Back to Archived Journals » Botanics: Targets and Therapy » Volume 6
Bioactive ingredients of rose hips (Rosa canina L) with special reference to antioxidative and anti-inflammatory properties: in vitro studies
Authors Winther K, Vinther Hansen AS, Campbell-Tofte J
Received 14 July 2015
Accepted for publication 2 October 2015
Published 29 February 2016 Volume 2016:6 Pages 11—23
DOI https://doi.org/10.2147/BTAT.S91385
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ayse Kuruuzum-Uz
Video abstract presented by Professor Kaj Winther.
Views: 21789
Kaj Winther,1 Anne Sophie Vinther Hansen,1 Joan Campbell-Tofte2
1Department of Nutrition, Exercise and Sports, University of Copenhagen, Frederiksberg C, Denmark; 2Coordinating Research Unit, Frederiksberg University Hospital, Copenhagen, Denmark
Abstract: Rosa canina pseudo fruits, often referred to as rose hips, have been used as herbal medicine for more than 2,000 years, yet research has only recently begun to clarify specific mechanisms by which this plant product affects human health. Numerous compounds have been identified, and speculations of their bioactivity have implicated flavonoids, carotenoids, and fatty acids (FAs). With more than 4,500 representatives, flavonoids have been subjected to comprehensive research, with results that suggest various individual structures may be health-promoting compounds, also in rose hips. The importance of carotenoids from R. canina is currently being debated, because the demonstration of specific bioactivity among this group is presently less clear. The benefits of specific FAs have been investigated for decades, and several types of FAs are termed “essential” for human health. The specific mechanisms for bioactivity associated with three FAs that are abundant in R. canina fruits have been clarified in research. For example, linoleic acid, α-linolenic acid (mostly present in the seeds from R. canina) and a galactolipid ((2S)-1,2-di-O-[(9Z,12Z,15Z)-octadeca-9-12-15-trienoyl]-3-O-β-d-galactopyranosyl glycerol), referred to as GOPO, have been shown to have anti-inflammatory properties. The aim of this review is to critically analyze the published literature on rose hip research, with emphasis on the broadness and varying significance of the publications. Initially, we describe the chemical ingredients of R. canina pseudo fruits, with some focus on what ingredients are found in the whole pseudo fruit and what we know is confined to the seeds (achene seeds), and/or the shells (hypanthium). Then, we evaluate important papers describing the in vitro investigations of the bioactivity and impacts of the constituents of rose hip.
Keywords: rose hip, Rosa canina, antioxidants, anti-inflammation, osteoarthritis, rheumatoid arthritis
Introduction
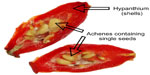
In recent years, the rising interest in herbal remedies has spawned numerous studies of a vast number of plants known and used in traditional medicine. This review aims to clarify the known bioactive constituents of one such plant, Rosa canina L, also termed “dog rose” (Figure 1). Specifically, the anti-inflammatory properties in the plant as well as possible impacts of the plant constituents on obesity will be evaluated, as obesity on its own is a major trigger of osteoarthritis – the most common joint disease worldwide. In this review, much of the focus will be centered on the pseudo fruits of R. canina, which are often alluded to as “fruits” in general medical literature. The pseudo fruits, which are called rose hips, are aggregate fruits consisting of several achenes (the actual seed-containing fruits of rose hips) enclosed by an enlarged, red, fleshy floral cup (hypanthium) (Figures 1 and 2). While rose hips are not unique to R. canina, but rather present in many types of roses, the rose hips of R. canina are, to the knowledge of the authors, the only rose hips with proven medicinal activities. In fact, R. canina has been known as a medicinal plant for more than 2,000 years. It consists of several subspecies,1 and several explanations have been suggested for the plants’ health promoting properties. These include R. canina’s composition and characteristics of: 1) flavonoids, 2) carotenoids, 3) fatty acids (FAs), 4) high content of vitamins (especially vitamin C), 5) antioxidant properties, and 6) anti-inflammatory agents.
Rose hips contain vast numbers of ingredients, and are subject to seasonal variation in their specific composition (like all other plant products). For this reason, we have strived to review research on standardized rose hip products of R. canina, and have further chosen to focus our attention on bioactive constituents. In other words, when referring to phenolics, the emphasis will mainly be on bioactive flavonoids. A description of the known active ingredients and to what extent these components are present qualitatively and quantitatively in R. canina seeds (seeds of the achenes), which also contain oils in the seeds themselves or in the shells (hypanthium), is also given. Finally, there is an evaluation of the in vitro methodologies used (cell- and non-cell-based assays) to assess bioactivity of rose hip constituents in the laboratory.
There are more than 4,500 known flavonoids, making them an enormous class of naturally occurring phenolic compounds. Hence, the systematic screening of the flavonoids is only in its infancy. Interestingly, new research suggests that a kaempferol derivate (tiliroside), only present in the seeds of rose hip, may play a role in antiobesity activity in R. canina.2,3 The rich and diverse carotenoid composition in R. canina has been known for over 10 years, but so far, no significant bioactivity has been reported for this class of compounds. Lately, carotenoids have been mentioned as a part of a complex, alleged to be present in some Chilean versions of rose hip powder. However, the complex failed to show any potency when tested in a clinical trial.4 The FAs in R. canina have also been investigated, and three major bioactive FA compounds have been isolated: 1) a galactolipid, 2) linoleic acid (a ω-6 polyunsaturated FA [PUFA]), and 3) α-linolenic acid (a ω-3 PUFA). All three compounds have displayed anti-inflammatory properties.5–7 In addition, the galactolipid has also shown chondroprotective capacity in vitro.8
The evolution and history of R. canina
Dog rose (R. canina L) is thought to have evolved in the last European postglacial period from a different genus of wild-growing Rosa spp. and an extinct ancestral “Protocaninae”. The dog rose possesses a unique meiotic and reproductive system consisting of a heterogamous meiosis with tetraploid egg cells and haploid pollen forming a permanent pentaploid organism. The unique meiotic behaviour of R. canina gives the plant matroclinal characters because of the distribution of 80% maternal genomes and 20% paternal genes.9
The plant was first described as a medicinal plant by Pliny the Elder (23–79 BC), who encountered its use among French tribes in the treatment of dog bites.10 This description subsequently spawned the name of the species (R. canina). In Europe, it was also described by the well-known German nun Hildegard of Bingen (AD 1098–1179), who used it as a strengthening tea in her treatments.11 Some medical uses of R. canina are shown in Table 1. The plant has also been known by sailors as a means of protection against scurvy, due to its high concentration of vitamin C, and thus it spread to several continents. Indeed, the high concentration of vitamin C in R. canina is well documented, for during the Second World War, rose hips were the key source of vitamin C in Britain, and massive harvest of rose hips were organized by the government.12 In Scandinavia, it has been a tradition to use the fruits for making marmalades and soups, although this has not been associated with health promotion per se. An explanation may very well be that the key ingredients responsible for health effects are labile and disintegrate under high temperatures, and that the boiling involved in the preparation of soups and marmalades inactivates the bioactive components in the plant material. Another aspect is that only the flesh (not the seeds) is used in tea and soup. Details of the “anatomy” of a rose hip are presented in Figures 2 and 3.
