, et al. In vivo study of polyurethane and tannin-modified hydroxyapatite composites for calvarial regeneration. J Tissue Eng. 2020;11. doi:10.1177/2041731420968030
41. Smith AW. Biofilms and antibiotic therapy: is there a role for combating bacterial resistance by the use of novel drug delivery systems? Adv Drug Del Rev. 2005;57(10):1539–1550. doi:10.1016/j.addr.2005.04.007
42. Hafeman AE, Zienkiewicz KJ, Carney E, et al. Local delivery of tobramycin from injectable biodegradable polyurethane scaffolds. J Biomater Sci Polym Ed. 2010;21(1):95–112. doi:10.1163/156856209x410256
43. Xie J, Yao Y, Wang S, et al. Alleviating oxidative injury of myocardial infarction by a fibrous polyurethane patch with condensed ROS-scavenging backbone units. Adv Healthc Mater. 2022;11(4). doi:10.1002/adhm.202101855
44. Luketich SK, Cosentino F, Di Giuseppe M, et al. Engineering in-plane mechanics of electrospun polyurethane scaffolds for cardiovascular tissue applications. J Mech Behav Biomed Mater. 2022;128. doi:10.1016/j.jmbbm.2022.105126
45. Jing X, Mi H-Y, Salick MR, et al. Electrospinning thermoplastic polyurethane/graphene oxide scaffolds for small diameter vascular graft applications. Mater Sci Eng C Mater Biol Appl. 2015;49:40–50. doi:10.1016/j.msec.2014.12.060
46. Staniszewski Z, Piegat A, Okroj W, et al. The effect of carbon nanoparticles on biological properties of polyester nanocomposites. J Biomater Appl. 2017;31(10):1328–1336. doi:10.1177/0885328217706193
47. Gostev AA, Karpenko AA, Laktionov PP. Polyurethanes in cardiovascular prosthetics. Polym Bull. 2018;75(9):4311–4325. doi:10.1007/s00289-017-2266-x
48. Xie F, Zhang T, Bryant P, et al. Degradation and stabilization of polyurethane elastomers. Prog Polym Sci. 2019;90:211–268. doi:10.1016/j.progpolymsci.2018.12.003
49. Ahn CB, Son KH, Yu YS, et al. Development of a flexible 3D printed scaffold with a cell-adhesive surface for artificial trachea. Biomed Mater. 2019;14(5):055001. doi:10.1088/1748-605X/ab2a6c
50. Li Y, Rehbock C, Nachev M, et al. Matrix-specific mechanism of Fe ion release from laser-generated 3D-printable nanoparticle-polymer composites and their protein adsorption properties. Nanotechnology. 2020;31:40. doi:10.1088/1361-6528/ab94da
51. Major TC, Brant DO, Burney CP, et al. The hemocompatibility of a nitric oxide generating polymer that catalyzes S-nitrosothiol decomposition in an extracorporeal circulation model. Biomaterials. 2011;32(26):5957–5969. doi:10.1016/j.biomaterials.2011.03.036
52. Malone-Povolny MJ, Schoenfisch MH. Extended Nitric oxide-releasing polyurethanes via S-nitrosothiol-modified mesoporous silica nanoparticles. ACS Appl Mater Interfaces. 2019;11(13):12216–12223. doi:10.1021/acsami.8b19236
53. Hasan SM, Harmon G, Zhou F, et al. Tungsten-loaded SMP foam nanocomposites with inherent radiopacity and tunable thermo-mechanical properties. Polym Adv Technol. 2016;27(2):195–203. doi:10.1002/pat.3621
54. Song E-H, Jeong S-H, Park J-U, et al. Polyurethane-silica hybrid foams from a one-step foaming reaction, coupled with a sol-gel process, for enhanced wound healing. Mater Sci Eng C Mater Biol Appl. 2017;79:866–874. doi:10.1016/j.msec.2017.05.041
55. Kim JH, Unnithan AR, Kim HJ, et al. Electrospun badger (Meles meles) oil/Ag nanoparticle based anti-bacterial mats for biomedical applications. J Ind Eng Chem. 2015;30:254–260. doi:10.1016/j.jiec.2015.05.030
56. Esmaeili E, Eslami-Arshaghi T, Hosseinzadeh S, et al. The biomedical potential of cellulose acetate/polyurethane nanofibrous mats containing reduced graphene oxide/silver nanocomposites and curcumin: antimicrobial performance and cutaneous wound healing. Int J Biol Macromol. 2020;152:418–427. doi:10.1016/j.ijbiomac.2020.02.295
57. Rembe J-D, Fromm-Dornieden C, Boehm J, Stuermer EK. Influence of human acute wound fluid on the antibacterial efficacy of different antiseptic polyurethane foam dressings: an in vitro analysis. Wound Repair Regen. 2018;26(1):27–35. doi:10.1111/wrr.12612
58. Buzarovska A, Dinescu S, Lazar AD, et al. Nanocomposite foams based on flexible biobased thermoplastic polyurethane and ZnO nanoparticles as potential wound dressing materials. Mater Sci Eng C Mater Biol Appl. 2019;104. doi:10.1016/j.msec.2019.109893
59. Shin YC, Kang SH, Lee JH, et al. Three-dimensional graphene oxide-coated polyurethane foams beneficial to myogenesis. J Biomater Sci Polym Ed. 2018;29(7–9):762–774. doi:10.1080/09205063.2017.1348738
