Back to Journals » Journal of Experimental Pharmacology » Volume 6
Biochronomer™ technology and the development of APF530, a sustained release formulation of granisetron
Authors Ottoboni T, Gelder M, O’Boyle E
Received 5 June 2014
Accepted for publication 14 August 2014
Published 9 December 2014 Volume 2014:6 Pages 15—21
DOI https://doi.org/10.2147/JEP.S68880
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Bal Lokeshwar
Thomas Ottoboni,1 Mark S Gelder,1 Erin O’Boyle2
1Heron Therapeutics, Inc., Redwood City, CA, USA; 2FibroGen, Inc., San Francisco, CA, USA
Abstract: Granisetron and other 5-hydroxytryptamine type 3 (5-HT3) receptor antagonists are first-line agents for preventing chemotherapy-induced nausea and vomiting (CINV). Current treatment guidelines prefer the longer-acting agent, palonosetron, for CINV prevention in some chemotherapy regimens. A new granisetron formulation, APF530, has been developed as an alternative long-acting agent. APF530 utilizes Biochronomer™ technology to formulate a viscous tri(ethylene glycol) poly(orthoester)-based formulation that delivers – by single subcutaneous (SC) injection – therapeutic granisetron concentrations over 5 days. The poly(orthoester) polymer family contain an orthoester linkage; these bioerodible polymer systems are specifically designed for controlled, sustained drug delivery. Pharmacokinetics and pharmacodynamics of APF530 250, 500, or 750 mg SC (granisetron 5, 10, or 15 mg, respectively) administered 30–60 minutes before chemotherapy were evaluated in two Phase II trials in cancer patients receiving moderately (MEC) or highly (HEC) emetogenic chemotherapy. Pharmacokinetics were dose proportional, with slow granisetron absorption and elimination. Both trials demonstrated similar results for median half-life, time to maximum concentration, and exposure for APF530 250 and 500 mg, with no differences between patients receiving MEC or HEC. A randomized Phase III trial demonstrated noninferiority of APF530 500 mg SC (granisetron 10 mg) to intravenous palonosetron 0.25 mg in preventing CINV in patients receiving MEC or HEC in acute (0–24 hours) and delayed (24–120 hours) settings, with activity over 120 hours. Mean maximum granisetron plasma concentrations were 10.8 and 17.8 ng/mL, and mean half-lives were 30.8 and 35.9 hours after SC administration of APF530 250 and 500 mg, respectively. Therapeutic granisetron concentrations were maintained for greater than 120 hours (5 days) in both APF530 dose groups. These data suggest that APF530 – an SC-administered formulation of granisetron delivered via Biochronomer technology – represents an effective treatment option for the prevention of both acute and delayed CINV in patients receiving either MEC or HEC.
Keywords: sustained release, poly(orthoester), granisetron, formulation, APF530
Introduction
Chemotherapy-induced nausea and vomiting (CINV) is associated with significant adverse effects on patient quality of life and can result in decreased compliance with further chemotherapy.1,2 Highly emetogenic chemotherapy (HEC; eg, cisplatin-based regimens) can produce acute CINV in virtually all patients, and moderately emetogenic chemotherapy (MEC; eg, carboplatin) can induce CINV in 30%–90% of patients.2,3 Even with the administration of antiemetic therapy, patients can continue to experience both acute CINV (0–24 hours after chemotherapy) and delayed CINV (24–120 hours after chemotherapy), and the incidence is often underestimated by physicians and nurses.4 Serotonin (5-hydroxytryptamine type 3 [5-HT3]) receptor antagonists have become an integral component of treatment, along with other antiemetic agents, for the prevention of acute and delayed CINV caused by either MEC or HEC agents.1,2,5 However, differences in pharmacokinetics and pharmacodynamics between the available 5-HT3 receptor antagonists can affect their efficacy in different clinical situations. Using an agent with a long duration of action and a good safety profile is important for ensuring effective prevention of CINV and simplifying management, especially in patients with comorbidities who are receiving multiple therapies or patients who are older and/or have cognitive impairment.6
Granisetron – one of several 5-HT3 receptor antagonists – is an effective treatment option for the prevention of CINV7,8 but has a relatively short half-life (t1/2; approximately 8 hours), so is administered daily on each day of chemotherapy.1,7,8 In contrast, the 5-HT3 receptor antagonist palonosetron has a longer t1/2 (~40 hours), so can be administered less frequently, and is indicated for prevention of acute and delayed CINV associated with MEC, and acute CINV associated with HEC.9–11 The control of delayed CINV, particularly in patients receiving HEC, is challenging. It has been reported that dexamethasone alone or a combination of dexamethasone and ondansetron can effectively control CINV in the MEC setting, but neither is as effective in the HEC setting.12 A formulation able to prolong therapeutically effective granisetron concentrations could provide an alternative option for control of both acute and delayed CINV in both MEC and HEC settings. This paper reports on a new formulation that provides sustained delivery of granisetron – designated APF530.