  | Figure 3 Separated seeds and shells from the hips of Rosa canina. |
Some medical utilization of R. canina
In European literature, the medicinal uses of R. canina are not well described; however, The Complete German Commission E Monographs: Therapeutic Guide to Herbal Medicines does list it as a medicinal plant, suggesting the use of the pseudo fruit’s seeds (seed of the achenes) and shells (the hypanthium surrounding the achenes of the pseudo fruit) for treating ailments, such as arthritic conditions, gout, sciatica, and diseases of the kidney and lower urinary tract.13 In Turkish folk medicine, however, R. canina is a very valued plant, such that the roots, leaves, branches, and fruits are used in the treatment of a number of ailments (Table 1).14–16 However, it should be emphasized that, in this review, the focus will only be on papers describing research on rose hip seeds and shells, for R. canina roots, leaves, and branches are at present the only species of academic interest.
In 2002, an extensive paper was published evaluating the total antioxidant properties in dietary plants (vegetables and fruits) from geographical locations all over the world.17 This paper demonstrated that there is a more than 1,000-fold difference in total antioxidants in dietary plants – R. canina was scored as containing the highest amount of antioxidant of all the plants examined.17
Known compounds of R. canina fruits
In recent years, several investigations have been launched to determine the compounds contained in R. canina fruits using high-performance liquid chromatography, thin-layer chromatography, tandem mass spectrometry, gas chromatography, and diode-array detection.18–21 From these investigations, numerous compounds have been identified in R. canina fruits. However, future investigations should be made to further identify additional compounds, which may in fact be responsible for some of the bioactive properties of the plant shown in recent years’ corresponding medical research. In addition, quantitative investigations of the constituents are lacking, and such studies may prove helpful in medicinal treatments, as well as for developing proper control of the breeding and postharvesting techniques for R. canina. Some compounds with bioactive properties are shown in Table 2.
Vitamins
Vitamins are defined as organic compounds synthesized in plants and in some lower animals, which are necessary in the diets of higher animals, in minute amounts. Vitamins have a diverse array of functions in the organism, such as coenzyme activity, precursor activity, antioxidative effect, regulation of calcium and phosphorus uptake, and regulation of coagulation (blood clotting). Vitamin deficiency in humans leads to numerous diseases and ailments (Table 3), and it is interesting to note that sailors for centuries used rose hip as a remedy against scurvy and brought the plant from Europe to South America, not knowing that vitamin C was a key factor for the relief of the disease.
  | Table 3 The function of vitamins and deficiency-related diseases |
Vitamins comprise a group of very different compounds with very different chemical properties. Their solubility varies, as some of the compounds have large numbers of functional groups capable of forming hydrogen bonds with water, while other structures are nonpolar. Water-soluble vitamins like the vitamins C and B are not stored in the body, but constantly need to be supplied through the diet. Unused water-soluble vitamins are excreted. Water-insoluble vitamins like vitamins A and E are storable and are therefore not excreted when consumed in excessive amounts. This situation can, unfortunately, lead to illness.22–24 All vitamins are bioactive, and research on the subject is extensive. In particular, the antioxidant potential of vitamins C and E has been subjected to numerous studies in recent years.17,25,26
Apart from its protection against scurvy, which is explained by its participation in the synthesis of collagen, vitamin C plays a part in several important enzymatic syntheses. For example, vitamin C is important in the synthesis of dopamine, carnitine, a number of neuroendocrine peptides, and in the transformation of cholesterol into bile acids.25 For its part, vitamin E is thought to mainly act as an antioxidant, protecting PUFAs within plasma membrane phospholipids and in plasma lipoproteins. Current research has revealed that vitamin E inhibits protein kinase C activity, although the physiological significance of this effect is yet to be clarified.25
Carotenoids
Carotenoids are tetraterpenoids, which absorb light between 400 and 500 nm in wavelength. As a result, carotenoids are evident as red, orange, and yellow colors in plants, imparting these colors to fruits and flowers. In addition, they are important light-harvesting molecules that transfer energy to reaction centers during photosynthesis and that suppress damaging photochemical reactions, particularly oxidations (radical scavengers). Animals are unable to synthesize carotenoids, and are therefore dependent on acquiring carotenoids through their diet. Most carotenoids are 40-carbon structures with isoprene as their basic structural unit. Carotenoids are generally divided into two subgroups: 1) xanthophylls, which are molecules containing oxygen (eg, lutein, zeaxanthin, and cryptoxanthin) and 2) carotenes, which are non-hydroxylated hydrocarbons (ie, alpha-carotene, beta-carotene, and lycopene). The colors of carotenoids are linked directly to their structure (the number of conjugated double bonds and presence or absence of oxygen). Xanthophylls, which contain oxygen, are often yellow, while carotenes, which lack oxygen, are orange or red (BL Møller, University of Copenhagen, personal communication, June, 2008).26
Studies in carotenoids have shown bioactivity, as carotenoids are associated with antioxidation both in vitro and in vivo.27–30 The antioxidant activities of carotenoids are a direct consequence of their structure, as they consist of a highly reactive electron-rich system of conjugated double bonds, enabling them to form radicals stabilized from attacks by electrophilic reagents.31 However, the use of animal models for studying carotenoids is limited, since most animals do not absorb or metabolize carotenoids like humans do.32
Dietary carotenoids have been associated with induction of apoptosis, inhibition of mammary cell proliferation, and inhibition of angina pectoris.33–37 Carotenoids have also been suggested to prevent prostate cancer in humans.38 However, a recent meta-analysis of 68 reliable antioxidant supplementation experiments involving a total of 232,606 individuals suggests that the consumption of additional beta-carotene from supplements is unlikely to be beneficial, and may actually be harmful.39 This may be due to the high doses of a single carotenoid (beta-carotene). Any reported positive effects of carotenoids may therefore suggest a “sparring effect”, due to the fact that carotenoids are suited better as markers of a high intake of vegetables and fruit,40 or that the epidemiological results reported are caused by compounds with no relation to carotenoids. However, a few carotenoids have been reported as exhibiting interesting bioactivity. For instance, studies of lutein and zeaxanthin in humans show that these compounds are found in high concentrations in the macula of the human retina, and may play a role in protecting the macula and photoreceptor outer segments of the retina from oxidative stress.41 Interestingly, lutein and zeaxanthin are also constituents of rose hip,42 and volunteers with macular degeneration taking rose hip seed and shell product have claimed visual improvement (K Winther, University of Copenhagen, personal communication, August, 2014).
