Back to Journals » International Journal of General Medicine » Volume 13
Biochemical and Ultrasound Characteristics of Subclinical Hypothyroid Patients in North of Jordan: Who Was Treated?
Authors Saadeh NA , Saadeh R, Rousan LA , Rawashdeh D, Obeidat A, Saadeh AM
Received 2 March 2020
Accepted for publication 28 May 2020
Published 15 June 2020 Volume 2020:13 Pages 305—310
DOI https://doi.org/10.2147/IJGM.S252114
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Nesreen A Saadeh,1 Rami Saadeh,2 Liqa A Rousan,3 Dalia Rawashdeh,1 Aya Obeidat,1 Abdullah M Saadeh1
1Department of Internal Medicine, Faculty of Medicine, Jordan University of Science and Technology, Irbid, Jordan; 2Department of Public Health, Faculty of Medicine, Jordan University of Science and Technology, Irbid, Jordan; 3Department of Radiology, Faculty of Medicine, Jordan University of Science and Technology, Irbid, Jordan
Correspondence: Nesreen A Saadeh P.O.Box 1214, Irbid 21110, Jordan
Tel +962 7970 23157
Email [email protected]
Background: Early diagnosis and management of subclinical hypothyroidism (SCH) are important to avoid the risk of developing overt hypothyroidism. This study aimed to evaluate patients with subclinical hypothyroidism (SCH), in regard to their biochemical characteristics, and ultrasound features, and factors associated with initiating treatment for this condition.
Methods: This was a retrospective study, which reviewed the data of patients who visited the Endocrinology clinic of a tertiary hospital in Jordan, King Abdullah University Hospital. Patients who visited the clinic with SCH over 1 year, Jan 2016–Dec 2016, were included. The charts were reviewed again 2 years later to check patients who started L-thyroxine for the different indications of treatment of SCH. Thyroid function tests (free T3, free T4, and TSH) and thyroid peroxidase antibodies (TPO-Ab) were found to be measured for all cases (n=287), among whom, thyroid ultrasound was done for 43 patients.
Results: Most patients were females (88.1%). Mean age was 42.36 (± 15.36 years). Positive TPO-Ab status was associated with higher TSH (p=0.056), lower free T4 levels (p= 0.012), and more patients treated with L- thyroxine for SCH in 2 years (p=0.001). On ultrasound, hypoechogenicity was more predominant among TPO-Ab positive patients than TPO-Ab negative patients (78% vs 30%).
Conclusion: SCH patients with positive TPO-Abs were more likely to be treated for this condition based on the various indications, and more likely to have had hypoechogenicity on ultrasound. Hence, thyroid ultrasonography and TPO-Ab status should be implemented early in evaluating and treating patients with SCH.
Keywords: subclinical hypothyroidism, thyroid peroxidase ab, thyroid ultrasound, Jordan
Introduction
Subclinical hypothyroidism (SCH) is defined as elevated serum levels of thyrotropin (TSH) combined with normal serum thyroid hormone levels.1
According to the National Health and Nutrition Survey (NHANES III), the prevalence of subclinical hypothyroidism was 4.3% with a greater prevalence for the female gender.2
The prevalence of subclinical hypothyroidism in Jordan was 5.98% among females and 4.40% among males, as shown in a recent cross-sectional study conducted in three representative areas of Jordan by Abu-Helalah et al 2019.3
The significance of SCH is largely due to its potential risk for developing into overt hypothyroidism.
SCH has been associated with infertility.4 In pregnancy, several studies have reported a higher incidence of placental abruption, preterm delivery, miscarriages and preeclampsia in SCH.5–10
As for the adverse outcomes of SCH in the fetus, they include perinatal morbidity and mortality, as well as subsequent neurologic and psychomotor delays.11–14
Treatment of subclinical hypothyroidism is considered in patients with pregnancy, infertility, associated symptoms of hypothyroidism, or high risk of progression to overt hypothyroidism.15
Many endocrine societies endorse treatment for SCH when TSH becomes >10 µIU/mL at any time during follow up.16,17
Through several prospective studies, initial high serum TSH and high serum anti-thyroid peroxidase antibody (TPO-Ab) concentrations in patients with SCH have been strongly associated with progression to overt hypothyroidism.18,19
Ultrasonography (US) findings of thyroiditis and the association between echogenicity of the thyroid gland and thyroid function were observed in many studies. More specifically, decreased echogenicity of the thyroid gland is associated with overt hypothyroidism.19–21
Several studies also demonstrated an association between hypoechogenicity in thyroid US and higher levels of serum TSH even in subjects without overt thyroid disease.20,21
Thyroid US in conjunction with TPO-Ab assay for the initial assessment of a patient with subclinical hypothyroidism appears to be more helpful than TPO-Ab alone for predicting the progression to overt hypothyroidism.22
Research is still exploring factors contributing to the progression of SCH to overt hypothyroidism, and the benefits of treating this condition versus the risks.