60. Namviriyachote N, Muangman P, Chinaroonchai K, Chuntrasakul C, Ritthidej GC. Polyurethane-biomacromolecule combined foam dressing containing asiaticoside: fabrication, characterization and clinical efficacy for traumatic dermal wound treatment. Int J Biol Macromol. 2020;143:510–520. doi:10.1016/j.ijbiomac.2019.10.166
61. Ding MM, Song NJ, He XL, et al. Toward the next-generation nanomedicines: design of multifunctional multiblock polyurethanes for effective cancer treatment. Acs Nano. 2013;7(3):1918–1928. doi:10.1021/nn4002769
62. Bhattacharyya A, Mukherjee D, Mishra R, Kundu PP. Preparation of polyurethane-alginate/chitosan core shell nanoparticles for the purpose of oral insulin delivery. Eur Polym J. 2017;92:294–313. doi:10.1016/j.eurpolymj.2017.05.015
63. Pan Z, He X, Song N, et al. Albumin-modified cationic nanocarriers to potentially create a new platform for drug delivery systems. ACS Appl Mater Interfaces. 2019;11(18):16421–16429. doi:10.1021/acsami.9b05599
64. da Silva GR, da Silva-cunha A, Behar-Cohen F, Ayres E, Orefice RL. Biodegradable polyurethane nanocomposites containing dexamethasone for ocular route. Mater Sci Eng C Mater Biol Appl. 2011;31(2):414–422. doi:10.1016/j.msec.2010.10.019
65. Irani M, Sadeghi GMM, Haririan I. A novel biocompatible drug delivery system of chitosan/temozolomide nanoparticles loaded PCL-PU nanofibers for sustained delivery of temozolomide. Int J Biol Macromol. 2017;97:744–751. doi:10.1016/j.ijbiomac.2017.01.073
66. Chen Y-P, S-h H. Preparation and characterization of novel water-based biodegradable polyurethane nanoparticles encapsulating superparamagnetic iron oxide and hydrophobic drugs. J Mater Chem B. 2014;2(21):3391–3401. doi:10.1039/c4tb00069b
67. Mohammadi A, Barikani M, Barmar M. Effect of surface modification of Fe3O4 nanoparticles on thermal and mechanical properties of magnetic polyurethane elastomer nanocomposites. J Mater Sci. 2013;48(21):7493–7502. doi:10.1007/s10853-013-7563-7
68. Mohammadi A, Barikani M, Barmar M. Effect of polyol structure on the properties of the resultant magnetic polyurethane elastomer nanocomposites. Polym Adv Technol. 2013;24(11):978–985. doi:10.1002/pat.3173
69. Cheng K-W, S-h H. A facile method to prepare superparamagnetic iron oxide and hydrophobic drug-encapsulated biodegradable polyurethane nanoparticles. Int J Nanomedicine. 2017;12:1775–1789. doi:10.2147/ijn.S120290
70. Xu S, Shi J, Feng D, Yang L, Cao S. Hollow hierarchical hydroxyapatite/Au/polyelectrolyte hybrid microparticles for multi-responsive drug delivery. J Mater Chem B. 2014;2(38):6500–6507. doi:10.1039/c4tb01066c
71. Lin T-W, Hsu S-H. Self-healing hydrogels and cryogels from biodegradable polyurethane nanoparticle crosslinked chitosan. Adv Sci. 2020;7(3). doi:10.1002/advs.201901388
72. Lee JM, Kang WS, Lee KG, et al. Combinatorial biophysical cue sensor array for controlling neural stem cell fate. Biosens Bioelectron. 2020;156. doi:10.1016/j.bios.2020.112125
73. Xing G, Li M, Sun Y, et al. Neurexin-Neuroligin 1 regulates synaptic morphology and functions via the WAVE regulatory complex in Drosophila neuromuscular junction. Elife. 2018;7. doi:10.7554/eLife.30457
74. Vigneault P, Parent S, Kanda P, et al. Electrophysiological engineering of heart-derived cells with calcium-dependent potassium channels improves cell therapy efficacy for cardioprotection. Nat Commun. 2021;12(1). doi:10.1038/s41467-021-25180-8
75. Sartori S, Chiono V, Tonda-Turo C, Mattu C, Gianluca C. Biomimetic polyurethanes in nano and regenerative medicine. J Mater Chem B. 2014;2(32):5128–5144. doi:10.1039/c4tb00525b
76. Demir US, Shahbazi R, Calamak S, et al. Gold nano-decorated aligned polyurethane nanofibers for enhancement of neurite outgrowth and elongation. J Biomed Mater Res A. 2018;106(6):1604–1613. doi:10.1002/jbm.a.36365
77. Jaswal R, Shrestha S, Shrestha BK, et al. Nanographene enfolded AuNPs sophisticatedly synchronized polycaprolactone based electrospun nanofibre scaffold for peripheral nerve regeneration. Mater Sci Eng C Mater Biol Appl. 2020;116. doi:10.1016/j.msec.2020.111213
78. Shrestha S, Shrestha BK, Lee J, et al. A conducting neural interface of polyurethane/silk-functionalized multiwall carbon nanotubes with enhanced mechanical strength for neuroregeneration. Mater Sci Eng C Mater Biol Appl. 2019;102:511–523. doi:10.1016/j.msec.2019.04.053
79. Shahrousvand M, Hoseinian MS, Ghollasi M, et al. Flexible magnetic polyurethane/Fe2O3 nanoparticles as organic-inorganic nanocomposites for biomedical applications: properties and cell behavior. Mater Sci Eng C Mater Biol Appl. 2017;74:556–567. doi:10.1016/j.msec.2016.12.117