Diets rich in lutein and zeaxanthin have been moderately associated with decreased prevalence of nuclear cataracts in elderly women,43 as well as in the prevention of age-related macular degeneration. Yet, there is no direct evidence that there are more antioxidant protections of the macula apart from the absorption of blue light.44 A previous study have shown that incorporation of carotenoids in lipophilic membranes and subsequent exposure to blue light reveals a filter efficacy in the order of lutein > zeaxanthin > beta-carotene > lycopene.45 Furthermore, lycopene has been suggested to promote health when given as a tomato extract or paste in cases of prostate cancer46 and in cases of non-eosinophilic airway inflammation.47 These findings are, however, problematic, because they are the result of the administration of a mixture of compounds. Indeed, a review written by Glovannucci concluded that a link between lycopene and prostate cancer is doubtful.48
Flavonoids
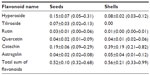
Flavonoids are secondary plant metabolites belonging to the phenylpropanoid group of compounds. The basic flavonoid skeleton consists of two aromatic rings joined by a three-carbon bridge. Two separate biosynthetic pathways contribute to the formation of this skeletal structure; the three-carbon bridge and one aromatic ring are derived from the shikimic acid pathway via phenylalanine, and the other aromatic ring comes from the condensation of three acetate units produced in the malonate pathway. Flavonoids may, however, have diverse substituents, the commonest being sugars, as most flavonoids exist naturally as glycosides. Other common substitutions are methylations. Examples of flavonoids are presented in Table 4.49
  | Table 4 Different flavonoids present in Rosa canina |
Flavonoids can be divided into the following six subclasses: 1) anthocyanins, 2) flavones, 3) flavanols, 4) isoflavones, 5) flavanols, and 6) flavanones. The most widespread group of the colored flavonoids is anthocyanins, which are beta-glycosides that have sugars in position 3. Anthocyanins act as attraction agents, luring animals (ie, pollinators) to flowers and fruits with visual signals. They may also serve as deterrents of microbes and insects. Another main function of flavonoids is in protecting cells from ultraviolet B (UV-B) radiation. They accumulate in epidermal layers and harness damaging UV-B radiation, while allowing visible wavelengths to pass through. Indeed, rose hip seed oil, which is also rich in flavonoids, is used as protection of the skin from sunburns in many countries. Flavones and flavanols absorb light at shorter wavelengths than wavelengths utilized by anthocyanins and carotenoids. As a result, flavones and flavanols are not visible to the human eye. They may, however, be visible to insects that see UV tones in the light spectrum, as they have been associated with UV patterns in flowers called “nectar guides”. Isoflavones have been shown to have several biological activities, which also include antimicrobial and insecticidal properties.50–52
Some of the health-promoting effects of fruit and vegetable intake have been attributed to their content of polyphenols and flavonoids. However, research has yet to clarify the specific mechanism(s) by which these compounds affect human health. In vitro data obtained from screening bioactivity of the flavonoids have often conflicted with results obtained from in vivo studies on the antioxidant capacity of plasma or on the resistance of plasma and lipoproteins to oxidation ex vivo after the consumption of flavonoid-rich foods by human subjects.53 Consumption of flavonoid-rich foods, in particular fruits and vegetables, has been associated with a lower incidence of diseases such as cancer, inflammation, heart disease, ischemic stroke, atherosclerosis, and other chronic diseases.54–60 Quercetin is one of the few flavonoids that shows interesting bioactive properties in vitro and in some in vivo tests. For example, rutin and its glycoside (rutin and quercitrin) have shown anti-inflammatory properties in models of intestinal inflammation, possibly through down-regulation of the nuclear factor-kappa beta pathway.61 Quercetin has also been suggested to modify eicosanoid biosynthesis, to protect low-density lipoprotein (LDL) from oxidation, to have antithrombotic effects, and to relax the cardiovascular smooth muscles.62 Interestingly, R. canina contains the flavonoid, tiliroside (kaempferol 3-O-β-d-(6-p-coumaryl)-glycopyranoside)2 that inhibits the oxidation of human LDL in vitro and has been shown to possess significant antiobesity, antioxidant, cytotoxic, and anticomplement properties in humans.3,63 The molecular structures of tiliroside and hyperoside are shown in Figure 4.
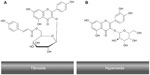  | Figure 4 The molecular structures of tiliroside (A) and hyperoside (B). |
Conversely, some flavonoids have been associated with a decrease in the nutritional value of some foods and fodders. Explanations for the negative effect have been based on their ability to form complexes with proteins, essential amino acids, carbohydrates, and digestive enzymes.59,60
Triterpene acids
Triterpenes are one of the most numerous and diverse groups of natural phytochemicals. These include more than 4,000 different complex molecules that are, for the most part, beyond the reach of chemical synthesis. Simple triterpenes are components of surface waxes and specialized membranes of plants. Some simple triterpenes may act as signaling molecules, whereas complex glycosylated triterpenes or the saponins provide protection against pathogens and pests. Hence, the triterpenes have a wide range of applications in food, health, and industrial biotechnology sectors.18,64 Animals and plants make triterpenes that are precursors to sterols. Sterols are important structural components of membranes, and they also have a role in cell signaling as steroidal hormones. However, triterpenes are not regarded as essential for normal growth and development. Both simple and conjugated triterpenes are well represented among rose hip constituents. Triterpene acids and other FAs are listed in Table 2.