In this study, we examined patients with SCH; their characteristics, their TPO-Ab status and the signs of thyroid disease on US examination; aiming to find more features predictive of progression to overt hypothyroidism, and the subset of patients requiring treatment for SCH.
Methods
Design and Settings
Patients were referred to the endocrinology clinic at King Abdullah University Hospital (KAUH), which is a tertiary Hospital in north of Jordan, for the evaluation of abnormally elevated serum TSH levels.
Review of the medical records was done after the approval of the institution Review Board (IRB) at King Abdullah University Hospital in accordance with the Helsinki Declaration. The Ethics Committee of the IRB waived the need to obtain consent for the collection, analysis and publication of the retrospectively obtained and anonymized data in this study.
It was conducted in the period from January 2016 to December 2016.
The charts were reviewed again 2 years later to detect patients who started L-thyroxine within those years (Jan. 2017 -Dec.2018).
Subjects
A total of 299 patients visited the endocrinology clinic in 2016 for the evaluation of SCH. The patients selected were adults aged 18–65 years. Their Thyroid function tests at the time of initial evaluation showed subclinical hypothyroidism.
Subjects who had history of previous thyroid diseases, pregnant females, and patients already on thyroxine were excluded. Further exclusion included subjects with any record of medication history that could influence thyroid function eg, amiodarone, and those with pituitary and hypothalamic disorders. A total of 287 patients were thus included in the analysis.
The charts were reviewed once more at the end of 2018 to check the number and characteristics of patients who required treatment with L-thyroxine.
Patients started treatment with L-thyroxine as indicated for SCH by their treating physician. Indications of treatment mainly included TSH >10 µIU/mL at any time during follow-up visits, becoming pregnant or seeking pregnancy, or having persistent symptoms suggestive of hypothyroidism in the context of persistent evidence of SCH on testing.
Thyroid Function Test and Thyroid Autoantibodies
Serum TSH, freeT4, and freeT3 were measured by electrochemiluminescence immunoassay “ECLIA” on cobas e-601 analyzer immunoassay analyzers (Roche Diagnostics); the intra- and inter-assay coefficients of variants (CVs) were (4.0%, 4.4%), (2.9%, 3.3%), and (7.2%, 4.2%) for free T3, free T4 and TSH, respectively. Serum TSH has a laboratory reference range of 0.270–4.20 µIU/mL, and the laboratory reference range for free T4 is 12–22 pmol/L. Serum-free T3 (laboratory reference range 3.1–6.8 pmol/L) was also measured.
Subclinical hypothyroidism was defined as free T3 and free T4 in the normal reference range, while TSH values were >4.6 and <10 µIU/mL.
Anti-TPO was also measured in all subjects included in the study. Anti-TPO is an ELISA-based, automated, in-vitro test system for the quantitative determination of autoantibodies (IgG) against thyroperoxidase (TPO) in human serum or plasma (ORGENTEC). The intra- and inter-assay coefficients of variants (CVs) were 2.9% and 3.5%, and laboratory reference range 0–3000 IU/mL with 75 IU/mL as cut-off.
Ultrasound Evaluation of the Thyroid Gland
When reviewing the charts of these SCH patients, it was found that Thyroid Ultrasound with color-flow Doppler was done as part of the workup for 43 patients only. Thyroid US, using a multifrequency (10 MHz) linear transducer, was done by different radiologists, but were later reviewed and interpreted at the time of retrospective chart review by one experienced radiologist, who was blind to the status of the patient. Real-time sonography features suggestive of thyroid disease, including echogenicity, echotexture, AP diameter, vascularity, glandular margin, and the presence of scattered microcalcifications was reported.