80. Sheikh FA, Macossay J, Cantu T, et al. Imaging, spectroscopy, mechanical, alignment and biocompatibility studies of electrospun medical grade polyurethane (Carbothane(TM) 3575A) nanofibers and composite nanofibers containing multiwalled carbon nanotubes. J Mech Behav Biomed Mater. 2015;41:189–198. doi:10.1016/j.jmbbm.2014.10.012
81. Macossay J, Sheikh FA, Cantu T, et al. Imaging, spectroscopic, mechanical and biocompatibility studies of electrospun Tecoflex (R) EG 80A nanofibers and composites thereof containing multiwalled carbon nanotubes. Appl Surf Sci. 2014;321:205–213. doi:10.1016/j.apsusc.2014.09.198
82. Xu J, Wong C-W, Hsu S-H. An injectable, electroconductive hydrogel/scaffold for neural repair and motion sensing. Chem Mater. 2020;32(24):10407–10422. doi:10.1021/acs.chemmater.0c02906
83. Wang J, Zuo Y, Zhao M, et al. Physicochemical and biological properties of a novel injectable polyurethane system for root canal filling. Int J Nanomedicine. 2015;10:697–709. doi:10.2147/ijn.S74025
84. Samuel U, Guggenbichler JP. Prevention of catheter-related infections: the potential of a new nano-silver impregnated catheter. Int J Antimicrob Agents. 2004;23(Suppl 1):S75–S78.
85. Rtimi S, Sanjines R, Pulgarin C, Kiwi J. Microstructure of Cu-Ag uniform nanoparticulate films on polyurethane 3D catheters: surface properties. ACS Appl Mater Interfaces. 2016;8(1):56–63. doi:10.1021/acsami.5b09738
86. Hosny AE-DMS, Farrag HA, Helmy OM, Hagras SAA, El-Hag Ali A. In-vitro evaluation of antibacterial and antibiofilm efficiency of radiation-modified polyurethane-ZnO nanocomposite to be used as a self-disinfecting catheter. J Radiat Res Appl Sci. 2020;13(1):215–225. doi:10.1080/16878507.2020.1719328
87. Cheng Q, Cao D, Liu X, et al. Superhydrophobic coatings with self-cleaning and antibacterial adhesion properties for denture base. J Mech Behav Biomed Mater. 2019;98:148–156. doi:10.1016/j.jmbbm.2019.06.006
88. Khan AS, Aamer S, Chaudhry AA, Wong FSL, Rehman IU. Synthesis and characterizations of a fluoride-releasing dental restorative material. Mater Sci Eng C Mater Biol Appl. 2013;33(6):3458–3464. doi:10.1016/j.msec.2013.04.029
89. Khan AS, Hassan KR, Bukhari SF, Wong FSL, Rehman IU. Structural and in vitro adhesion analysis of a novel covalently coupled bioactive composite. J Biomed Mater Res B Appl Biomater. 2012;100B(1):239–248. doi:10.1002/jbm.b.31945
90. Garces IT, Aslanzadeh S, Boluk Y, Ayranci C. Cellulose nanocrystals (CNC) reinforced shape memory polyurethane ribbons for future biomedical applications and design. J Thermoplast Compos Mater. 2020;33(3):377–392. doi:10.1177/0892705718806334
91. Hernandez-Gomora AE, Lara-Carrillo E, Robles-Navarro JB, et al. Biosynthesis of silver nanoparticles on orthodontic elastomeric modules: evaluation of mechanical and antibacterial properties. Molecules. 2017;22(9). doi:10.3390/molecules22091407
92. Zhang X, Wang W, Yu D. Synthesis of waterborne polyurethane-silver nanoparticle antibacterial coating for synthetic leather. J Coat Technol Res. 2018;15(2):415–423. doi:10.1007/s11998-017-9997-3
93. Li X, Xie J, Liao L, Jiang X, Fu H. UV-curable polyurethane acrylate-Ag/TiO2 nanocomposites with superior UV light antibacterial activity. Int J Polym Mater Polym Biomater. 2017;66(16):835–843. doi:10.1080/00914037.2016.1276063
94. Bhattacharyya A, Nasim F, Mishra R, Bharti RP, Kundu PP. Polyurethane-incorporated chitosan/alginate core-shell nano-particles for controlled oral insulin delivery. J Appl Polym Sci. 2018;135(26). doi:10.1002/app.46365
95. Wang Y-J, Jeng US, Hsu S-H. Biodegradable water-based polyurethane shape memory elastomers for bone tissue engineering. ACS Biomater Sci Eng. 2018;4(4):1397–1406. doi:10.1021/acsbiomaterials.8b00091
96. Xie H, Shao J, Ma Y, et al. Biodegradable near-infrared-photoresponsive shape memory implants based on black phosphorus nanofillers. Biomaterials. 2018;164:11–21. doi:10.1016/j.biomaterials.2018.02.040
97. Rodriguez-Tobias H, Morales G, Grande D. Comprehensive review on electrospinning techniques as versatile approaches toward antimicrobial biopolymeric composite fibers. Mater Sci Eng C Mater Biol Appl. 2019;101:306–322. doi:10.1016/j.msec.2019.03.099
98. Fischer M, Vahdatzadeh M, Konradi R, et al. Multilayer hydrogel coatings to combine hemocompatibility and antimicrobial activity. Biomaterials. 2015;56:198–205. doi:10.1016/j.biomaterials.2015.03.056
