Back to Journals » Cancer Management and Research » Volume 12
Biochemical Markers of Colorectal Cancer – Present and Future
Received 10 March 2020
Accepted for publication 22 May 2020
Published 22 June 2020 Volume 2020:12 Pages 4789—4797
DOI https://doi.org/10.2147/CMAR.S253369
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Eileen O'Reilly
Wojciech Jelski,1 Barbara Mroczko1,2
1Department of Biochemical Diagnostics, Medical University of Bialystok, Bialystok, Poland; 2Department of Neurodegeneration Diagnostics, Medical University of Bialystok, Bialystok, Poland
Correspondence: Wojciech Jelski
Department of Biochemical Diagnostics, Medical University of Bialystok, Waszyngtona 15 A, Bialystok 15– 269, Poland
Tel +48 85 746 8587
Fax +48 85 746 8585
Email [email protected]
Abstract: According to a report by the National Cancer Institute, colorectal cancer (CRC) is one of the most common types of cancer worldwide. CRC is often recognized too late for successful therapy. Tumor markers have been sought for a number of years to detect the transformation of malignant cells at the earliest possible stage. They are usually proteins associated with a malignancy and might be clinically useful in patients with cancer. Several classical markers have been used to recognize colorectal cancer, including carcinoembryonic antigen (CEA), carbohydrate antigen (CA 19.9), tissue polypeptide specific antigen (TPS) and tumor-associated glycoprotein-72 (TAG-72). None of these tests, however, have excellent diagnostic accuracy. Recent studies have been conducted on the use of hematopoietic growth factors (HGFs) and various enzymes in the diagnosis and prognosis of colorectal cancer. These include macrophage-colony stimulating factor (M-CSF) and granulocyte-macrophage-colony stimulating factor (GM-CSF), interleukin-3, interleukin-6 and enzymes (alcohol dehydrogenase and lysosomal exoglycosidases). Significantly, most cancer deaths are not caused by the primary tumor itself but by its spread. Analysis of circulating cancer cells (CTCs), ie, factors responsible for metastasis, may be a source of information useful in the treatment of patients with colorectal cancer. Currently available markers have significant limitations.
Keywords: tumor markers, colorectal cancer
Introduction
Colorectal cancer (CRC) is one of the most common cancers worldwide, with over one million new cases per year. CRC is the second leading cause of cancer deaths in the United States.1 In recent years, an increase in colorectal cancer incidence has occurred in younger people (aged<50 years). Beginning in the early 1990s, incidence rates increased among younger adults from 8.6 per 100,000 in 1992 to 12.5 per 100,000 in 2015, an overall increase of 45%.2,3 Over time, the incidence of CRC increases in younger patients. In China, due to changes in diet and lifestyle, morbidity associated with CRC is on the rise, and CRC has recently begun to affect younger people. One of the primary risk factors for colorectal cancer is obesity, a condition typically assessed using a scale known as the body mass index (BMI).4 The underlying etiology of CRC includes both genetic variation and environmental exposure. It has been suggested that the interplay between genetic variants and environmental risk factors, known as gene–environment interaction, may also contribute to an increase of CRC risk.5 The majority of cases are due to poor dietary patterns, host immunity, and lifestyle factors such as smoking, low physical activity levels, and obesity. Other gastrointestinal disorders, such as inflammatory bowel disease characterized by chronic inflammation, mucosa disruption, and the excessive production of reactive oxygen species, act as risk factors in the onset of cancer. In recent years, a new and remarkable factor in the development of cancer and other related intestinal diseases has emerged; the gastrointestinal tract microbiota.6 Carcinogenesis is a long, complex and gradual process. The prognosis for patients with colon cancer is correlated with the pathological stage at the time of detection and it is very important to find markers that would detect a malignant tumor as early as possible.7 This is why the search for new biochemical markers in blood is necessary. Colorectal cancer is a serious disease that is characterized by rapid progression, invasiveness and high resistance to treatment. Diagnosing CRC at an early stage is not easy, as cancer is often asymptomatic. Screening requires tools and methods that are both highly sensitive and specific when diagnosing the early stages of cancer. They must be safe, cheap and widely accepted. A tumor marker can be detected in a solid tumor tissue, in a lymph node, bone marrow, peripheral blood, or other biological materials (urine, ascites, and stool).8 Several markers of colorectal cancer, including carcinoembryonic antigen (CEA), carbohydrate antigen (CA 19.9), tissue polypeptide specific antigen (TPS), tumor-associated glycoprotein-72 (TAG-72), and hematopoietic growth factors (HGF-s) have been recognized and are accepted in routine clinical practice.9 The first diagnostic examination is frequently a simple, noninvasive and inexpensive fecal occult blood test (Figure 1). However, fecal blood is a nonspecific indicator of colorectal cancer, as it can not only come from cancerous lesions but also from polyps. Distal endoscopy, which is the gold standard in diagnosing CRC, allows the diagnosis of changes in real time and enables physicians to perform a target biopsy and histopathological analysis. Endoscopic ultrasound, computed tomography and magnetic resonance imaging (MRI) with full clinical assessment make the choice of therapeutic treatment possible.