FAs and galactolipids
FAs contain hydrocarbon chains of various lengths and degrees of saturation, terminating with a carboxylic acid group. FAs are key constituents of lipids, which by definition are water-insoluble biomolecules that are highly soluble in organic solvents. Lipids are key constituents of cell membranes, serve as fuel molecules or highly concentrated energy stores, act as signal molecules, and are also messengers in signal-transduction pathways. Triglycerides are the major storage lipids of both plants and animals. In animal triglycerides, FAs often are saturated (do not contain double bonds), resulting in molecules with linear chains that pack tightly and generate solid fats. By contrast, FAs are often unsaturated in plants. This prevents close packing, such that the resulting lipid molecules tend to be liquid at room temperature and are therefore termed “oils”. Fats and oils play vital roles in nutrition and in the food industry, where they are divided into groups according to their degree of saturation.51,65,66
In general, the consensus is that saturated FAs are abundant in many average western meat-based diets, while PUFAs, such as ω-3 and ω-6 FAs, are lacking. As earlier mentioned in Table 2, the seeds of R. canina fruits are rich in the ω-3 and ω-6 PUFAs; the extensive body of research into the physiological significance of PUFAs show their numerous health benefits, which include decreasing triglycerides and cholesterol in blood, inhibition of thrombosis, dilatation of blood vessels, enhancement of blood fluidity, increased plasticity of erythrocytes, reduced cardiovascular disease, and inhibition of inflammation.67–69 Furthermore, other PUFAs, such as linoleic and α-linolenic acids isolated from R. canina seeds, are also rich in oils and have been demonstrated to inhibit cyclooxygenase (COX)-1 and COX-2, thus revealing anti-inflammatory activity.6,7 Other FAs from plants and fish have also been demonstrated to have similar anti-inflammatory properties.67,70–72 Galactolipids are glycolipids in which the sugar molecule, galactose, is attached to the lipid backbone glycerol. Galactolipids are especially abundant in thylakoid membranes in plants. The galactolipid (2S)-1,2-di-O-[(9Z,12Z,15Z)-octadeca-9-12-15-trienoyl]-3-O-β-d-galactopyranosyl glycerol, also known as GOPO, has been isolated from R. canina and has shown strong anti-inflammatory activities.5 Taking rose hip seed and rose hip seed shell powder into account, FAs and galactolipid GOPO can explain some of the improvement observed in patients with inflammatory diseases.73
Other compounds including dietary fibers
Beta-sitosterol is a phytosterol present in rose hip and is thought to inhibit the absorption of dietary cholesterol. Several reports54,55 have appeared in the literature indicating that phytosterols have immunological and anti-inflammatory activities in in vitro and in vivo models of cancer (colorectal and breast cancer). However, it is only in the last 10 years that their direct immune-modulatory activity on human lymphocytes and their mechanism of action in cancer cells have been investigated.74
Rose hips, and in particular their seeds, have high amounts of dietary fibers, which include pectin. Although mammals are unable to digest vegetable fibers, dietary fibers are very important in human diets because they slow down the movement of food through the intestinal tract, promoting better digestion and the increased absorption of nutrients.76
Comparison of active ingredients in seeds and shells
Schwager et al compared two rose hip products, a pure rose hip shell (hypanthium) product and a product containing rose hip shell (hypanthium) combined with a seed (seed of the rose hip achenes), respectively.73 FA content in the seed-only product was more than four times higher than that of the product without seeds. Likewise, PUFA linoleic acid content was more than seven times higher in the rose hip product containing seeds as compared to the product made from the shells alone, indicating that FAs are predominantly found in rose hip seeds.73 In contrast, vitamin C and beta-carotene contents were nearly identical in both products, while the amounts of triterpenoids, galactolipids, lycopene, and vitamin E were higher in the shell-only product, indicating dominance of these constituents in this part of the fruit. Flavonoids are present to the same extent in rose hip seeds and shells. However, the distribution of flavonoids is different in rose hip seeds as compared to the distribution found in the shells. Details of these differences are presented in Table 4.
In rose hip seeds, linoleic and α-linolenic acids are found as part of the triglycerides and are therefore not free FAs. Generally, triglycerides of long-chain FAs have very low solubility (Merck Index), while free FAs can easily form salts and so possess improved solubility. These properties may explain some of the inconsistencies experienced in bioassays when extracts from seeds are compared to extracts from rose hip shells or from combined seed and shell preparations.73,76 Consequently, when evaluating bioassay studies based on extracts, one should always consider the relevant physiologically active form of the investigational medicines and the environment in which the test is being conducted.
Differences in active ingredients between species
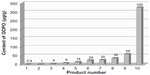
The biological variation in phytochemicals of rose hip from different species of R. canina is pronounced. Ten different commercially available rose hip products were tested for their contents of hydrolysable polyphenols in shells and in seeds.49 As can be seen from the range of species (Table 4), the variation of hyperoside in shells is above 600% and the variation in seeds is approximately 400%. The variation of rutin in seeds is likewise 600%, while for shells, this flavonoid was detectible in only one out of nine products. Certain tannins were not found in the seeds at all, and tiliroside, an important flavonoid in obesity research, was only present in the seeds; hyperoside in shells the varied more than 600%49 (Table 4). In a different study, the amount of alpha-linoleic and linolenic acids were tested in eight commercially available seed oils, and the variation between products was 30%–70% which is much lower than what was reported for flavonoids in Table 4 (A Guzman, Faculty of Pharmaceutical Science, University of Copenhagen, personal communication, June, 2012). However, when the galactolipid GOPO content was determined in ten different commercial rose hip powders that are available in Denmark, GOPO content varied from less than 1% to 20% of that found in the combined seed and shell powder based on R. canina lito, that is produced using standardized and patented methodology (Product no. 10, Figure 5).
The variation of active ingredients within the different species of R. canina, the environments they grow in, eg, number of hours with sun, altitude, soil, and amount of rain, influence the biochemical composition of the plant and thereby the quality of the product produced. In addition, active ingredient variations are influenced by rose hip drying methodology, including the drying temperature and time point of harvesting.77–81 It is interesting to note that in countries where rose hip has been used as a tea (France) or a soup (Sweden), for centuries there were, until recently, hardly any reports on anti-inflammatory action. This may be due to the fact that cooking destroys some active elements of rose hip and perhaps, also due to the fact that the seeds were never used in tea, soup, or marmalade. Rose hip powders also exhibit huge differences in color and smell, depending on the method of production, quality, and quantity of the different ingredients. Hence, the powders may be brownish (possibly caused by exposure to high temperatures during production), as some drying facilities use temperatures as high as 800°C. Some rose hip powders are orange, especially when kept at lower processing temperatures (Figure 6). It is important to note here that the color is also determined by the amount of seeds and shells. Variations in active ingredients are explored in Figure 4.
In summary, the quality and amount of active ingredients in rose hip powders vary widely depending on the subspecies of R. canina used, method of production, growth environment, and time of harvest. As of now, it is very difficult for the consumer to rely on the product information provided by the store or on the Internet. It is therefore very important that there is proper product regulation and quality control put in place by the government or regulating agencies.