Statistical Analysis
The characteristics of patients with SCH were classified into TPO-Ab positive and TPO-Ab negative patients. For categorical variables, chi-square test of independence was used to examine the association of TPO-Ab status with demographic information and with ultrasound features. Comparison between TPO-Ab positive and TPO-Ab negative patients for continuous variables such as age, free T3, free T4, and TSH were done using Student’s t-test. Further comparisons were done to examine if the decision of treatment with L-thyroxine medication was associated with echogenicity on ultrasound using chi- square test. Using t-tests, compared differences in free T3, free T4, and TSH levels among those who received L-thyroxine and those who did not. ANOVA tests were used to compare levels of freeT3, freeT4, and TSH among Isoechoic, hypoechoic, and hyperechoic patients. Analyses were performed using the Statistical Package for the Social Sciences “SPSS” software version 23. The level of significance equal to.05 was used for all statistical tests.
Results
The mean age of study participants was 42.36 (±15.36) years old. Female patients constituted (88.1%). 173 (60.2%) patients did not have any history of chronic medical illness while 58 (20.2%) patients reported having diabetes (Table 1).
 |
Table 1 Demographic Features of Patients |
The number of patients positive for TPO-Ab was 126 (43.9%) and TPO-Ab negative patients were 161 (56.1%). TPO-Ab positive patients had lower levels of free T3 and free T4 and higher levels of TSH. Free T4 levels were significantly lower among TPO-Ab positive patients (p=.012), as shown in Table 2.
 |
Table 2 Thyroid Function of Patients (N= 287) at Initial Evaluation |
Furthermore, the number of patients who were treated with L-thyroxine for SCH within 2 years of initial evaluation was significantly higher among TPO-Ab positive than TPO-Ab negative patients (p=.001) as shown in Table 3.
 |
Table 3 TPO-Ab Status of Patients Treated with L-Thyroxine Within 2 Years |
75.7% of those patients were females under the age of 45 years.
Ultrasound features of patients who had subclinical hypothyroidism are summarized in Table 4. Hypoechogenicity among TPO-Ab positive patients was significantly predominant (78%) compared to TPO-Ab negative patients (30.4%), (p=.005). Other ultrasound features of patients with subclinical hypothyroidism illustrated course echotexture; most of whom were TPO-Ab negative (55.6%). Furthermore, most patients with subclinical hypothyroidism had an increase in vascularity (78.8%) and smooth margins (59.5%), though not statistically significant. Isoechogenicity, a fine echotexture and a large AP > 2 cm were the least common detected features in ultrasound of patients with subclinical hypothyroidism.
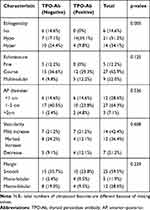 |
Table 4 Ultrasound Features of Subclinical Hypothyroid Patients |
Discussion
In about 60–80% of cases, subclinical hypothyroidism is associated with TPO-Ab, a marker of chronic lymphocytic (Hashimoto’s) thyroiditis. Hashimoto’s thyroiditis is more common in women, and the overall incidence increases with age in both sexes.1,15
Up to 46% of our patients had elevated titers of TPO-Ab. Most of the patients who visited the endocrine clinic were female aged under 45 years. This is well explained by the higher prevalence of autoimmune thyroid disease in females. Also, postpartum thyroiditis is a cause of thyroid dysfunction in females of childbearing age; more likely so in TPO positive than TPO negative women.23
Our results revealed an association between type 2 diabetes and SCH in 19.7% of patients.
The association between type 1 diabetes and SCH can be explained by autoimmunity as a common denominator.