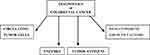 |
Figure 1 Division of colorectal cancer markers. |
Recent technological and analytic advances have boosted scientific biomarker research. In the near future, the advent of novel urinary assays with high efficacy that would reduce CRC mortality is expected. In colorectal cancer, molecular (eg mutations in the KRAS, NRAS, BRAF, PIK3CA genes) and immunohistochemical markers (eg TS, P21, PTEN proteins) are used to assess predictive goals. Molecular markers in colorectal cancer can be divided into somatic mutations and microsatellite instability (MSI).
Classical Tumor Markers
Carcinoembryonic antigen (CEA) is a glycoprotein oncofetal antigen that is expressed in many epithelial tumors. This relatively inexpensive blood test, first described by Gold and Freedman in 1965, was part of most recommended surveillance strategies.10 CEA is a glycoprotein which is formed in the cells of the large bowel. Seventy percent of patients with CRC have high CEA levels during diagnosis, which makes it a very good marker for the treatment and monitoring of the disease after resection. Although CEA is usually considered a cancer marker, its concentrations may also be elevated in a variety of benign conditions, including hepatitis, pancreatitis, obstructive pulmonary disease and inflammatory bowel disease. According to commonly accepted units of measurement values of up to 5 ng/mL are considered normal antigen level in blood. It has been observed that these values in smokers, in cases of ulcer colitis or liver cirrhosis, can be increased up to 10 ng/mL11. Tan et al conducted a quantitative meta-analysis of 20 studies involving 4285 patients and investigated CEA performance characteristics when used to detect recurrence of colorectal cancer. Overall sensitivity was found to be 0.64 and specificity 0.90.12 The study by Chen et al in Taiwan examined whether rising CEA was an added value in detecting postoperative relapses. In a study of 4841 patients, 999 had elevated CEA (defined at >5 ng/mL) and a relapse. About three-quarters of these patients had recurrence detected by other means at the same time as the first increase in CEA.13 Patients treated for colorectal cancer should have CEA levels monitored every 3 months. Unfortunately, an increase of CEA concentration is only sometimes observed during the first stage of CRC. This mostly happens in the advanced stages of cancer. An increased concentration of CEA prior to operation may correlate with an adverse prognosis.
CA 19.9 (carbohydrate antigen) is a glycoprotein characterized by a high molecular weight which may be released to the blood. This marker is used in the diagnostics of pancreatic, colorectal and gastric cancers. Like CEA, it is not specific to a particular histological type of carcinoma and the organ which it comes from. Vukobrat-Bijedic et al showed that CA 19.9 is less sensitive than CEA.14 The combined assays of CEA and CA 19.9 may increase diagnostic sensitivity in colorectal cancer detection. Moreover, the determination of both of these markers is used as a postoperative prognostic factor in the evaluation of the stage of the disease and survival rate.15 Nakatani et al in their research from 2012 provided data that colon cancer located in the region of sigma had extremely high concentrations of CEA and CA19.9.16 There is no significant increase in sensitivity by combining CEA and CA 19.9 determinations. Both CA 19.9 concentration and sensitivity increase with higher Dukes’ stage of disease, but do not correlate with the tumor location and number of positive lymph nodes. Patients with Dukes’ C tumors with preoperative CA 19.9 concentrations higher than 37 U/mL had a shorter disease-free survival period.17
Tissue polypeptide specific antigen (TPS) has been described as a useful tumor marker in many malignant cancers and as a response factor in monitoring chemotherapy in different advanced gastrointestinal carcinomas.18 It is a singular conjugated chain of polypeptide, which is produced in different phases of the molecular cycle (S or G2) and subsequently released to tissue after mitotic division. Tissue-polypeptide-specific antigen (TPS) is a soluble fragment derived from the carboxy-terminal end of cytokeratin 18. High TPS concentration is a marker of tumor activity, but not necessarily mass of tumor. The level of TPS in blood, strongly associated with proliferation of cancer cells, is a function of the cell division rate. Estimation of tissue polypeptide specific antigen may be applicable in the early stages of cancers. A high level of tissue polypeptide specific antigen occurs in about 60–80% of patients with colorectal cancer.19 The survival rate was significantly lower in patients with initially higher concentrations of TPS. Repeated determination of TPS concentration during therapy may be of clinical importance, especially as a marker of non-response. Therefore, TPS is superior to the commonly used CEA.18 In asymptomatic patients that require active treatment due to a generally poor prognosis, changes in elevated TPS levels appear to be useful in determining the length of treatment.20
Tumor-associated glycoprotein-72 (TAG-72) is a glycoprotein formed in bile duct endothelial cells, gastric epithelium or renal pelvis cells. It is a mucin-like molecule with a molar mass of over 1000 kDa. TAG-72 is found on the surface of many cancer cells, including colon, ovary, breast, and pancreatic cells.7 Guadagni et al showed that serum concentrations of TAG-72, CEA, CA 19.9 were elevated in 43%, 43% and 27% of patients with colorectal cancer, respectively. It is advisable to determine TAG-72 together with other markers, primarily CEA. Sixty-one percent of patients had at least one marker with elevated levels when measuring these three markers.21