In vitro studies of the effects of rose hip
One of the first publications to show that rose hip might be of relevance as an anti-inflammatory agent reported that a water extract of rose hip inhibited chemotaxis of polymorphonucleated (PMN) cells isolated from healthy humans at a dosage of 500 μg/mL.79 In the same study, a water extract of rose hip shells alone was shown to be superior in reducing chemotaxis of PMN cells, as compared to the effects achieved with extracts of the whole fruit, ie, from both shells and seeds.79
As the 1999 study79 did not include extraction of FAs that are abundant in the seeds, the authors may have arrived at the wrong conclusion that R. canina shells are the most important part of the fruit as regards chemotaxis and antioxidative activity. This deduction can be made because subsequent studies have revealed high levels of fat-soluble elements in rose hip, including earlier mentioned FAs (in the section FAs and galactolipids), with anti-inflammatory and antioxidative activity.5–7,80,81 Polyphenolics (proanthocyanidins and flavonoids) with antioxidative properties, as demonstrated by their inhibition of chemotaxis in human PMN cells, were found in rose hip extracted with lipophilic solvents.80 This extract could inhibit reactive oxygen species in both cellular and cell-free systems, with half maximal inhibitory concentration (IC50) values ranging from 5.73 to 1.33 mg/L. Furthermore, the antioxidative effects were clearly shown not to be due to vitamin C alone, but were also due to substantial contributions from polyphenols.80
Isolation of GOPO
Motivated by the earlier findings, a group of Danish scientists5 decided to search for the biochemical background of the anti-inflammatory properties reported in R. canina. Thus, starting from milled powder of R. canina lito, which contained the natural amount of shells and seeds, both water and lipophilic extractions were made, fractionated, and the resulting extracts and fractions were tested in in vitro PMN bioassays. The fraction that showed high bioactivity in inhibiting chemotaxis of PMNs and monocytes in vitro contained only one compound, GOPO (Figure 7). With the aid of nuclear magnetic resonance (NMR), optical analysis, basic methanolysis, and acidic hydrolysis, the fraction was found to contain GOPO with a purity of >98%. The authors have indicated their readiness to provide a detailed description of the isolation procedure and identification data in the form of tabulated hydrogen-1 (1H) and carbon-13 (13C) NMR data.
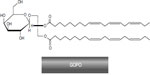  | Figure 7 The molecular structure of the galactolipid GOPO. |
The molecule was later been tested in different settings to confirm its strong anti-inflammatory and chondroprotective effects.8 From the Larsen et al publication,5 it is not clear if the galactolipid, GOPO, is mainly associated with rose hip shells or the seeds. However, data from Schwager et al73 specify that GOPO is present mostly in the shells.
COX inhibition by linoleic- and α-linolenic acid-containing rose hip extract
With the aid of COX enzyme in vitro assay kits, extracts of powdered whole fruits (seeds and shells) were tested for their impacts on COX-1 and COX-2 enzyme activity.6 Water extracts did not show activity in the COX-1/COX-2 assay,6,7 whereas methanol, dichloromethane, and hexane extracts all showed dose-dependent inhibition of COX-1 and COX-2 enzyme activity. The lowest IC50 values for methanol extracts of COX-1 and COX-2 inhibition were 12 μg/mL and 19 μg/mL, respectively. The data suggest that elements that are soluble in organic solvents must account for some of the effects on COX-1 and COX-2.6,7
Jäger et al have showed that GOPO is not the only FA involved in anti-inflammation, as extracts of whole rose hip fruits (containing seeds and shells) made with petroleum ether, dichloromethane, or methanol all exhibited dose-dependent inhibition of both COX-1 and COX-2 enzymes, as opposed to the water extract, which did not show any activity.7 The IC50 value for linoleic acid was 85 μM for COX-1 and 0.6 μM for COX-2. For α-linolenic acid, the values were 52 μM for COX-1 and 12 μM for COX-2. The COX-2/COX-1 ratio for linolenic and α-linolenic acids were 0.007 and 0.2, respectively, indicating that both acids are selective COX-2 inhibitors. Authentic standard linoleic acid was also tested, and this test confirmed previous results.7 As COX-2 inhibitors do not affect platelet aggregation in humans, the data from the Jäger et al,7 study are in agreement with an earlier report from Rein et al,81,82 which showed that platelet aggregation was not affected by rose hip powder when compared with the effects of acetylsalicylic acid, a non-steroidal anti-inflammatory drug that broadly inhibits the arachidonic acid pathway.
In a Korean study that tested the effect of rose hip extracts on the expression of the COX enzymes in isolated cartilage cells, it was demonstrated that some of the active ingredients in rose hips may be both heat stable and soluble in hot water.83 This result was fully supported, because extracts made by heating the shells alone or the whole fruits (containing seeds and shells) in boiling water inhibited COX-2 protein expression dose-dependently, whereas COX-1 expression remained unaffected. As the heat treatment involved keeping the plant materials at 1,000°C for more than 4 hours, one could argue that the preparation of the extract had been harsh. However, European cell-based studies showed that as it is lipophilic compounds that inhibit COX-2 enzymes,7,76 it is conceivable that the boiling water treatment that lasted several hours also dissolved some additional active ingredients. However, boiling for several hours is far from what happens in the living organism; by boiling for so long, many active ingredients from shells and from seeds may have been destroyed in making the two preparations, which initially were very different, the same.
The triterpene acids – ursolic acid, oleanolic acid, and betulinic acid – have also been identified in R. canina, although only in minute amounts.76 In the same study and as expected, linoleic and α-linolenic acids were also identified. However, there were no clear correlations between the amount of unsaturated FAs and COX-1 or COX-2 enzyme activity, which led the authors to suggest that possibly, other yet-undescribed lipophilic constituents might play a role in the observed in vitro inhibition of arachidonic acid metabolism. In particular, the methanolic extract exhibited potent radical scavenger activity, which may be correlated to the relatively high phenolic content of the preparation. The general conclusion was that extracts derived from powdered rose hip shells (without seeds) were more effective in the assays, as compared to extracts derived from powder consisting of both shells and seeds.76 It should, however, be borne in mind that FAs, especially those present in the seeds, are strongly bound to triglycerides. The n-hexane and dichloromethane extraction used in the study may have been less optimal for obtaining the total amount of bioactive lipid-soluble elements. This may also explain the difference in the amount of extracted FAs from seeds and shells as compared to the data from Wenzig et al76 and Schwager et al.73 What happens in the gastrointestinal tract in animals or in humans ingesting the rose hip powders may be very different from the biodata obtained when testing extracts and fractions of rose hip in a laboratory setting. Wenzig et al, however, demonstrated that there seem to be other lipophilic elements than the known FAs, including GOPO, which are responsible for observed anti-inflammatory activity.76
Anti-inflammatory triterpenes and interleukins
The Mono Mac 6 cell line resembles mature human monocytes and expresses interleukin (IL)-6 in a dose-dependent manner following activation with lipopolysaccharides (LPSs).18 Standardized rose hip powder containing the natural amount of seeds and shells (R. canina lito) was extracted with petroleum ether, dichloromethane, ethyl acetate, and water. The dichloromethane extract significantly inhibited the release of IL-6 from the cell line at the level of 10 μg/mL. Oleanic, betulinic, and ursolic acid were isolated from this dichloromethane extract. While only oleanolic and ursolic acids exhibited concentration-dependent inhibition of IL-6 release from LPS-activated cells, a mixture of the three triterpene acids exhibited an even stronger inhibition of IL-6, with an IC50 value of 21±6 μM.18 Thus, it has been shown that bioactivity of a plant product is often dependent on the interplay between many different ingredients – and not a single molecule.