99. Clarke SA, Choi SY, McKechnie M, et al. Osteogenic cell response to 3-D hydroxyapatite scaffolds developed via replication of natural marine sponges. J Mater Sci Mater Med. 2016;27(2). doi:10.1007/s10856-015-5630-0
100. Hess C, Schwenke A, Wagener P, et al. Dose-dependent surface endothelialization and biocompatibility of polyurethane noble metal nanocomposites. J Biomed Mater Res A. 2014;102(6):1909–1920. doi:10.1002/jbm.a.34860
101. Eftekhari A, Ahmadian E, Salatin S, et al. Current analytical approaches in diagnosis of melanoma. Trac Trends Analyt Chem. 2019;116:122–135. doi:10.1016/j.trac.2019.05.004
102. Yu J, Xia H, Teramoto A, Ni -Q-Q. The effect of hydroxyapatite nanoparticles on mechanical behavior and biological performance of porous shape memory polyurethane scaffolds. J Biomed Mater Res A. 2018;106(1):244–254. doi:10.1002/jbm.a.36214
103. Mehrban N, Bowen J, Tait A, et al. Silsesquioxane polymer as a potential scaffold for laryngeal reconstruction. Mater Sci Eng C Mater Biol Appl. 2018;92:565–574. doi:10.1016/j.msec.2018.07.003
104. Liu M, Liu T, Chen X, et al. Nano-silver-incorporated biomimetic polydopamine coating on a thermoplastic polyurethane porous nanocomposite as an efficient antibacterial wound dressing. J Nanobiotechnology. 2018;16. doi:10.1186/s12951-018-0416-4
105. Mandegary A, Saeedi A, Eftekhari A, Montazeri V, Sharif E. Hepatoprotective effect of silyamarin in individuals chronically exposed to hydrogen sulfide; modulating influence of TNF-alpha cytokine genetic polymorphism. J Pharm Sci. 2013;21. doi:10.1186/2008-2231-21-28
106. Dai -W-W, Guo H-F, Qian D-H, et al. Improving endothelialization by the combined application of polyethylene glycol coated cerium oxide nanoparticles and VEGF in electrospun polyurethane scaffolds. J Mater Chem B. 2017;5(5):1053–1061. doi:10.1039/c6tb02391f
107. Du C, Fikhman DA, Monroe MBB. Shape memory polymer foams with phenolic acid-based antioxidant properties. Antioxidants. 2022;11(6). doi:10.3390/antiox11061105
108. Qin X, Zhang J, Wang B, et al. Ferritinophagy is involved in the zinc oxide nanoparticles-induced ferroptosis of vascular endothelial cells. Autophagy. 2021;17(12):4266–4285. doi:10.1080/15548627.2021.1911016
109. Zhao J, Bowman L, Zhang X, et al. Titanium dioxide (TiO2) nanoparticles induce JB6 cell apoptosis through activation of the caspase-8/Bid and mitochondrial pathways. J Toxicol Environ Health A. 2009;72(19):1141–1149. doi:10.1080/15287390903091764
110. Loredo-Trevino A, Gutierrez-Sanchez G, Rodriguez-Herrera R, Aguilar CN. Microbial enzymes involved in polyurethane biodegradation: a review. J Polym Environ. 2012;20(1):258–265. doi:10.1007/s10924-011-0390-5
111. Mahajan N, Gupta P. New insights into the microbial degradation of polyurethanes. RSC Adv. 2015;5(52):41839–41854. doi:10.1039/c5ra04589d
112. Sun G, Ge H, Luo J, Liu R. Highly wear-resistant UV-curing antibacterial coatings via nanoparticle self-migration to the top surface. Prog Org Coat. 2019;135:19–26. doi:10.1016/j.porgcoat.2019.05.018
113. Soto-Quintero A, Romo-Uribe A, Bermudez-Morales VH, Quijada-Garrido I, Guarrotxena N. 3D-hydrogel based polymeric nanoreactors for silver nano-antimicrobial composites generation. Nanomaterials. 2017;7(8). doi:10.3390/nano7080209
114. Qiao Z, Yao Y, Song S, Yin M, Luo J. Silver nanoparticles with pH induced surface charge switchable properties for antibacterial and antibiofilm applications. J Mater Chem B. 2019;7(5):830–840. doi:10.1039/c8tb02917b
115. Wattanodorn Y, Jenkan R, Atorngitjawat P, Wirasate S. Antibacterial anionic waterborne polyurethanes/Ag nanocomposites with enhanced mechanical properties. Polym Test. 2014;40:163–169. doi:10.1016/j.polymertesting.2014.09.004
116. Mtimet I, Lecamp L, Kebir N, Burel F, Jouenne T. Green synthesis process of a polyurethane-silver nanocomposite having biocide surfaces. Polym J. 2012;44(12):1230–1237. doi:10.1038/pj.2012.90
117. Yan K, Liu C, Ma J. Dendritic fibrous nanosilica loaded chitosan for improving water vapor permeability and antibacterial properties of waterborne polyurethane acrylate membranes. J Clean Prod. 2021;291. doi:10.1016/j.jclepro.2021.125922
118. Guo Y, Zhang H, Duan S, et al. Bulk modification of thermoplastic polyurethanes for self-sterilization of trachea intubation. Macromol Biosci. 2021;21(2). doi:10.1002/mabi.202000318