Analysis of ctDNA in peripheral blood samples, so-called liquid biopsies, has the potential to discern early-stage detection of CRC and serve as a prognostic, monitoring and predictive tool. A number of studies describe the use of ctDNA methylation markers for the diagnosis and prognosis of colorectal cancer. So far, the highest accuracy for CRC detection has been obtained by SEPT9 hypermethylation analysis, especially in combined panels. The high sensitivities of up to 100% and specificities of up to 97% of SEPT9 methylation ctDNA analysis suggest a diagnostic role for this candidate marker.22 In addition, Lou et al have shown that a single ctDNA methylation marker, cg10673833, could yield high sensitivity (89.7%) and specificity (86.8%) for the detection of CRC and precancerous lesions in a high-risk population in a prospective cohort study.23 Several studies found that abnormal methylation of septin9 (mSEPT9) in the blood can be used as an early diagnostic marker for colorectal cancer. Using the latest second-generation mSEPT9 assay, Zhi Yao Ma et al found a significantly higher sensitivity of mSEPT9 than CEA for the diagnosis of CRC (73.2% vs 48.2%; P < 0.001), especially for patients with stage II and III cancer.24 Toth et al reported similar results, with respective sensitivities of 95.6% (88/92) and 51.8% (14/27), and specificities of 84.8% and 85.2% for mSEPT9 and CEA20.25 In another recent study, mSEPT9 was also shown to have a higher diagnostic value than CEA for both sensitivity (61.8% vs 35.0%) and specificity (89.6% vs 62.6%).26
Insulin-like growth-factor binding protein 2 (IGFBP-2) is an extracellular protein that binds insulin-like growth factor 2 (IGF-2) and, with a smaller affinity, insulin-like growth factor 1 (IGF-1). IGFBP-2 plays an important role in heat shock protein 27-mediated cancer progression and metastasis. IGFBP-2 serum levels were reported to be significantly elevated in patients with colon cancer in three studies.27,28
Recently, several inflammatory markers including pretreatment neutrophil to lymphocyte ratio (NLR) have been used as prognostic factors, since host inflammatory response to cancer is believed to determine disease progression.29 Dimitriou et al have found that in patients with CRC, a pretreatment NLR above 4.7 is a poor prognostic factor for disease-free survival, 5-year survival and overall survival. The poor prognostic effect of NRL is magnified in stage II CRC patients.30
The concentration of IGFBP-2 appears to be a prognostic factor that strongly correlates with overall survival.27 Heat shock protein 60 (HSP60) is a key factor involved in inflammation, and serum HSP60 levels might also be increased in patients with inflammatory pathologies such as ulcerative colitis and Crohn’s disease.31 Vocka et al indicated that serum HSP60 could be used as an effective prognostic biomarker of CRC with the same sensitivity as CEA and better sensitivity than CA19-9.27
Hematopoietic Growth Factors
Colorectal cancer cells are capable of producing hematopoietic growth factors (HGFs). Stem cell factor (SCF), macrophage-colony stimulating factor (M-CSF) and granulocyte-macrophage-colony stimulating factor (GM-CSF) are members of glycoprotein cytokines called colony-stimulating factors (CSFs) or HGFs. Hematopoietic growth factors are involved in the regulation of growth and spread of cancer. HGFs regulate the proliferation of hematopoietic progenitor cells and can also affect the proliferation of nonhematopoietic cells (Figure 2). Cell surface receptors for HGF have been detected in colon cancer cell lines and the stimulation of tumor cells proliferations occurs via these receptors. Several studies have shown that HGFs can also stimulate the proliferation of nonhematopoietic cells and the effect of these cytokines is not limited to bone marrow cells.32 HGFs can act on cancer tissue in an autocrine manner or on supporting tissues and blood vessels to produce an environment conducive to the development of cancer. Receptors of HGFs have been detected in colorectal cancer cell lines and stimulation of CSFs receptors induced the proliferation of tumor cells. HGFs may also induce normal cells, such as tumor-associated macrophages (TAM) and endothelial cells, to produce additional cytokines that support the malignant process. Several cell lines of a malignant tumor have been demonstrated to secrete large amounts of CSFs. Mroczko et al found that blood concentration of M-CSF and granulocyte-colony stimulating factor (G-CSF) were significantly higher in colorectal cancer patients in comparison to controls.33 The level of both markers was dependent on the stage of the tumor, but only M-CSF showed significant differences. In addition, it was found that M-CSF serum levels were higher in patients with lymph node or distant metastases. The diagnostic specificity and sensitivity of M-CSF were 95% and 65%, respectively. All diagnostic criteria such as sensitivity, specificity and area under ROC curve were lower for G-CSF than for M-CSF. Therefore, M-CSF seems to be a better marker than G-CSF in the diagnosis and prognosis of colorectal cancer. Other studies showed elevated levels of several proinflammatory cytokines, such as interleukin-6 (IL 6), interleukin-8 (IL 8), tumor necrosis factor-α (TNF-α) and acute-phase proteins in patients with colorectal carcinoma and other malignancies.34,35 Mroczko et al showed a potential role for stem cell factor and interleukin-3 (IL 3) as tumor markers for colorectal cancer, especially in combination with CEA and CA19-9.36
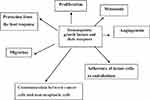 |
Figure 2 The role of hematopoietic growth factors and their receptors in tumor development. |
Enzymes