In another study, inflammatory processes were induced in murine macrophage cells or human peripheral blood leucocytes with LPS, and the level of inflammatory mediators such as nitric oxide, prostaglandin E2, and cytokines/chemokines that are released during activation was determined. In macrophages and in blood peripheral blood leucocytes, rose hip powder consisting of seeds and shells dissolved in dimethyl sulfoxide (DMSO), as well as the isolated molecule GOPO, inhibited the production of cytokines such as tumor necrosis factor (TNF)-alpha, IL-1 beta, IL-6, and IL-12.8
Schwager et al have definitively demonstrated that rose hip powder prepared from whole dried R. canina fruits and purified GOPO attenuates inflammatory responses in cellular systems such as PMNs and chondrocytes, in a way that mirrors the reduction of the catabolic processes associated with the breakdown of cartilage in osteoarthritis or rheumatoid arthritis.8,74 Specifically, the gene expression and secretion of cytokines CCL5/RANTES, CXCL10/IP-10, IL-6, and IL-12 were reduced in LPS/interferon-activated peripheral blood leukocytes treated with rose hip powder or GOPO. Likewise, rose hip preparations reduced the expression of matrix metalloproteinase (MMP)-1, MMP-3, and MMP-13, and ADAMTS-4 in IL-1-treated normal chondrocytes.8
In summary, the results from testing extracts of rose hip powder on different cell-based and non-cell-based bioassay systems indicate that rose hip powder based on shells alone may be more active than that which is made from grinding whole rose hip fruits (containing seeds and shells). The fact that certain elements with lipophilic characteristics are not extracted from seeds by using the common laboratory extractants may explain why the processes triggered in in vitro assays may be very different from what goes on in a living organism, which ingests the entire powdered rose hip that is further exposed to influences from gastric acid and numerous digestive enzymes in the gastrointestinal tract.
Limitations
The literature search included articles from 1975 onward to identify studies on rose hip, R. canina, or dog rose. The search was restricted to English language articles. We further searched the authors’ own files to improve the number of relevant papers. Finally, relevant papers were extracted independently by the three authors.
Conclusion
Much research has been conducted to investigate the health-enhancing properties of R. canina pseudo fruits, and the current review addresses only a small portion of those enquiries, particularly those that are directed at understanding the beneficial effects of rose hip on pain and inflammation in joint diseases. From the current review, it is clear that active research continues to be conducted worldwide on various bioactive properties of the compounds found in R. canina pseudo fruits (rose hips).
Although several active ingredients have been suggested, it is too early to give a definite answer as to what is the most important active ingredient in rose hip in clinical settings. Current findings suggest that flavonoids (especially tiliroside),2,3 GOPO,5 and the PUFAs, linoleic and α-linolenic acids,6,7 are among the more interesting ingredients. However, it is also evident that there are other very important lipid-soluble compounds in rose hip that are still unknown.76
There is a great variability in the different bioactive ingredients in different species of R. canina. In addition, the growth environment, period of harvesting, and the mode of production of the final rose hip powder play important roles in determining the quality of the powders that consumers get. More quality control is therefore needed. As it stands today, with so many different powders and capsules available on the shelves of stores, there is a lack of adequate quality declaration. For example, it is difficult to ascertain if the rose hip preparation one is considering buying contains only rose hip shell/husk powder or is a combination of seed plus shell/husk powder.
In vitro studies carried out in non-cell and in cell-based systems are very interesting and informative, and they teach us much about the effects of rose hip constituents in important and relevant cellular mechanisms. However, such studies are normally based on extractions presented to cells in a test tube – a situation that can be very far from what actually occurs in humans or in animals ingesting rose hip powder. Thus, we should be critical of in vitro studies. Future animal and human studies are therefore strongly warranted.
Disclosure
K Winther has been a consultant to Hyben Vital on veterinarian products. The authors report no other conflicts of interest in this work.