As for Type 2 diabetes, several studies have shown that the most frequent thyroid disorders in these patients were subclinical hypothyroidism and overt hypothyroidism.24–26
Latin American Thyroid Society (LATS) recommends aggressive case finding of hypothyroidism in type 2 diabetes mellitus (T2DM) patients, based on the review of studies that have shown that the prevalence of hypothyroidism is higher in T2DM patients (odds ratio 3.45; 95% confidence interval [CI], 2.5 to 4.7) and diabetic complications are more prevalent in SCH.27
In a systemic review by Han et al, the adjusted pooled prevalence of SCH in T2DM patients was 10.2%. Also, T2DM was associated with a 1.93-fold increase in the risk of SCH (95% CI: 1.66, 2.24).28
On the contrary, recommendations of the Polish Society of endocrinology and Polish diabetes association for the management of thyroid dysfunction in type 1 and type 2 diabetes stated that autoimmune diabetes, but not type 2 diabetes, was strongly and gender neutrally associated with an increased prevalence of hypo- and hyperthyroidism and the presence of thyroid peroxidase antibodies. Also, increased surveillance for hypothyroidism appears not necessary in patients with type 2 diabetes.29
While other associations of endocrinology have not issued specific recommendations about thyroid disease screening in T2DM.30,31
Furthermore, our results show that TPO-Ab positive SCH patients were more likely to have lower free T4 and higher TSH. They were also more likely to be treated within 2 years with L-thyroxine for SCH. These findings are concordant with other studies which showed that serum TSH of more than 2·5 mU/L and presence of thyroid autoantibodies were predictors of long-term risk of hypothyroidism.22,32–35 In these studies, the annual rate of progression to overt disease was about 4% in women with raised serum TSH and positive antithyroid antibodies, and less likely so in those with only raised serum TSH concentrations or with only anti-thyroid antibodies present.22,33
Ultrasound can reveal the typical pattern of hypoechogenicity, heterogeneity, and increased blood flow seen in autoimmune thyroiditis.36 In our study, hypoechogenicity among TPO-Ab positive SCH patients was significantly predominant.
In a study by Premawardhana et al, the authors concluded that hypothyroid form of postpartum thyroiditis, high TPO-Ab levels, and a hypoechogenic US pattern lead to a high risk (relative risk, 32) of long-term thyroid dysfunction.34
Shin et al, investigated the value of ultrasonographic examination compared to the measurement of serum TPO-Ab for the evaluation of levothyroxine treatment in SCH. Ultrasonographic findings of diffuse parenchymal hypoechogenicity or a heterogeneous echo pattern of the thyroid gland had a higher negative predictive value compared with the absence of TPO-Ab and a similar positive predictive value to that of thyroid autoantibodies in predicting the outcome of SCH.37
As well, Rosário et al, concluded in their study that most adult women less than 60 years old in whom there is mild elevation of serum TSH, ranging from 5 to 10 mIU̸ L, do not progress to overt hypothyroidism, at least in a 3-year period. However, the presence of TPO-Ab and hypoechographic US patterns increases the risk of progression.21
One of the limitations of this study is the retrospective design. This has limited the number of available ultrasound examinations for analysis. Nonetheless, the significance of hypoechogenicity was still evident. This points to the importance of having thyroid ultrasound as a routine assessment tool in subclinical hypothyroid patients.
Other limitations include the inability to determine the rate of progression to overt hypothyroidism in the relatively short 2 years of follow up and the lack of proper documentation of indication to start treatment with L-thyroxine in the charts. However, positive TPO-Ab status and hypoechogenicity proved significantly predominant among patients treated for the different indications of SCH.