Newly conducted research by Jelski et al on the use of enzymes as markers for colorectal cancer, including alcohol dehydrogenase (ADH), cathepsin D and lysosomal exoglycosidases reported that the activity of alcohol dehydrogenase is significantly higher in cancerous cells than that in healthy tissue and the activity of aldehyde dehydrogenase (ALDH) is not different between healthy and cancer tissues. ADH activity seems to be disproportionally higher compared to the activity of ALDH in cancer tissue. This would suggest that cancer cells have a greater capability for ethanol oxidation and considerably less ability to remove acetaldehyde than healthy tissues. Acetaldehyde concentration may increase in cancer tissue and intensify the carcinogenesis. Moreover, the same studies showed that only the activity of ADH I (the most important colon isoenzymes of alcohol dehydrogenase) is markedly higher in colorectal cancer than in healthy colon cells.37 The high activity of enzymes in cancer tissues is reflected in an increase in their level in the blood. The serum total ADH activity has been changed in the course of CRC. The increase of total activity of alcohol dehydrogenase was positively correlated with isoenzyme class I of ADH, so the cause of the increase of serum total alcohol dehydrogenase in the course of colorectal cancer is an elevation of class I ADH isoenzymes.38 Moreover, the total serum activity of ADH and ADH I tended to be higher in colorectal cancer patients with more advanced stages. The diagnostic sensitivity for ADH I was 76%, specificity 82%, positive and negative predictive values were 85% and 74%, respectively. Area under Receiver Operating Characteristic (ROC) curve for ADH I was 0.72. These results suggest a potential role for ADH (especially ADH I) as markers for colorectal cancer, but further investigation and confirmation through a prospective study is necessary.39 Estimation of alcohol dehydrogenase activity may be conducted in the majority of laboratories.
Development of colorectal cancer and its metastases can be supported by exoglycosidases released by macrophages.40,41 Szajda et al showed a marked increase of N-acetyl-β-D-hexosaminidase, its isoenzymes A and B activity in the blood and urine of CRC patients.42 Waszkiewicz et al reported that the high level of cathepsin D is due to increased breakdown and nest restoration of glycoconjugates in colorectal adenocarcinoma.31 The lysosomal exoglycosidases are unspecific. Their activity is also high in other cancers, such as thyroid, renal, pancreatic, ovarian, as well as such diseases as idiopathic arthritis hypertension, glomerulonephritis or following liver transplantation.43–46
Ornithine decarboxylase (ODC) activity is higher in colorectal cancer and increases gradually from normal, through adenomatous, to cancerous. It has been shown that ODC activity in microscopically normal colon tissue from patients with CRC is higher than in the normal colon of patients without CRC.47
Circulating Tumor Cells (CTCs)
In the case of cancer (including colorectal cancer), death is rarely caused by the primary tumor itself but is due to determination, ie the distant metastases, that may develop years after the primary tumor resection. Circulating tumor cells (CTC) have been reported in patients with metastatic CRC as an independent predictor of overall and progression-free survival. There are at least three advantages to CTCs. The first is the monitoring of the treatment efficacy of CRC patients. The second is the molecular characterization of captured CTCs for targeted treatment, and the third is the cultivation of captured CTCs for drug sensitivity testing. All of these approaches allow researchers to recognize and respond to changes of the phenotype of cancer cells during disease progression and introduce personalized medicine into clinical practice. Despite promising results, decisions regarding disease stage and adjuvant treatment still do not include CTC results. This is largely due to the lack of standardized and automated CTC detection systems, such as CellSearch, which currently holds a dominant position in the field of CTC detection devices.48 The role of CTCs as prognostic markers for primary colorectal cancer has been described in many studies.49,50 Detection of CTC in the serum of patients after resection of colorectal, liver or many other metastases is associated with the prognosis of the disease. In 2008, the CellSearchTM system (Veridex LCC, Raritan, NJ, USA) was cleared by the US Food and Drug Administration (FDA) as a diagnostic tool for identifying and counting CTCs in blood samples in patients with metastatic colon cancer. Compared with other techniques such as reverse transcription-polymerase chain reaction (RT-PCR), the CellSearchTM system is an excellent platform for the detection of CTC in a clinical setting. The FDA approved CEllSearchTM system and two panels of antibodies against cytokeratins: cytokeratin 8, 18, and 19 (CK8/18/19) and CK8/18/19/20, were used for the detection of CTCs. Cytokeratin 20 (CK20) is a well-established marker for colon epithelium. Welinder et al suggest that CK20 is a biomarker for CTCs in patients with metastatic colorectal cancer.51 The importance of the CTC topic becomes evident in the context of the rapid integration of the evaluation of K-ras mutations in the daily practice of oncologists. Assessment of the presence of K-ras mutations in cancer cells in patients treated with epidermal growth-factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) was conducted by Pao et al.52 This study suggested an association between K-ras mutations and an absence of response to EGFR-TKIs treatment. In addition to determining the status of CTC K-ras, assessing other genes in captured CTC may improve predictive response to treatment. Gazzaniga et al determined the expression profile of multidrug resistance-related proteins (MRPs) of patients with a diagnosis of CRC in CTCs isolated from peripheral blood.53
K-RAS Mutations