119. Zhao Y-Q, Sun Y, Zhang Y, et al. Well-defined gold nanorod/polymer hybrid coating with inherent antifouling and photothermal bactericidal properties for treating an infected hernia. Acs Nano. 2020;14(2):2265–2275. doi:10.1021/acsnano.9b09282
120. Sehmi SK, Noimark S, Bear JC, et al. Lethal photosensitisation of Staphylococcus aureus and Escherichia coli using crystal violet and zinc oxide-encapsulated polyurethane. J Mater Chem B. 2015;3(31):6490–6500. doi:10.1039/c5tb00971e
121. Jia L, Prabhakaran MP, Qin X, Ramakrishna S. Guiding the orientation of smooth muscle cells on random and aligned polyurethane/collagen nanofibers. J Biomater Appl. 2014;29(3):364–377. doi:10.1177/0885328214529002
122. Pant B, Park M, Park S-J. One-step synthesis of silver nanoparticles embedded polyurethane nano-fiber/net structured membrane as an effective antibacterial medium. Polymers. 2019;11(7). doi:10.3390/polym11071185
123. Yazdani I, Movahedi B, Naeimi M, Sattary M, Rafienia M. Novel electrospun polyurethane scaffolds containing bioactive glass nanoparticles. Bioinspired Biomimetic Nanobiomater. 2020;9(3):175–183. doi:10.1680/jbibn.18.00004
124. Lee JH, Kim SH. Fabrication of silane-grafted graphene oxide and its effect on the structural, thermal, mechanical, and hysteretic behavior of polyurethane. Sci Rep. 2020;10(1). doi:10.1038/s41598-020-76153-8
125. Wlodarczyk D, Urban M, Strankowski M. Chemical modifications of graphene and their influence on properties of polyurethane composites: a review. Phys Scr. 2016;91(10). doi:10.1088/0031-8949/91/10/104003
126. Bhanushali H, Amrutkar S, Mestry S, Mhaske ST. Shape memory polymer nanocomposite: a review on structure-property relationship. Polym Bull. 2022;79(6):3437–3493. doi:10.1007/s00289-021-03686-x
127. Kausar A. Shape memory polyurethane/graphene nanocomposites: structures, properties, and applications. J Plast Film Sheeting. 2020;36(2):151–166. doi:10.1177/8756087919865296
128. Duarah R, Singh YP, Gupta P, Mandal BB, Karak N. High performance bio-based hyperbranched polyurethane/carbon dot-silver nanocomposite: a rapid self-expandable stent. Biofabrication. 2016;8(4). doi:10.1088/1758-5090/8/4/045013
129. Mohammadi A, Barikani M, Lakouraj MM. Biocompatible polyurethane/thiacalix 4 arenes functionalized Fe3O4 magnetic nanocomposites: synthesis and properties. Mater Sci Eng C Mater Biol Appl. 2016;66:106–118. doi:10.1016/j.msec.2016.04.064
130. Gabriel LP, Dos Santos MEM, Jardini AL, et al. Bio-based polyurethane for tissue engineering applications: how hydroxyapatite nanoparticles influence the structure, thermal and biological behavior of polyurethane composites. Nanomedicine. 2017;13(1):201–208. doi:10.1016/j.nano.2016.09.008
131. Li B, Shao W, Wang Y, et al. Synthesis and morphological control of biocompatible fluorescent/magnetic janus nanoparticles based on the self-assembly of fluorescent polyurethane and Fe3O4 nanoparticles. Polymers. 2019;11(2). doi:10.3390/polym11020272
132. Kausar A. Polylactic acid-based polyurethane/polyamide 6,12 and graphene nanocomposite: structure and physical property study for packaging application. Int J Polym Anal Charact. 2017;22(5):394–407. doi:10.1080/1023666x.2017.1312746
133. Kianpour G, Bagheri R, Pourjavadi A, Ghanbari H. In situ synthesized TiO2-polyurethane nanocomposite for bypass graft application: in vitro endothelialization and degradation. Mater Sci Eng C Mater Biol Appl. 2020;114. doi:10.1016/j.msec.2020.111043
134. Sabbah IA, Zaky MF, Hendawy ME, Negm NA. Synthesis, characterization and antimicrobial activity of colloidal copper nanoparticles stabilized by cationic thiol polyurethane surfactants. J Polym Res. 2018;25(12). doi:10.1007/s10965-018-1649-5
135. Palza H. Antimicrobial polymers with metal nanoparticles. Int J Mol Sci. 2015;16(1):2099–2116. doi:10.3390/ijms16012099
136. Hsu S-H, Tseng H-J, Lin Y-C. The biocompatibility and antibacterial properties of waterborne polyurethane-silver nanocomposites. Biomaterials. 2010;31(26):6796–6808. doi:10.1016/j.biomaterials.2010.05.015
137. Hwang B-U, Lee J-H, Tran Quang T, et al. Transparent stretchable self-powered patchable sensor platform with ultrasensitive recognition of human activities. Acs Nano. 2015;9(9):8801–8810. doi:10.1021/acsnano.5b01835
138. Macdonald TJ, Wu K, Sehmi SK, et al. Thiol-capped gold nanoparticles swell-encapsulated into polyurethane as powerful antibacterial surfaces under dark and light conditions. Sci Rep. 2016;6. doi:10.1038/srep39272
139. Hwang GB, Noimark S, Page K, et al. White light-activated antimicrobial surfaces: effect of nanoparticles type on activity. J Mater Chem B. 2016;4(12):2199–2207. doi:10.1039/c6tb00189k