Conclusion
Patients referred for evaluation of SCH were more likely to be treated with L-thyroxine if they were TPO-Ab positive. Free T4 levels were significantly lower among TPO-Ab positive SCH patients, and hypoechogenicity was significantly predominant among TPO-Ab positive patients as well. These findings can help identify patients with SCH requiring treatment with L-thyroxine without delay, but more studies are definitely needed to characterize this subset of subclinical hypothyroid patients further.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Cooper DS. Clinical practice. Subclinical hypothyroidism. N Engl J Med. 2001;345:260–265. doi:10.1056/NEJM200107263450406
2. Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): national health and nutrition examination survey (NHANES III). J Clin Endocrinol Metab. 2002;87:489–499. doi:10.1210/jcem.87.2.8182
3. Abu-Helalah M, Alshraideh HA, Al-Sarayreh SA, et al. A cross-sectional study to assess the prevalence of adult thyroid dysfunction disorders in Jordan. Thyroid. 2019;29:1052–1059. doi:10.1089/thy.2018.0579
4. Abalovich M, Mitelberg L, Allami C, et al. Subclinical hypothyroidism and thyroid autoimmunity in women with infertility. Gynecol Endocrinol. 2007;23(5):279–283. doi:10.1080/09513590701259542
5. Casey BM, Dashe JS, Wells CE, et al. Subclinical hypothyroidism and pregnancy outcomes. Obstet Gynecol. 2005;105:239–245. doi:10.1097/01.AOG.0000152345.99421.22
6. Benhadi N, Wiersinga WM, Reitsma JB, Vrijkotte TG, Bonsel GJ. Higher maternal TSH levels in pregnancy are associated with increased risk for miscarriage, fetal or neonatal death. Eur J Endocrinol. 2009;160:985–991. doi:10.1530/EJE-08-0953
7. Allan WC, Haddow JE, Palomaki GE, et al. Maternal thyroid deficiency and pregnancy complications: implications for population screening. J Med Screen. 2000;7:127–130. doi:10.1136/jms.7.3.127
8. Negro R, Schwartz A, Gismondi R, et al. Increased pregnancy loss rate in thyroid antibody negative women with TSH levels between 2.5 and 5.0 in the first trimester of pregnancy. J Clin Endocrinol Metab. 2010;95:E44–8. doi:10.1210/jc.2010-0340
9. Liu H, Shan Z, Li C, et al. Maternal subclinical hypothyroidism, thyroid autoimmunity, and the risk of miscarriage: a prospective cohort study. Thyroid. 2014;24:1642–1649. doi:10.1089/thy.2014.0029
10. Wilson KL, Casey BM, McIntire DD, Halvorson LM, Cunningham FG. Subclinical thyroid disease and the incidence of hypertension in pregnancy. Obstet Gynecol. 2012;119(2, Part 1):315–320. doi:10.1097/AOG.0b013e318240de6a
11. Saki F, Dabbaghmanesh MH, Ghaemi SZ, et al. Thyroid function in pregnancy and its influences on maternal and fetal outcomes. Int J Endocrinol Metab. 2014;12:e19378. doi:10.5812/ijem.19378
12. Smit BJ, Kok JH, Vulsma T, et al. Neurologic development of the newborn and young child in relation to maternal thyroid function. Acta Paediatr. 2000;89:291–295. doi:10.1111/j.1651-2227.2000.tb18424.x
13. Li Y, Shan Z, Teng W, et al. Abnormalities of maternal thyroid function during pregnancy affect neuropsychological development of their children at 25–30 months. Clin Endocrinol (Oxf). 2010;72:825–829. doi:10.1111/j.1365-2265.2009.03743.x
14. Haddow JE, Palomaki GE, Allan WC, et al. Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. N Engl J Med. 1999;341:549–555. doi:10.1056/NEJM199908193410801
15. Biondi B, Cappola AR, Cooper DS. Subclinical hypothyroidism: a review. JAMA. 2019;322:153–160. doi:10.1001/jama.2019.9052
16. Jonklaas J, Bianco AC, Bauer AJ, et al. Guidelines for the treatment of hypothyroidism: prepared by the american thyroid association task force on thyroid hormone replacement. Thyroid. 2014;24:1670–1751. doi:10.1089/thy.2014.0028
17. Garber JR, Cobin RH, Gharib H; American Association of Clinical Endocrinologists, et al. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the american association of clinical endocrinologists and the american thyroid association. Endocrine Practice. 18;2012:988–1028. doi:10.4158/EP12280.GL
18. Huber G, Staub JJ, Meier C, et al. Prospective study of the spontaneous course of subclinical hypothyroidism: prognostic value of thyrotropin, thyroid reserve, and thyroid antibodies. J Clin Endocrinol Metab. 2002;87:3221–3226. doi:10.1210/jcem.87.7.8678
19. Pedersen OM, Aardal NP, Larssen TB, Varhaug JE, Myking O, Vik-Mo H. The value of ultrasonography in predicting autoimmune thyroid disease. Thyroid. 2000;10:251–259. doi:10.1089/thy.2000.10.251
20. Vejbjerg P, Knudsen N, Perrild H, et al. The association between hypoechogenicity or irregular echo pattern at thyroid ultrasonography and thyroid function in the general population. Eur J Endocrinol. 2006;155:547–552. doi:10.1530/eje.1.02255
21. Rosario PW, Bessa B, Valadao MM, Purisch S. Natural history of mild subclinical hypothyroidism: prognostic value of ultrasound. Thyroid. 2009;19:9–12. doi:10.1089/thy.2008.0221
22. Vanderpump MP, Tunbridge WM, French JM, et al. The incidence of thyroid disorders in the community: a twenty-year follow-up of the Whickham Survey. Clin Endocrinol (Oxf). 1995;43:55–68. doi:10.1111/j.1365-2265.1995.tb01894.x
23. Keely EJ. Postpartum thyroiditis: an autoimmune thyroid disorder which predicts future thyroid health. Obstet Med. 2011;4:7–11. doi:10.1258/om.2010.100041
24. Khan NZ, Muttalib MA, Sultana GS, Mishu FA, Nesa A. Study of thyroid disorders among type 2 diabetic patients attending a tertiary care hospital. Mymensingh Med J. 2017;26(4):874–878.