Evaluation of mutations in KRAS is an example of the application of the molecular test needed to introduce targeted therapy in a specific group of patients, in this case, colorectal cancer patients. Nowadays, this research is necessary in order to make a decision about the treatment of these patient groups. The KRAS gene codes a small protein that is involved in the activation of the cascade of signal paths, including receptor signaling pathway for epidermal function growth factor (epidermal growth-factor receptor – EGFR), which is considered fundamental in the regulation of life, growth and cancer transformation epithelial cells.54 The RAS protein functions as a signal transducer from activated EGFR. EGFR activation (by linking to its ligand) leads to the activation of RAS RAF/MAPK and PI3K/AKT, and the increased proliferation and inhibition of cancer cell apoptosis. As a result of the mutation in RAS, a protein encoded by the mutated gene is formed, which due to difficult hydrolysis still remains in an active form (RAS-GTP). In the cells with a mutation in KRAS, there is a constant signal transduction that induces mitogenesis, regardless of whether the EGF receptor is activated. Analysis of mutations in KRAS allows stratification of patients with metastatic colorectal cancer for therapy with anti-EGFR mAb and mutations in KRAS are a negative predictor of this therapy.55 Mutations in KRAS in colorectal cancer most often occur in codons 12, 13 of exon 2 (in nearly 40% of colorectal cancers), less often activating KRAS mutations in codons 59, 61, 117 and 146. The association of colorectal cancer location and metastasis site with the presence of mutations in KRAS was found. Patients with mutations in codons 12 and 13 were more likely to have colorectal cancer located on the right side of the colon compared to patients without the KRAS mutation.56
Summary – CRC Diagnostics in the Future
The use of tumor markers in screening examinations and intervention in the first stages of colorectal cancer can significantly reduce mortality from colorectal cancer. Numerous studies on colorectal cancer use animal models.57–59 Of all animals, the mouse is the most used animal model in the study of carcinogenesis, and the main model in biomedical research. By comparing the human genome with an animal genome, it is possible to understand the structure and function of human genes better and apply that knowledge to studying human diseases in order to develop new strategies and mechanisms to prevent, detect, and treat CRC. The availability of recombinant inbred mouse panels and the existence of transgenic, knock-out and knock-in genetic models further increase the value of animal studies. The current management of mCRC involves various active drugs, either in combination or as single agents, but the effects of available treatment strategies for mCRC are often temporary, with resistance and disease progression developing in most patients.60 Thus, new treatment strategies are urgently needed. Targeted therapies, based on the use of monoclonal antibodies directed against the epidermal growth-factor receptor (EGFR) and vascular endothelial growth factor (VEGF), have been shown as promising treatments. On the basis of the presence of specific receptors for hypothalamic peptides on various human cancers including CRC, Engel et al developed targeted cytotoxic analogs of somatostatin (SST) and LHRH linked to doxorubicin or 2-pyrrolinodoxorubicin.61
The actual understanding of the basic biology of cancer initiation and development confirmed that suppressor gene mutations and oncogenes can be identified in body fluids that drain from the organs affected by the tumor. The analysis of single markers in the recognition and prognosis of the disease is applicable, but often associated with low sensitivity and specificity in routine medical practice (Table 1). The overall findings of multiple authors, as presented above, suggest the usefulness of serum HGFs, enzymes and especially classical tumor markers in the diagnosis and prognosis of CRC patients.
 |
Table 1 Diagnostic Criteria for Markers of Colorectal Cancer |
The best way seems to be to determine at least two or more markers simultaneously to increase their diagnostic utility. Circulating tumor cells analysis could be a part of an integrative medical approach of the multimodal diagnostics, individual patient profiles, disease-specific biomarker patterns, and person-specific treatment. Recently, technological and analytic advances have boosted scientific biomarker research. In the near future, we expect the advent of novel urinary assays with high efficacy that would reduce CRC mortality. In colorectal cancer, molecular (eg mutations in the KRAS, NRAS, BRAF, PIK3CA genes) and immunohistochemical markers (eg TS, P21, PTEN proteins) are used to assess predictive goals. Molecular markers in colorectal cancer can be divided into somatic mutations and microsatellite instability (MSI). Liquid biopsies could improve the diagnosis, prognostication, and monitoring of colorectal cancer (CRC). Mutation, chromosomal copy number alteration, and methylation analysis in circulating tumor DNA (ctDNA) from plasma or serum have gained great interest. However, the literature on preferred candidate markers is inconsistent, hampering a clear direction for further studies and clinical translation. A comprehensive review of candidate ctDNA markers shows that SEPT9 methylation analysis is promising in detecting CRC, and KRAS mutation analysis can help in forecasting and monitoring. Prospective assessment of marker panels in clinical decision-making should implement ctDNA analysis.
Disclosure
Wojciech Jelski has received consultation honoraria from Wiener Lab and Abbott. Barbara Mroczko has received consultation honoraria from Wiener Lab, Roche, Cormay, Abbott and Biameditek. The authors report no other conflicts of interest in this work.