APF530
Product description and physicochemical properties
APF530 is a viscous tri(ethylene glycol) poly(orthoester) (TEG-POE)-based formulation designed to deliver, by a single subcutaneous (SC) injection, therapeutic concentrations of granisetron over a 5-day period. POEs are a family of bioerodible polymers that contain an orthoester linkage, and the use of these polymer systems is specifically designed for sustained release drug delivery applications.13 Biochronomer™ is a fourth-generation POE proprietary technology developed by Heron Therapeutics, Inc. (formerly AP Pharma, Inc.; Redwood City, CA, USA) that is synthesized by the addition of diols to a diketene acetal (Figure 1). Exposure to an aqueous environment results in the cleavage of the ester bond to create polymer fragments, which are rapidly cleared from the body. The diols used in this composition incorporate short segments containing glycolic acid esters (latent acid) that, when hydrolyzed, allow accurate control of the erosion rate. The composition takes advantage of the acid-labile nature of the polymer, which leads to controlled polymer hydrolysis and release of the active compound.13
With the proper selection of monomers, Biochronomers can be prepared in a variety of physical forms, ranging from hard, glassy materials to semi-solids, allowing the production of various dose forms such as injectable gels, microspheres, coatings, and strands. A significant advantage of the Biochronomer semi-solids technology is that drugs can be incorporated by simple mixing procedures, resulting in formulations that are injectable at room temperature (Ottoboni, unpublished data, 2013).
APF530 is a viscous TEG-POE-based formulation intended to provide controlled and sustained release of the free-base form of granisetron. The structure of the Biochronomer in APF530 used in clinical trials is shown in Figure 2. The formulation of APF530 uses the hydrophilic diol TEG. The molecular weight of the bioerodible polymer was limited to approximately 6 kDa, and methoxypoly(ethylene glycol) (molecular weight 550 Da) was used as an excipient to reduce viscosity. Overall, the composition of APF530 is 78.4% polymer, 19.6% methoxypoly(ethylene glycol) 550, and 2% (by weight) granisetron. Thus, APF530 250 mg contains 5 mg of granisetron and APF530 500 mg contains 10 mg of granisetron (Heron Therapeutics, Inc., data on file, 2013).
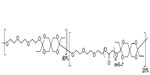  | Figure 2 Structure of Biochronomer™ used in APF530 clinical trials. |
Early-phase development
The pharmacodynamics and pharmacokinetics of granisetron are well established.7,8,14,15 The drug is a selective 5-HT3 receptor antagonist with little or no affinity for other serotonin receptors; α1-, α2-, or β-adrenoreceptors; or dopamine-D2, histamine-H1, benzodiazepine, or opioid receptors.8,15 Granisetron has been extensively evaluated in the prevention of acute and delayed CINV among patients receiving MEC or HEC.14 However, a significant proportion of patients continue to experience delayed CINV when using 5-HT3 receptor antagonists,4 suggesting that there is still a need for an improvement in treatment options.