140. Wierzbicki M, Jaworski S, Sawosz E, et al. Graphene oxide in a composite with silver nanoparticles reduces the fibroblast and endothelial cell cytotoxicity of an antibacterial nanoplatform. Nanoscale Res Lett. 2019;14(1). doi:10.1186/s11671-019-3166-9
141. Marycz K, Alicka M, Kornicka-Garbowska K, et al. Promotion through external magnetic field of osteogenic differentiation potential in adipose-derived mesenchymal stem cells: design of polyurethane/poly(lactic) acid sponges doped with iron oxide nanoparticles. J Biomed Mater Res B Appl Biomater. 2020;108(4):1398–1411. doi:10.1002/jbm.b.34488
142. Fallahiarezoudar E, Ahmadipourroudposht M, Yusof NM, Idris A, Ngadiman NHA. 3D biofabrication of Thermoplastic Polyurethane (TPU)/Poly-l-lactic Acid (PLLA) Electrospun Nanofibers Containing Maghemite (-Fe2O3) for tissue engineering aortic heart valve. Polymers. 2017;9(11). doi:10.3390/polym9110584
143. Qiao Y, Wan J, Zhou L, et al. Stimuli-responsive nanotherapeutics for precision drug delivery and cancer therapy. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2019;11(1). doi:10.1002/wnan.1527
144. Charpentier PA, Burgess K, Wang L, et al. Nano-TiO2/polyurethane composites for antibacterial and self-cleaning coatings. Nanotechnology. 2012;23:42. doi:10.1088/0957-4484/23/42/425606
145. Villani M, Consonni R, Canetti M, et al. Polyurethane-based composites: effects of antibacterial fillers on the physical-mechanical behavior of thermoplastic polyurethanes. Polymers. 2020;12(2). doi:10.3390/polym12020362
146. Jena KK, Narayan R, Raju KVSN. Investigation of the effect of ZnO nanoparticles on the thermomechanical and microbial properties of hyperbranched polyurethane-urea hybrid composites. Polym Int. 2012;61(8):1309–1317. doi:10.1002/pi.4209
147. Singha P, Workman CD, Pant J, Hopkins SP, Handa H. Zinc-oxide nanoparticles act catalytically and synergistically with nitric oxide donors to enhance antimicrobial efficacy. J Biomed Mater Res A. 2019;107(7):1425–1433. doi:10.1002/jbm.a.36657
148. Du J, Zuo Y, Lin L, et al. Effect of hydroxyapatite fillers on the mechanical properties and osteogenesis capacity of bio-based polyurethane composite scaffolds. J Mech Behav Biomed Mater. 2018;88:150–159. doi:10.1016/j.jmbbm.2018.08.028
149. Rashti A, Yahyaei H, Firoozi S, et al. Development of novel biocompatible hybrid nanocomposites based on polyurethane-silica prepared by sol gel process. Mater Sci Eng C Mater Biol Appl. 2016;69:1248–1255. doi:10.1016/j.msec.2016.08.037
150. Lee D, Lee SJ, Moon J-H, et al. Preparation of antibacterial chitosan membranes containing silver nanoparticles for dental barrier membrane applications. J Ind Eng Chem. 2018;66:196–202. doi:10.1016/j.jiec.2018.05.030
151. Lei W, Zhou X, Fang C, et al. New approach to recycle office waste paper: reinforcement for polyurethane with nano cellulose crystals extracted from waste paper. Waste Manage. 2019;95:59–69. doi:10.1016/j.wasman.2019.06.003
152. Cheng L, Ren S, Lu X. Application of eco-friendly waterborne polyurethane composite coating incorporated with nano cellulose crystalline and silver nano particles on wood antibacterial board. Polymers. 2020;12(2). doi:10.3390/polym12020407
153. Cui Z, Marcelle S, Zhao M, et al. Thermoplastic polyurethane/titania/polydopamine(TPU/TiO2/PDA) 3-D porous composite foam with outstanding oil/water separation performance and photocatalytic dye degradation. Adv Compos Hybrid Mater. 2022. doi:10.1007/s42114-022-00503-5
154. Xu D, Su Y, Zhao L, et al. Antibacterial and antifouling properties of a polyurethane surface modified with perfluoroalkyl and silver nanoparticles. J Biomed Mater Res A. 2017;105(2):531–538. doi:10.1002/jbm.a.35929
155. Borges I, Henriques PC, Gomes RN, et al. Exposure of smaller and oxidized graphene on polyurethane surface improves its antimicrobial performance. Nanomaterials. 2020;10(2). doi:10.3390/nano10020349
156. Jian Z, Wang H, Liu M, et al. Polyurethane-modified graphene oxide composite bilayer wound dressing with long-lasting antibacterial effect. Mater Sci Eng C Mater Biol Appl. 2020;111. doi:10.1016/j.msec.2020.110833
157. Barbucci R, Magnani A. Physiochemical characterization and coating of polyurethane with a new heparin-adsorbing material. Biomaterials. 1989;10(6):429–431. doi:10.1016/0142-9612(89)90136-1