References
1. Ferlay J, Shin HR, Bray F, Forman D, Marhers C, Parkin DM. Estimates of world wide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2000;127(12):2893–2917. doi:10.1002/ijc.25516
2. Murphy CC, Wallace K, Sandler RS, Baron JA. Racial disparities in incidence of young-onset colorectal cancer and patient survival. Gastroenterology. 2019;156(4):958–965. doi:10.1053/j.gastro.2018.11.060
3. Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014;64(2):104–117. doi:10.3322/caac.21220
4. Harriss DJ, Atkinson G, George K, et al. C-CLEAR group. Lifestyle factors and colorectal cancer risk (1): systematic review and meta-analysis of associations with body mass index. Colorectal Dis. 2009;11(6):547–563. doi:10.1111/j.1463-1318.2009.01766.x
5. Yang T, Li X, Farrington SM, et al. A systematic analysis of interactions between environmental risk factors and genetic variation in susceptibility to colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2020:
6. Cueva C, Silva M, Pinillos I, Bartolomé B, Moreno-Arribas MV. Interplay between dietary polyphenols and oral and gut microbiota in the development of colorectal cancer. Nutrients. 2020;12(3):
7. Negm RS, Verma M, Srivastava S. The promise of biomarkers in cancer screening and detection. Trends Mol Med. 2002;8(6):288–293. doi:10.1016/S1471-4914(02)02353-5
8. Legolvan MP, Taliano RJ, Resnick MB. Application of molecular techniques in the diagnosis, prognosis and management of patients with colorectal cancer: a practical approach. Human Pathol. 2012;8(8):1157–1168. doi:10.1016/j.humpath.2012.03.003
9. Świderska M, Choromańska B, Dąbrowska E, et al. The diagnostics of colorectal cancer. Contemp Oncol. 2014;18:1–6.
10. Quentmeier A, Moller P, Schwarz V, Abel U, Schlag P. Carcinoembryonic antigen, CA 19.9, and CA 125 in normal and carcinomatous human colorectal tissue. Cancer. 1987;60(9):2261–2266. doi:10.1002/1097-0142(19871101)60:9<2261::AID-CNCR2820600926>3.0.CO;2-P
11. Koness RJ. CEA: is it of value in colorectal cancer? RI Med. 1995;78:164–166.
12. Tan E, Gouvas N, Nicholls RJ, Ziprin P, Xynos E, Tekkis PP. Diagnostic precision of carcinoembryonic antigen in the detection of recurrence of colorectal cancer. Surg Oncol. 2009;18(1):15–24. doi:10.1016/j.suronc.2008.05.008
13. Chen JS, Chen KT, Fan WC, Yu JS, Chang YS, Chan EC. Combined analysis of surviving autoantibody and carcinoembryonic antigen biomarkers for improved detection of colorectal cancer. Clin Chem Lab Med. 2010;48(5):719–725. doi:10.1515/CCLM.2010.123
14. Vukobrat-Bijedic Z, Husic-Selimovic A, Sofic A, et al. Cancer antigens (CEA and CA 19-9) as markers of advanced stage of colorectal carcinoma. Med Arch. 2013;67(6):397–401. doi:10.5455/medarh.2013.67.397-401
15. Stiksma J, Grootendorst DC, van der Linden PW. CA 19-9 as a marker in addition to CEA to monitor colorectal cancer. Clin Colorectal Cancer. 2014;13(4):239–244. doi:10.1016/j.clcc.2014.09.004
16. Nakatani H, Kumon T, Kumon M. High serum levels of both carcinoembryonic antigen and carbohydrate antigen 19-9 in a patient with sigmoid colon cancer without metastasis. J Med Invest. 2012;59(3.4):280–283. doi:10.2152/jmi.59.280
17. Filella X, Molina R, Grau JJ, et al. Prognostic value of CA 19.9 levels in colorectal cancer. Ann Surg. 1992;216(1):55–59. doi:10.1097/00000658-199207000-00008
18. Rupert K, Holubec L, Nosek J, Houdek K, Topolcan O, Treska V. Significance of the TPS cytokeratin marker in the postoperative follow up of colorectal carcinoma patients. Rozhl Chir. 2009;88(8):428–433.
19. Mishaeli M, Klein B, Sadikov E, et al. Initial TPS serum level as an indicator of relapse and survival in colorectal cancer. Anticancer Res. 1998;18(3B):2101–2105.
20. Levy M, Visokai V, Lipska L, Topolcan O. Tumor markers in staging and prognosis of colorectal cancer. Neoplasma. 2008;55(2):138–142.