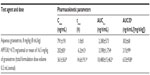
Findings from in vitro and nonclinical pharmacology and pharmacokinetics studies with APF530 supported the investigation of this formulation in clinical trials. In vitro studies investigated the release of granisetron from APF530 by incubating approximately 50–60 mg aliquots of APF530 in a vial with a biologically relevant medium (phosphate-buffered saline). Samples of the medium were taken at 0, 2, 4, 6, and 24 hours, and then approximately every 24 hours until total release of granisetron from the polymer vehicle. The medium was analyzed for the presence of granisetron using high-performance liquid chromatography. In one in vitro study, the mean release amounts of granisetron from AFP530 at 52, 95, and 142 hours were approximately 25%, 60%, and 90%, respectively. Total release of granisetron from the polymer was typically achieved within 200 hours (Figure 3; Heron Therapeutics, Inc., data on file, 2013). In an in vivo study in rats, the administration of APF530 0.2 mL SC (equivalent to granisetron 16.5 mg/kg) was associated with prolonged exposure to granisetron, compared with the administration of the commercially available saline formulation of granisetron at a lower dose of 8 mg/kg SC (Heron Therapeutics, Inc., data on file, 2013). This was characterized by a maximum plasma concentration (Cmax) two to four times lower and a time to Cmax (tmax) approximately six times longer than those with aqueous granisetron (Heron Therapeutics, Inc., data on file, 2013), even though the granisetron dose was higher with APF530, due to the delayed release characteristics of the APF530 formulation. The area under the concentration–time curves – corrected for dose – were similar or increased for APF530, compared with aqueous granisetron (Table 1). In other preclinical studies, APF530 was negative for genotoxicity in mouse models, and there were no granisetron-associated adverse effects on fertility in male and female rats at oral doses of granisetron up to 100 mg/kg/day and SC doses of APF530 up to 6 mg/kg/day. On the basis of these and other preclinical studies and early studies in healthy volunteers, three doses of APF530 were selected for further investigation: 250 mg (containing granisetron 5 mg), 500 mg (granisetron 10 mg), and 750 mg (granisetron 15 mg) to be administered as a single SC injection in the abdomen.
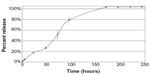  | Figure 3 In vitro percentage release of granisetron from APF530. |
Efficacy and safety
In two Phase II trials, one conducted in the US (N=45) and one in Europe (N=35), single-dose APF530 was evaluated in patients with cancer receiving single-day MEC or HEC.16 In the US trial, patients received APF530 250, 500, or 750 mg SC (granisetron 5, 10, and 15 mg, respectively) 30–60 minutes prior to chemotherapy, and patients in the European trial received 250 or 500 mg SC. Complete response (CR) – defined as no emesis and no rescue medication, and assessed over the 168-hour period after chemotherapy – was obtained in ≥80% and ≥75% of patients, in both trials combined, with APF530 250 mg and 500 mg, respectively.16 Thus, the 250 and 500 mg SC doses of APF530 were investigated in a subsequent Phase III trial.17
This randomized, multicenter, double-dummy, parallel-group Phase III trial evaluated the efficacy and safety of APF530 in chemotherapy-naïve and non-naïve patients with cancer receiving single-day administrations of either MEC or HEC (as defined by Hesketh).18 Patients were randomized to receive palonosetron 0.25 mg intravenously (IV), APF530 250 mg SC, or APF530 500 mg SC, all in combination with matching placebo and standardized doses of dexamethasone during their first cycle of chemotherapy in this study. During chemotherapy cycles two through four, all patients received either APF530 250 mg SC or APF530 500 mg SC. The primary efficacy endpoint was CR (no emesis and no rescue medication). Noninferiority was determined by the lower bound of the confidence interval (CI) calculated using the difference in CR rate between APF530 and palonosetron in relation to the lower bound of the