References
Celik F, Kazankaya A, Ercisli S. Fruit characteristics of some selected promising rose hip (Rosa spp.) genotypes from Van region of Turkey. Afr J Agric Res. 2009;4(3):236–240. | |
Ninomiya K, Matsuda H, Kubo M, Morikawa T, Nishida N, Yoshikawa M. Potent antiobese principle from Rosa canina: structural requirements and mode of action of trans-tiliroside. Bioorg Med Chem Lett. 2007;17:3059–3064. | |
Nagatomo A, Nishida N, Fukuhara I, et al. Daily intake of rosehip extract decreases abdominal visceral fat in preobese subjects: a randomized, double-blind, placebo-controlled clinical trial. Diabetes Metab Syndr Obes. 2015;8:147–156. | |
Christensen R, Tarp S, Altman RD, et al. Comparing different preparations and doses of rosehip powder in patients with osteoarthritis of the knee: an exploratory randomized active-controlled trial. Int J Clin Rheumatol. 2014;9(3):267–278. | |
Larsen E, Kharazmi A, Christensen LP, Christensen SB. An antiinflammatory galactolipid from rose hip (Rosa canina) that inhibits chemotaxis of human peripheral blood neutrophils in vitro. J Nat Prod. 2003;7:994–995. | |
Jäger AK, Eldeen IM, van Staden J. COX-1 and -2 activity of rose hip. Phyother Res. 2007;21(12):1251–1252. | |
Jäger AK, Petersen KN, Thomasen G, Christensen SB. Isolation of linolenic and alpha-linolenic acids as COX-1 and -2 inhibitors in rose hip. Phytother Res. 2008;22:982–984. | |
Schwager J, Hoeller U, Wolfram S, Richard N. Rose hip and its constituent galactolipids confer cartilage protection by modulating cytokine and chemochine expression. BMC Complement Altern Med. 2011;11:105. | |
Ritz CM, Schmuths H, Wissemann V. Evolution by Reticulation: Evolution by reticulation: European dogroses originated by multiple hybridization across the genus rosa. J Hered. 2005;96(1):4–14. | |
Heinemann W. The ilder Pliny. In: Natural History VII: Books XXIV–XXVII. London: Pearson; 1962:149. | |
Strehlow W, Herzka G. Hildegard of Bingen’s Medicine. Rochester, VT: Bear and Company; 1988:63. | |
Haas LF. Rosa canina (dog rose). J Neurol Neurosurg Psychiatry. 1995;59(5):470. | |
Blumenthal M, Busse WR, Goldberg A, et al. The Complete German Commission E Monographs: Therapeutic Guide to Herbal Medicines. 2nd ed. Riggins C, editor. Elsevier Health Sciences. Austin (TX): American Botanical Council; 1998. | |
Kültür S. Medicinal plants used in Kirklareli Province (Turkey). J Ethnopharmacol. 2007;111:341–364. | |
Yeşilada E, Ustün O, Sezik E, Takaishi Y, Ono Y, Honda G. Inhibitory effects of Turkish folk remedies on inflammatory cytokines: interleukin-1 alpha, interleukin-1 beta and tumor necrosis factor alpha. J Ethnopharmacol. 1997;58:59–73. | |
Blumenthal M, Busse WR, Goldberg A, et al. The Complete German Commission E Monographs: Therapeutic Guide to Herbal Medicines. 1st ed. Riggins C, editor. Elsevier Health Sciences. Austin (TX): American Botanical Council; 1990. | |
Halvorsen BL, Holte K, Myhrstad MC, et al. A systematic screening of total antioxidants in dietary plants. J Nutr. 2002;132(3):461–471. | |
Saaby L, Jäger AK, Moesby L, Hansen EW, Christensen SB. Isolation of immunomodulatory triterpene acids from a standardized rosehip powder (Rosa canina L.). Phytotherapy Res. 2011;25:195–201. | |
Ercisli S. Chemical composition of fruit in some rose (Rosa ssp.) species. Food Chem. 2007;104:1379–1384. | |
Zlatanov MD. Lipid composition of Bulgarian chokeberry, black current and rose hip seed oils. J Sci Food Agric. 1999;79:1620–1624. | |
Ozcan M. Nutrient composition of rose (Rosa canina L.) seed and oils. J Med Food. 2002;5(3):137–140. | |
Ouellette RJ. Organic Chemistry: A Brief Introduction. 2nd ed. Upper Saddle River, NJ: Prentice Hall; 1998. | |
Lawrence E, editor. Henderson’s Dictionary of Biology. 13th ed. London: Pearon Plc; 2005. | |
Berg JM, Tymoczko JL, Stryer L. Biochemistry. 6th ed. New York, NY: WH Freeman; 2007. | |
Hemilä H. Do Vitamin C and E Affect Respiratory Infection? Helsinki: University of Helsinki; 2006. | |
Kiokas S, Varzakas T, Oreopoulou V. In vitro activity of vitamins, flavonoids, and natural phenolic antioxidants against the oxidative deterioration of oil-based systems. Crit Rev Food Sci Nutr. 2008;48:78–93. | |
Hill TJ, Land EJ, McGarvey DJ, Schalch W, Tinkler JH, Truscott TG. Interactions between carotenoids and the CCl3O2 radical. J Am Chem Soc. 1995;117:8322–8326. | |
Kiokias S, Gordon MH. Dietary supplementation with a natural carotenoid mixture decreases oxidative stress. Eur J Clin Nutr. 2003;57:1135–1140. | |
Bub A, Watzl B, Abrahamse L, et al. Moderate intervention with carotenoid-rich vegetable products reduces lipid peroxidation in men. J Nutr. 2000;18:2200–2206. | |
Matos HR, Di Mascio P, Medeiros MH. Protective effect of lycopene on lipid peroxidation and oxidative damage in cell culture. Arch Biochem Biophys. 2000;383:56–59. | |
Mortensen A, Skibsted LH. Reactivity of beta-carotene towards peroxyl radicals studied by laser flash and steady-state photolysis. FEBS Lett. 1998;426:392–396. | |
Pavia SA, Russell RM. Beta-carotene and other carotenoids as antioxidants. J Am Coll Nutr. 1999;18(5):426–433. | |
Sumantran VN, Zhang R, Lee DS, Wicha MS. Differential regulation of apoptosis in normal versus transformed mammary epithelium by lutein and retinoic acid. Cancer Epidemiol Biomarkers Prev. 2000;9:257–263. | |
Prakash P, Krinsky NI, Russel RM. Retinoids, carotenoids, and human breast cancer cell cultures: a review of differential effects. Nutr Rev. 2000;58:17–76. | |
Terry P, Terry JB, Wolk A. Fruit and vegetable consumption in the prevention of cancer: an update. J Intern Med. 2001;250:280–290. | |
Borek C. Dietary antioxidants and human cancer. Integr Cancer Ther. 2004;3:333–341. | |
Ford ES, Giles WH. Serum vitamins, carotenoids, and angina pectoris: findings from the National Health and Nutrition Examination Survey III. Ann Epidemiol. 2000;10(2):106–116. | |
Wigle DT, Turner MC, Gomes J. Parent ME. Role of hormonal and other factors in human prostate cancer. J Toxicol Environ Health B Crit Rev. 2008;11(3–4):242–259. | |
Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. JAMA. 2007;297(8):842–857. | |