21. Guadagni F, Roselli M, Cosimelli M. TAG-72 (CA 72-4 assay) as a complementary serum tumor antigen to carcinoembryonic antigen in monitoring patients with colorectal cancer. Cancer. 1993;72(7):2098–2106. doi:10.1002/1097-0142(19931001)72:7<2098::AID-CNCR2820720707>3.0.CO;2-G
22. Bach S, Sluiter NR, Beagan JJ, et al. Circulating tumor DNA analysis: clinical implications for colorectal cancer patients. A systematic review. JNCI Cancer Spectr. 2019;3(3):pkz042. doi:10.1093/jncics/pkz042
23. Luo H, Zhao Q, Wei W, et al. Circulating tumor DNA methylation profiles enable early diagnosis, prognosis prediction, and screening for colorectal cancer. Sci Transl Med. 2020;12(524):
24. Ma ZY, Law WL, Ng EKO, et al. Methylated septin 9 and carcinoembryonic antigen for serological diagnosis and monitoring of patients with colorectal cancer after surgery. Sci Rep. 2019;9(1):10326–10334. doi:10.1038/s41598-019-46876-4
25. Tóth K, Sipos F, Kalmar A, et al. Detection of methylated SEPT9 in plasma is a reliable screening method for both left- and right-sided colon cancers. PLoS One. 2012;7(9):e46000. doi:10.1371/journal.pone.0046000
26. Xie L, Jiang X, li Q, et al. Diagnostic value of methylated septin9 for colorectal cancer detection. Front Oncol. 2018;8:247–254. doi:10.3389/fonc.2018.00247
27. Vocka M, Langer D, Fryba V, et al. Novel serum markers HSP60, CHI3L1, and IGFBP-2 in metastatic colorectal cancer. Oncol Lett. 2019;18(6):6284–6292. doi:10.3892/ol.2019.10925
28. Kushlinskii NE, Gershtein ES, Nikolaev AA, et al. Insulin-like growth factors (IGF), IGF-binding proteins (IGFBP), and vascular endothelial growth factor (VEGF) in blood serum of patients with colorectal cancer. Bull Exp Biol Med. 2014;156(5):684–688. doi:10.1007/s10517-014-2425-0
29. Maeda K, Shibutani M, Otani H, et al. Inflammation-based factors and prognosis in patients with colorectal cancer. World J Gastrointest Oncol. 2015;7(8):111–117. doi:10.4251/wjgo.v7.i8.111
30. Dimitriou N, Felekouras E, Karavokyros I, Alexandrou A, Pikoulis E, Griniatsos J. Neutrophils to lymphocytes ratio as a useful prognosticator for stage II colorectal cancer patients. BMC Cancer. 2018;18(1):1202–1216. doi:10.1186/s12885-018-5042-x
31. Rodolico V, Tomasello G, Zerilli M, et al. Hsp60 and Hsp10 increase in colon mucosa of Crohn’s disease and ulcerative colitis. Cell Stress Chaperones. 2015;15(6):877–884. doi:10.1007/s12192-010-0196-8
32. Mroczko B, Szmitkowski M, Okulczyk B. Hematopoietic growth factors in colorectal cancer patients. Clin Chem Lab Med. 2003;41(5):646–651. doi:10.1515/CCLM.2003.098
33. Mroczko B, Szmitkowski M, Okulczyk B. Granulocyte-colony stimulating factor (G-CSF) and macrophage-colony stimulating factor (M-CSF) in colorectal cancer patients. Clin Chem Lab Med. 2002;40(4):351–355. doi:10.1515/CCLM.2002.056
34. Groblewska M, Mroczko B, Wereszczyńska-Siemiatkowska U, et al. Serum interleukin 6 (IL-6) and C-reactive protein (CRP) levels in colorectal adenoma and cancer patients. Clin Chem Lab Med. 2008;46(10):351–355. doi:10.1515/CCLM.2008.278
35. Chechlińska M, Kowalska M, Kamińska J. Cytokines as potential tumour markers. Expert Opin Med Diagn. 2008;2(6):691–711. doi:10.1517/17530059.2.6.691
36. Mroczko B, Szmitkowski M, Wereszczyńska-Siemiatkowska U, Okulczyk B. Stem cell factor (SCF) and interleukin 3 (IL-3) in the sera of patients with colorectal cancer. Dig Dis Sci. 2005;50(6):1019–1024. doi:10.1007/s10620-005-2697-3
37. Jelski W, Zalewski B, Chrostek L, Szmitkowski M. The activity of class I, II, III and IV of alcohol dehydrogenase (ADH) isoenzymes and aldehyde dehydrogenase (ALDH) in the colorectal cancer. Dig Dis Sci. 2004;49(6):977–981. doi:10.1023/B:DDAS.0000034557.23322.e0
38. Jelski W, Zalewski B, Chrostek L, Szmitkowski M. The activity of alcohol dehydrogenase (ADH) isoenzymes and aldehyde dehydrogenase (ALDH) in the sera of patients with colorectal cancer. Clin Exp Med. 2007;7(4):154–157. doi:10.1007/s10238-007-0140-0
39. Jelski W, Mroczko B, Szmitkowski M. The diagnostic value of alcohol dehydrogenase (ADH) isoenzymes and aldehyde dehydrogenase (ALDH) measurement in the sera of colorectal cancer patients. Dig Dis Sci. 2010;55(10):2953–2957. doi:10.1007/s10620-009-1098-4
40. Szajda SD, Borzym-Kluczyk M, Snarska J, Puchalski Z, Zwierz K. N-acetyl-beta-D-hexosaminidase and its isoenzymes A and B in blond serum and urine as a potential colon cancer markers. Hepatogastroenterology. 2009;56(94–95):1287–1298.