predefined 15% noninferiority margin. Noninferiority and superiority were demonstrated if the lower bound of the CI was above 15% and 0%, respectively. Within each emetogenic stratum, the type I error rate was adjusted for the two APF530 doses and two endpoints using Hochberg’s Bonferroni procedure.19 Treatment comparisons were based on Fisher’s exact test. A total of 1,395 patients received study drug at 103 sites in three countries. In the modified intent-to-treat population, 707 patients (53%) received HEC and 634 (47%) received MEC.17 The most common MEC and HEC regimens were doxorubicin/cyclophosphamide (54% of patients) and a carboplatin combination (49% of patients), respectively.20
During cycle one, both the APF530 250 mg and 500 mg SC doses were noninferior to palonosetron for the prevention of acute CINV following MEC and HEC and APF530 500 mg was noninferior to palonosetron for the prevention of delayed CINV following MEC. Both doses of APF530 elicited CR rates comparable to that of palonosetron during the delayed CINV phase following HEC (Table 2).17 In the combined MEC and HEC populations, there was no significant difference between APF530 and palonosetron in CR rate for chemotherapy-naïve patients (APF530, 55% versus IV palonosetron, 58%) and non-naïve patients (APF530, 63% versus IV palonosetron, 55%) over the entire 120-hour period.20
  | Table 2 Complete response during acute and delayed CINV with APF530 250 mg subcutaneously, APF530 500 mg subcutaneously, and palonosetron 0.25 mg intravenously after administration of moderately emetogenic chemotherapy and highly emetogenic chemotherapy in cycle one (modified intent-to-treat population) (according to Hesketh criteria18) |
APF530 was generally well tolerated in these three clinical trials, with adverse events consistent with those previously reported for granisetron.17 In the Phase II trials, the most common adverse events were injection-site reactions (eg, erythema and induration) that were predominantly mild and occurred in fewer than 20% of patients.16 In the Phase III trial, most adverse events were mild, and most were considered unrelated to treatment by the investigator.17 The most commonly reported adverse events during cycle one were constipation, nausea, diarrhea, headache, and abdominal pain (Table 3). Apart from injection-site reactions, there were no differences in the occurrence of adverse events between the two doses of APF530 and between APF530 and palonosetron.17 Moreover, in a separate definitive QT study in healthy volunteers, APF530 at a dose of 1 g SC did not induce any clinically significant changes in QTc interval prolongation or changes in other electrocardiogram intervals.21
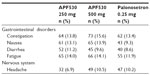  | Table 3 Treatment-emergent adverse events with APF530 250 mg subcutaneously, APF530 500 mg subcutaneously, and palonosetron 0.25 mg intravenously after administration of moderately emetogenic chemotherapy and highly emetogenic chemotherapy in cycle one |
A comparison of data from the previously untreated patients in this Phase III trial of APF530 (500 mg SC) and data from a similar population of patients receiving HEC in a separate comparative randomized double-blind Phase III trial of palonosetron (0.75 mg IV) and granisetron (40 μg/kg)10 showed that continuous exposure to a 5-HT3 receptor antagonist (all in combination with dexamethasone), using either a long-acting agent (palonosetron IV) or an extended-release formulation (APF530 SC) provided better emetic control than the standard IV formulation of granisetron.22 The benefit was particularly evident for delayed CINV and overall 5-day control. Control of delayed CINV was observed in 58%, 61%, 57%, and 45% of patients, respectively, in the APF530 500 mg SC, palonosetron 0.25 mg IV, palonosetron 0.75 mg IV, and granisetron 40 μg/kg IV groups; overall 5-day control was observed in 55%, 58%, 52%, and 40% of patients, respectively.22 These data support a role for long-acting or sustained release formulations of 5-HT3 antagonists, such as APF530, in the prevention of delayed CINV, in addition to palonosetron.