25. Papazafiropoulou A, Sotiropoulos A, Kokolaki A, Kardara M, Stamataki P, Pappas S. Prevalence of thyroid dysfunction among Greek type 2 diabetic patients attending an outpatient clinic. J Clin Med Res. 2010;2(2):75.
26. Subekti I, Pramono LA, Dewiasty E, Harbuwono DS. Thyroid dysfunction in type 2 diabetes mellitus patients. Acta Med Indones. 2017;49(4):314–323.
27. Brenta G, Caballero AS, Nunes MT. Case finding for hypothyroidism should include type 2 diabetes and metabolic syndrome patients: a Latin American thyroid society (LATS) position statement. Endocr Pract. 2019;25(1):101–105. doi:10.4158/EP-2018-0317
28. Han C, He X, Xia X, et al. Subclinical hypothyroidism and type 2 diabetes: a systematic review and meta-analysis. PLoS One. 2015;10(8).
29. Sowiński J, Czupryniak L, Milewicza A, et al. Polish society of endocrinology; Polish diabetes association. Recommendations of the Polish society of endocrinology and Polish diabetes association for the management of thyroid dysfunction in type 1 and type 2 diabetes. Endokrynol Pol. 2013;64:73–77.
30. Baskin HJ, Cobin RH, Duick DS, et al. American Association of clinical endocrinologists medical guidelines for clinical practice for the evaluation and treatment of hyperthyroidism and hypothyroidism. Endocr Pract. 2002;8(6):457–469. doi:10.4158/1934-2403-8.6.457
31. The Association for Clinical Biochemistry British Thyroid Association; British Thyroid Foundation. UK Guidelines for the Use of Thyroid Function Tests. 2006.
32. Diez JJ, Iglesias P. Spontaneous subclinical hypothyroidism in patients older than 55 years: an analysis of natural course and risk factors for the development of overt thyroid failure. J Clin Endocrinol Metab. 2004;89:4890–4897. doi:10.1210/jc.2003-032061
33. Li Y, Teng D, Shan Z, et al. Antithyroperoxidase and antithyroglobulin antibodies in a five-year follow-up survey of populations with different iodine intakes. J Clin Endocrinol Metab. 2008;93:1751–1757. doi:10.1210/jc.2007-2368
34. Premawardhana LD, Parkes AB, Ammari F, et al. Postpartum thyroiditis and long-term thyroid status: prognostic influence of thyroid peroxidase antibodies and ultrasound echogenicity. J Clin Endocrinol Metab. 2000;85:71–75. doi:10.1210/jcem.85.1.6227
35. Walsh JP, Bremner AP, Feddema P, Leedman PJ, Brown SJ, O’Leary P. Thyrotropin and thyroid antibodies as predictors of hypothyroidism: a 13-year, longitudinal study of a community-based cohort using current immunoassay techniques. J Clin Endocrinol Metab. 2010;95:1095–1104. doi:10.1210/jc.2009-1977
36. Anderson L, Middleton WD, Teefey SA, et al. Hashimoto thyroiditis: part 2, sonographic analysis of benign and malignant nodules in patients with diffuse Hashimoto thyroiditis. AJR Am J Roentgenol. 2010;195:216–222. doi:10.2214/AJR.09.3680
37. Shin DY, Kim EK, Lee EJ. Role of ultrasonography in outcome prediction in subclinical hypothyroid patients treated with levothyroxine. Endocr J. 2010;57:15–22. doi:10.1507/endocrj.K09E-154
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