158. Tan A, Farhatnia Y, Goh D, et al. Surface modification of a polyhedral oligomeric silsesquioxane poly(carbonate-urea) urethane (POSS-PCU) nanocomposite polymer as a stent coating for enhanced capture of endothelial progenitor cells. Biointerphases. 2013;8. doi:10.1186/1559-4106-8-23
159. Lackner JM, Waldhauser W, Hartmann P, et al. Hemocompatibility of inorganic Physical Vapor Deposition (PVD) coatings on thermoplastic polyurethane polymers. J Funct Biomater. 2012;3(2):283–297. doi:10.3390/jfb3020283
160. Meskinfam M, Bertoldi S, Albanese N, et al. Polyurethane foam/nano hydroxyapatite composite as a bone tissue regeneration. Mater Sci Eng C Mater Biol Appl. 2018;82:130–140. doi:10.1016/j.msec.2017.08.064
161. Prabhakar PK, Raj S, Anuradha PR, Sawant SN, Doble M. Biocompatibility studies on polyaniline and polyaniline-silver nanoparticle coated polyurethane composite. Colloids Surf B Biointerface. 2011;86(1):146–153. doi:10.1016/j.colsurfb.2011.03.033
162. Guo B, Ma PX. Synthetic biodegradable functional polymers for tissue engineering: a brief review. Sci China Chem. 2014;57(4):490–500. doi:10.1007/s11426-014-5086-y
163. Seitz BS, Plenagl N, Raschpichler M, et al. Nanoparticles and liposomes for the surface modification of implants: a comparative study of spraying and dipping techniques. Phys Status Solidi A. 2018;215:15. doi:10.1002/pssa.201700847
164. Huang Z, Ghasemi H. Hydrophilic polymer-based anti-biofouling coatings: preparation, mechanism, and durability. Adv Colloid Interface Sci. 2020;284. doi:10.1016/j.cis.2020.102264
165. Reczynska K, Major R, Kopernik M, et al. Surface modification of polyurethane with eptifibatide-loaded degradable nanoparticles reducing risk of blood coagulation. Colloids Surf B Biointerface. 2021;201. doi:10.1016/j.colsurfb.2021.111624
166. Yu D-G, Lin W-C, Yang M-C. Surface modification of poly(L-lactic acid) membrane via layer-by-layer assembly of silver nanoparticle-embedded polyelectrolyte multilayer. Bioconj Chem. 2007;18(5):1521–1529. doi:10.1021/bc060098s
167. Shen S, Chen J, Wang M, et al. Titanium dioxide nanostructures for photoelectrochemical applications. Prog Mater Sci. 2018;98:299–385. doi:10.1016/j.pmatsci.2018.07.006
168. Zhang X, Zhang X, Zhang X, et al. ZnSe nanoribbon/Si nanowire p-n heterojunction arrays and their photovoltaic application with graphene transparent electrodes. J Mater Chem. 2012;22(43):22873–22880. doi:10.1039/c2jm34720b
169. Xu C, Hong Y. Rational design of biodegradable thermoplastic polyurethanes for tissue repair. Bioact Mater. 2022;15:250–271. doi:10.1016/j.bioactmat.2021.11.029
170. Qiu S, Deng F, Xu S, et al. Degradation of pollutant and antibacterial activity of waterborne polyurethane/doped TiO2 nanoparticle hybrid films. J Wuhan Univ Technol Mater Sci Ed. 2015;30(3):447–451. doi:10.1007/s11595-015-1169-7
171. Zhao J, Du F, Cui W, et al. Effect of silica coating thickness on the thermal conductivity of polyurethane/SiO2 coated multiwalled carbon nanotube composites. Compos Part A-Appl S. 2014;58:1–6. doi:10.1016/j.compositesa.2013.11.008
172. Patel KK, Purohit R, Hashmi SAR, Gupta RK. Effect of the diameter of MWCNTs on shape memory and mechanical properties of polyurethane composites. J Polym Res. 2020;27(2). doi:10.1007/s10965-019-2003-2
173. Kumar P, Kumar V, Kumar R, Kumar R, Pruncu CI. Fabrication and characterization of ZrO2 incorporated SiO2-CaO-P2O5 bioactive glass scaffolds. J Mech Behav Biomed Mater. 2020;109. doi:10.1016/j.jmbbm.2020.103854
174. Maganty S, Roma MPC, Meschter SJ, et al. Enhanced mechanical properties of polyurethane composite coatings through nanosilica addition. Prog Org Coat. 2016;90:243–251. doi:10.1016/j.porgcoat.2015.10.016
175. Serkis M, Spirkova M, Kredatusova J, Hodan J, Bures R. Organic-inorganic nanocomposite films made from polyurethane dispersions and colloidal silica particles. Compos Interfaces. 2016;23(2):157–173. doi:10.1080/09276440.2016.1124666
176. Dimitry OIH, Abdeen ZI, Ismail EA, Saad ALG. Preparation and properties of elastomeric polyurethane/organically modified montmorillonite nanocomposites. J Polym Res. 2010;17(6):801–813. doi:10.1007/s10965-009-9371-y
177. Kennedy JP, McCandless SP, Lasher RA, Hitchcock RW. The mechanically enhanced phase separation of sprayed polyurethane scaffolds and their effect on the alignment of fibroblasts. Biomaterials. 2010;31(6):1126–1132. doi:10.1016/j.biomaterials.2009.10.024
178. Li Y, Zhang C, Zhu LF, et al. Elastic antibacterial membranes comprising particulate laden fibers for wound healing applications. J Appl Polym Sci. 2019;136(8). doi:10.1002/app.47105