41. Szajda SD, Jankowska A, Zwierz K. Carbohydrate markers in colon carcinoma. Dis Markers. 2008;25(4–5):233–242. doi:10.1155/2008/206510
42. Szajda SD, Snarska J, Puchalski Z, Zwierz K. Lysosomal exoglycosidase in serum and urine of patients with colon adenocarcinoma. Hepatogastroenterology. 2008;55(84):921–925.
43. Waszkiewicz N, Zalewska-Szajda B, Szajda SD. Lysosomal exoglycosidases and cathepsin D in colon adenocarcinoma. Pol Arch Med Wewn. 2012;122(11):551–556.
44. Choromańska B, Luto M, Szajda SD, et al. Activity of N-acetyl-β-D-hexosaminidase and its isoenzymes A and B in cancer. Post Hig Med Dosw. 2011;65:752–758. doi:10.5604/17322693.966833
45. Chuaire-Noack L, Rondon-Lagos S, Sanchez-Corredor M, Ibanez-Pinilla M, Ramirez-Clavijo S. Beta-galactosidase activity as a marker of senescence in primary cultures of the ovarian surface epithelium. Invest Clin. 2010;65:351–367.
46. Olszewska E, Olszewski S, Borzym-Kluczyk M, Zwierz K. Role of N-acetyl-beta-D-hexosaminidase in cholesteatoma tissue. Acta Biochim Pol. 2007;54(2):365–370. doi:10.18388/abp.2007_3258
47. Elitsur Y, Moshier JA, Murthy R, Barbish A, Luk GD. Polyamine levels, ornithine decarboxylase (ODC) activity, and ODC-mRNA expression in normal and cancerous human colonocytes. Life Sci. 1992;50(19):1417–1424. doi:10.1016/0024-3205(92)90260-V
48. Pesta M, Kulda V, Narsanska A, Fichtl J, Topolcan O. May CTC technologies promote better cancer management? EPMA J. 2015;6(1):1. doi:10.1186/s13167-014-0023-x
49. Galanzha EI, Zharov VP. Circulating tumor cell detection and capture by photoacoustic flow cytometry in vivo and ex vivo. Cancers. 2013;5(4):1691–1738. doi:10.3390/cancers5041691
50. Li P, Stratton ZS, Dao M, Ritz J, Huang TJ. Probing circulating tumor cells in microfluidics. Lab Chip. 2013;13(4):602–609. doi:10.1039/c2lc90148j
51. Welinder C, Jansson B, Lindell G, Wenner J. Cytokeratin 20 improves the detection of circulating tumor cells in patients with colorectal cancer. Cancer Lett. 2015;358(1):43–46. doi:10.1016/j.canlet.2014.12.024
52. Pao W, Wang TY, Riely GJ. KRAS mutations and primary resistance of lung adenocarcinomas to gefitinib or erlotinib. PLoS Med. 2005;2(1):e17. doi:10.1371/journal.pmed.0020017
53. Gazzaniga P, Naso G, Gradilone A. Chemosensitivity profile assay of circulating cancer cells: prognostic and predictive value in epithelial tumors. Int J Cancer. 2010;126(10):2437–2447. doi:10.1002/ijc.24953
54. Grossmann AH, Samowitz WS. Epidermal growth factor receptor pathway mutations and colorectal cancer therapy. Arch Pathol Lab Med. 2011;135(10):1278–1282. doi:10.5858/arpa.2011-0047-RA
55. Lievre A, Bachet JB, Le Corre D, et al. KRAS mutation status is predictive of response to cetuximab therapy in colorectal cancer. Cancer Res. 2006;66(8):3992–3995. doi:10.1158/0008-5472.CAN-06-0191
56. Morris VK, Lucas FA, Overman MJ, et al. Clinicopathologic characteristics and gene expression analyses of non-KRAS 12/13, RAS-mutated metastatic colorectal cancer. Ann Oncol. 2014;25(10):2008–2014. doi:10.1093/annonc/mdu252
57. Hite N, Klinger A, Hellmers L, et al. An optimal orthotopic mouse model for human colorectal cancer primary tumor growth and spontaneous metastasis. Dis Colon Rectum. 2018;61:
58. Liao HW, Hung MC. Intracaecal orthotopic colorectal cancer xenograft mouse model. Bio Protoc. 2017;7(11):e2311. doi:10.21769/BioProtoc.2311
59. Oliveira RC, Abrantes AM, Tralhão JG, Botelho MF. The role of mouse models in colorectal cancer research. The need and the importance of the orthotopic models. Animal Model Exp Med. 2020;3(1):1–8. doi:10.1002/ame2.12102
60. Hohla F, Winder T, Greil R, Rick FG, Block NL, Schally AV. Targeted therapy in advanced metastatic colorectal cancer: current concepts and perspectives. World J Gastroenterol. 2014;20(20):6102–6112. doi:10.3748/wjg.v20.i20.6102
61. Engel J, Emons G, Pinski J, Schally AV. AEZS-108: a targeted cytotoxic analog of LHRH for the treatment of cancers positive for LHRH receptors. Expert Opin Investig Drugs. 2012;21(6):891–899. doi:10.1517/13543784.2012.685128
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