Clinical pharmacokinetics
Following administration of APF530 SC, granisetron is slowly absorbed and eliminated. Plasma concentrations of granisetron after administration of single doses of APF530 125 mg, 250 mg, 500 mg, and 1 g SC show that the absolute bioavailability of granisetron is high (Figure 4). In the two open-label Phase II clinical trials, pharmacokinetic parameters were assessed in patients undergoing MEC or HEC who received single doses of APF530 250 or 500 mg SC 30–60 minutes before their chemotherapy. Plasma granisetron was measured from pre-dose to 168 hours, and open noncompartmental methods were used to derive the pharmacokinetic parameters, which appeared to be dose proportional. In these two trials, mean t1/2 and median tmax were similar for the 250 mg doses, as were those for the 500 mg doses (Table 4).16 In the Phase III trial, the median tmax values of granisetron were 23.8 and 22.7 hours, respectively, after administration of APF530 250 and 500 mg SC. After reaching a Cmax of 10.8 and 17.8 ng/mL following administration of APF530 250 and 500 mg SC, respectively, plasma granisetron concentrations declined, with mean t1/2 values of 30.8 and 35.9 hours, respectively (Heron Therapeutics, Inc., data on file, 2013). Sustained concentrations of granisetron were observed over the entire 120-hour period in both APF530 dose groups.17 In comparison, in patients with cancer receiving granisetron 40 μg/kg IV, Cmax was reported to be 63.8 ng/mL, with a t1/2 of 8.95 hours.8
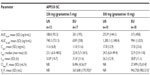  | Table 4 Pharmacokinetics of APF530 in two Phase II clinical trials in patients receiving moderately or highly emetogenic chemotherapy |
Place in therapy
The 5-HT3 receptor antagonists were a significant advance in the prevention of CINV when first introduced, and are now considered as first-line agents for the prevention and treatment of acute and delayed CINV.1,2,5 Palonosetron appears to be superior to other 5-HT3 receptor antagonists in preventing both acute and delayed CINV in patients receiving either MEC or HEC, presumably because of its prolonged duration of 5-HT3 antagonism.23 In current treatment guidelines, palonosetron is considered the preferred 5-HT3 antagonist for the prevention of CINV in patients receiving MEC or HEC, or in patients receiving MEC other than combinations of an anthracycline and cyclophosphamide.1,2 Results from a large randomized Phase III trial demonstrated that APF530 500 mg (granisetron 10 mg) is noninferior to palonosetron for the prevention of acute and delayed CINV in patients receiving MEC or HEC, with efficacy over a 120-hour period.17,22 These data suggest that APF530 – an SC-administered sustained release formulation of granisetron delivered via Biochronomer technology – provides an alternative treatment option for the prevention of CINV in patients receiving MEC or HEC. Specifically, the Biochronomer technology employed in AFP530, a formulation of the first-generation 5-HT3 antagonist granisetron in combination with the TEG-POE polymer, provides a long-acting treatment that can be administered by a single SC injection to control both acute and delayed CINV in patients receiving MEC and HEC with antiemetic efficacy comparable to that of palonosetron. The utility of APF530 in other clinical scenarios requiring sustained antiemetic activity, such as in patients receiving multiday chemotherapy or radiation therapy, or to control postoperative CINV, is yet to be determined. Future studies may also consider the use of AFP530 in combination with other antiemetic agents, such as neurokinin-1 antagonists. The Biochronomer technology may also provide the opportunity to expand treatment options in clinical settings beyond that of CINV, where a sustained release formulation is required.
Acknowledgments
Medical writing support was provided by Yvonne E Yarker of SciStrategy Communications, and was funded by Heron Therapeutics, Inc.
Disclosure
Thomas Ottoboni and Mark S Gelder are employees of Heron Therapeutics, Inc. Erin O’Boyle was an employee of AP Pharma, Inc. (now Heron Therapeutics, Inc.) at the initiation of the manuscript.