179. Andrews KD, Hunt JA, Black RA. Technology of electrostatic spinning for the production of polyurethane tissue engineering scaffolds. Polym Int. 2008;57(2):203–210. doi:10.1002/pi.2317
180. Yin C, Rozet S, Okamoto R, et al. Physical properties and in vitro biocompatible evaluation of silicone-modified polyurethane nanofibers and films. Nanomaterials. 2019;9(3). doi:10.3390/nano9030367
181. Yang B, Zou Q, Lin L, et al. Synthesis and characterization of fluorescein-grafted polyurethane for potential application in biomedical tracing. J Biomater Appl. 2017;31(6):901–910. doi:10.1177/0885328216681893
182. Yuan WJ, Feng YK, Wang HY, et al. Hemocompatible surface of electrospun nanofibrous scaffolds by ATRP modification. Mater Sci Eng C Mater Biol Appl. 2013;33(7):3644–3651. doi:10.1016/j.msec.2013.04.048
183. Awad NK, Niu HT, Ali U, Morsi YS, Lin T. Electrospun fibrous scaffolds for small-diameter blood vessels: a review. Membranes. 2018;8(1):15. doi:10.3390/membranes8010015
184. Boffito M, Pontremoli C, Fiorilli S, et al. Injectable thermosensitive formulation based on polyurethane hydrogel/mesoporous glasses for sustained co-delivery of functional ions and drugs. Pharmaceutics. 2019;11(10). doi:10.3390/pharmaceutics11100501
185. Zhang C, Feng X, He L, Zhang Y, Shao L. The interrupted effect of autophagic flux and lysosomal function induced by graphene oxide in p62-dependent apoptosis of F98 cells. J Nanobiotechnology. 2020;18(1). doi:10.1186/s12951-020-00605-6
186. Eftekhari A, Azarmi Y, Parvizpur A, Eghbal MA. Involvement of oxidative stress and mitochondrial/lysosomal cross-talk in olanzapine cytotoxicity in freshly isolated rat hepatocytes. Xenobiotica. 2016;46(4):369–378. doi:10.3109/00498254.2015.1078522
187. Sanchez-Abella L, Ruiz V, Perez-San Vicente A, et al. Reactive oxygen species (ROS)-responsive biocompatible polyethylene glycol nanocomposite hydrogels with different graphene derivatives. J Mater Sci. 2021;56(16):10041–10052. doi:10.1007/s10853-021-05919-w
188. Liu J, Kang Y, Yin S, et al. Key role of microtubule and its acetylation in a zinc oxide nanoparticle-mediated lysosome-autophagy system. Small. 2019;15(25). doi:10.1002/smll.201901073
189. Zhao X, Toyooka T, Ibuki Y. Silver nanoparticle-induced phosphorylation of histone H3 at serine 10 is due to dynamic changes in actin filaments and the activation of Aurora kinases. Toxicol Lett. 2017;276:39–47. doi:10.1016/j.toxlet.2017.05.009
190. Mayer A, Vadon M, Rinner B, et al. The role of nanoparticle size in hemocompatibility. Toxicology. 2009;258(2–3):139–147. doi:10.1016/j.tox.2009.01.015
191. Dasgupta S, Auth T, Gompper G. Shape and orientation matter for the cellular uptake of nonspherical particles. Nano Lett. 2014;14(2):687–693. doi:10.1021/nl403949h
192. Bramini M, Sacchetti S, Armirotti A, et al. Graphene oxide nanosheets disrupt lipid composition, Ca2+ homeostasis, and synaptic transmission in primary cortical neurons. Acs Nano. 2016;10(7):7154–7171. doi:10.1021/acsnano.6b03438
193. Li Y, Liu C, Mo H, et al. Sodium triphosphate-capped silver nanoparticles on a decellularized scaffold-based polyurethane vascular patch for bacterial infection inhibition and rapid endothelialization. J Bioact Compat Polym. 2019;34(4–5):357–372. doi:10.1177/0883911519872779
194. Zhu H, Ji X, Guan H, et al. Tantalum nanoparticles reinforced polyetheretherketone shows enhanced bone formation. Mater Sci Eng C Mater Biol Appl. 2019;101:232–242. doi:10.1016/j.msec.2019.03.091
195. Gordon WJ, Conzemius MG, Birdsall E, et al. Chondroconductive potential of tantalum trabecular metal. J Biomed Mater Res B Appl Biomater. 2005;75B(2):229–233. doi:10.1002/jbm.b.30242
196. Huang Y, Zhai X, Ma T, et al. Rare earth-based materials for bone regeneration: breakthroughs and advantages. Coord Chem Rev. 2022;450. doi:10.1016/j.ccr.2021.214236
197. Choi J, Kim H-Y, Ju EJ, et al. Use of macrophages to deliver therapeutic and imaging contrast agents to tumors. Biomaterials. 2012;33(16):4195–4203. doi:10.1016/j.biomaterials.2012.02.022
198. Chen L, Hong W, Ren W, et al. Recent progress in targeted delivery vectors based on biomimetic nanoparticles. Signal Transduct Target Ther. 2021;6(1). doi:10.1038/s41392-021-00631-2
199. Molinaro R, Corbo C, Martinez JO, et al. Biomimetic proteolipid vesicles for targeting inflamed tissues. Nat Mater. 2016;15(9):1037–1046. doi:10.1038/nmat4644
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.