References
National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Antiemesis – v1.2014. Fort Washington, PA: National Comprehensive Cancer Network; 2013. Available from: http://www.nccn.org/professionals/physician_gls/pdf/antiemesis.pdf. Accessed September 4, 2014. | |
Roila F, Herrstedt J, Aapro M, et al. Guideline update for MASCC and ESMO in the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting: results of the Perugia consensus conference. Ann Oncol. 2010;21(Suppl 5):v232–v243. | |
Grunberg SM, Osoba D, Hesketh PJ, et al. Evaluation of new antiemetic agents and definition of antineoplastic agent emetogenicity – an update. Support Care Cancer. 2005;13(2):80–84. | |
Grunberg SM, Deuson RR, Mavros P, et al. Incidence of chemotherapy-induced nausea and emesis after modern antiemetics. Cancer. 2004;100(10):2261–2268. | |
Basch E, Prestrud AA, Hesketh PJ, et al. Antiemetics: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2011;29(31):4189–4198. | |
Aapro M, Johnson J. Chemotherapy-induced emesis in elderly cancer patients: the role of 5-HT3-receptor antagonists in the first 24 hours. Gerontology. 2005;51(5):287–296. | |
Kytril® (granisetron hydrochloride) tablets oral solution [prescribing information]. Basel: Hoffman-La Roche Ltd; 2010. | |
Kytril® (granisetron hydrochloride) injection [prescribing information]. South San Francisco, CA: Genentech, Inc.; 2011. | |
Stoltz R, Cyong JC, Shah A, Parisi S. Pharmacokinetic and safety evaluation of palonosetron, a 5-hydroxytryptamine-3 receptor antagonist, in US and Japanese healthy subjects. J Clin Pharmacol. 2004;44(5): 520–531. | |
Saito M, Aogi K, Sekine I, et al. Palonosetron plus dexamethasone versus granisetron plus dexamethasone for prevention of nausea and vomiting during chemotherapy: a double-blind, double-dummy, randomised, comparative Phase III trial. Lancet Oncol. 2009;10(2):115–124. | |
Aloxi® (palonosetron HCl) injection for intravenous use [prescribing information]. Woodcliff Lake, NJ: Eisai Inc.; 2014. | |
The Italian Group for Antiemetic Research. Dexamethasone alone or in combination with ondansetron for the prevention of delayed nausea and vomiting induced by chemotherapy. N Engl J Med. 2000;342(21):1554–1559. | |
Heller J, Barr J. Biochronomer technology. Expert Opin Drug Deliv. 2005;2(1):169–183. | |
Aapro M. Granisetron: an update on its clinical use in the management of nausea and vomiting. Oncologist. 2004;9(6):673–686. | |
Hsu ES. A review of granisetron, 5-hydroxytryptamine3 receptor antagonists, and other antiemetics. Am J Ther. 2010;17(5):476–486. | |
Gabrail N, Yanagihara RH, Cooper W, et al. Pharmacokinetics (PK), tolerability, and efficacy of APF530 in patients receiving moderately (MEC) and highly (HEC) emetogenic chemotherapy: Phase II trial results [abstract]. J Clin Oncol. 2013;31 Suppl:abstract e20518. | |
Raftopoulos H, Cooper W, O’Boyle E, Gabrail N, Boccia R, Gralla RJ. Comparison of an extended-release formulation of granisetron (APF530) versus palonosetron for the prevention of chemotherapy-induced nausea and vomiting associated with moderately or highly emetogenic chemotherapy: results of a prospective, randomized, double-blind, noninferiority phase 3 trial. Support Care Cancer. Epub 2014 September 2. | |
Hesketh PJ. Defining the emetogenicity of cancer chemotherapy regimens: relevance to clinical practice. Oncologist. 1999;4(3):191–196. | |
Hochberg J. A sharper Bonferroni procedure for multiple tests of significance. Biometrika. 1988;75(4):800–802. | |
Charu V, Clark-Snow R, Gabrail N, et al. Patient satisfaction with control of emesis following chemotherapy [abstract]. Support Care Cancer. 2012;20(Suppl 1):abstract 1109. | |
Mason JW, Moon TE, O’Boyle E, Dietz A. A randomized, placebo-controlled, four-period crossover, definitive QT study of the effects of APF530 exposure, high-dose intravenous granisetron, and moxifloxacin on QTc prolongation. Cancer Manag Res. 2014;6:181–190. | |
Raftopoulos H, O’Boyle E, Gralla RJ, Rosenberg M, Barr J. The effect of continuous exposure to serotonin receptor antagonism on delayed emesis: an analysis of 1,535 patients in two randomized clinical trials with granisetron (G), APF530, and palonosetron (palo) [abstract]. J Clin Oncol. 2012;30 Suppl:abstract e19635. | |
Botrel TE, Clark OA, Clark L, Paladini L, Faleiros E, Pegoretti B. Efficacy of palonosetron (PAL) compared to other serotonin inhibitors (5-HT3R) in preventing chemotherapy-induced nausea and vomiting (CINV) in patients receiving moderately or highly emetogenic (MoHE) treatment: systematic review and meta-analysis. Support Care Cancer. 2011;19(6):823–832. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.