Jansen MC, Van Kappel AL, Ocké MC, et al. Plasma carotenoid levels in Dutch men and women, and the relation with vegetable and fruit consumption. Eur J Clin Nutr. 2004;58:1386–1395. | |
Semba RD, Dagnelie G. Are lutein and zeaxanthin conditionally essential nutrients for eye health? Med Hypotheses. 2003;61(4):465–472. | |
Hodisan T, Socaciu C, Ropan I, Neamtu G. Carotenoid composition of Rosa canina fruits determined by thin-layer chromatography and high-performance liquid chromatography. J Pharm Biomed Anal. 1997;16:521–528. | |
Moeller SM, Voland R, Tinker L, et al; CAREDS Study Group; Women’s Helath Initiative. Associations between age-related nuclear cataract and lutein and zeaxanthin in the diet and serum in the carotenoids in the Age-Related Eye Disease Study, an ancillary study of the Women’s Health Initiative. Arch Ophthalmol. 2008;126(3):354–364. | |
Krinsky NI, Landrum JT, Bone RA. Biologic mechanisms of the protective role of lutein and zeaxanthin in the eye. Annu Rev Nutr. 2003;23:171–201. | |
Junghans A, Sies H, Stahl W. Macular pigments lutein and zeaxanthin as blue light filters studied in liposomes. Arch Biochem Biophys. 2001;391(2):160–164. | |
Giovannuci E, Ascherio A, Rimm EB, Stampfer MJ, Colditz GA, Willet WC. Intake of carotenoids and retinol in relation to risk of prostate cancer. J Natl Cancer Inst. 1995;87(23):1767–1776. | |
Wood LG, Garg ML, Powell H, Gibson PG. Lycopene-rich treatments modify noneosinophilic airway inflammation in asthma: proof of concept. Free Radic Res. 2008;42(1):94–102. | |
Giovannucci E. A review of epidemiologic studies of tomato, lycopene and prostate cancer. Exp. Biol. Med. 2002;227(10):852–859. | |
Fecka I. Qualitative and quantitative determination of hydrolysable tannins and other polyphenols in herbal products from meadowsweet and dog rose. Phytochem Anal. 2009;20:177–190. | |
Buchanan BB, Gruissem W, Jones RL, editors. Biochemistry and Molecular Biology of Plants. New York, NY: John Wiley & Sons; 2000. | |
Taiz L, Zeiger E. Plant Physiology. 2nd ed. Sunderland, MA: Sinauer Associates; 2002. | |
Dennis DT, Turpin DH, Lefebvre DD, Layzell DB, editors. Plant Metabolism. 2nd ed. The Hague: SPB Academic Publishing; 1997. | |
Lotito SB, Frei B. Consumption of flavonoid-rich foods and increased plasma antioxidant capacity in humans: cause, consequence, or epiphenomenon? Free Radic Biol Med. 2006;41(12):1727–1746. | |
Galati G, O’Brien PJ. Potential toxicity of flavonoids and other dietary phenolics:significance for their chemopreventative and anticancer properties. Free Radic Biol Med. 2004;37:287–303. | |
Riboli E, Norat T. Epidemiologic evidence of the protective effect of fruit and vegetables on cancer risk. Am J Clin Nutr. 2004;78:559S–569S. | |
Tribolo S, Lodi F, Connor C, et al. Comparative effects of quercetin and its predominant human metabolites on adhesion molecule expression in activated human vascular endothelial cells. Atherosclerosis. 2008;197:50–56. | |
Verlangieri AJ, Kapeghian, JC, el-Dean S, Bush M. Fruit and vegetable consumption and cardiovascular mortality. Med Hypotheses. 1985;16:7–15. | |
Joshipura, KJ, Ascherio A, Manson JE, et al. Fruit and vegetable intake in relation to risk of ischemic stroke. JAMA. 1999;282:1233–1239. | |
Haslam A. Practical Polyphenols: From Structure to Molecular Recognition and Physiological Action. Cambridge: Cambridge University Press; 1998. | |
Chung KT, Wong TY, Wei CI, Huang YW, Lin Y. Tannins and human health: a review. Crit Rev Food Sci Nutr. 1998;38:421–464. | |
Comalada M, Camuesco D, Sierra S, et al. In vivo quercetin anti-inflammation effect involves release of quercetin, which inhibits inflammation through down-regulation of the NF-kappaB pathway. Eur J Immunol. 2005;35(2):584–592. | |
Formica JV, Regelson W. Review of the biology of quercetin and related bioflavonoids. Food Chem Toxicol. 1995;33(12):1061–1080. | |
Rao YK, Geethanqili M, Fang SH, Tzeng YM. Antioxidant and cytotoxic activities of naturally occurring phenolic and related compounds: a comparative study. Food Chem Toxicol. 2007;45(9):1770–1776. | |
Thimmappa R, Geisler K, Louveau T, O’Maille P, Osbourn A. Triterpene biosynthesis in plants. Annu Rev Plant Biol. 2014;65:225–257. | |
Housecroft CE, Constable EC. Chemistry. 3rd ed. 2006. | |
Turner P, McLennan A, Bates A, White M. Molecular Biology. 3rd ed. London: Taylor & Francis; 2005. | |
Salden T. Allt om Omega-3 [Everything about Omega-3]. SwedeHealth Press, Sweden; 1997. Swedish. | |
Calder PC. N-3 polyunsatured fatty acids, inflammation, and inflammatory disease. Am J Clin Nutr. 2006;83:1515S–1519S. | |
Leaf A, Weber PC. Cardiovascular effects of n-3 fatty acids. N Engl J Med. 1988;318(9):549–557. | |
Zurier RB, Rossetti RG, Jacobson EW, et al. Gamma-linolenic acid treatment of rheumatoid arthritis. A randomized, placebo-controlled trial. Arthritis Rheum. 1996;39(11):1808–1817. | |
Leventhal LJ, Boyce EG, Zurier RB. Treatment of rheumatoid arthritis with gammalinolenic acid. Ann Intern Med. 1993;119(9):867–873. | |
Willich SN, Rossnagel K, Roll S, et al. Rose hip herbal remedy in patients with rheumatoid arthritis – a randomized controlled trial. Phytomedicine. 2010;17:87–93. | |
Schwager J, Richard N, Schoop R, Wolfram S. A novel rose hip preparation with enhanced anti-inflammatory and chondroprotective effects. Mediators Inflamm. 2014;2014:105710. | |
Bouic PJ. The role of phytosterol and phytosterolins in immune modulation: a review of the past 10 years. Opin Clin Nutr Metab Care. 2001;4:471–475. | |
Tuohy KM, Conterno L, Gasperotti M, Viola R. Up-regulating the human intestinal microbiome using whole plant foods, polyphenols, and/or fiber. J Agric Food Chem. 2012;60:8776–8782. | |
Wenzig EM, Widowitz U, Kunert O, et al. Phytochemical compositions and in vitro pharmacological activity of two rose hip (Rosa canina L.) preparations. Phytomedicine. 2008;15:826–835. | |
Roman I, Stănilă A, Stănilă S. Bioactive compounds and antioxidant activity of Rosa canina L. biotypes from spontaneous flora of Transylvania. Chem Cent J. 2013;7:73. | |
Demir F, Özcan M. Chemical and technological properties of rose (Rosa canina L.), fruits grown wild in Turkey. J Food Ing. 2001;7:333–336. | |
Kharazmi A, Winther K. Rose hip inhibits chemotaxis and chemiluminescence of human peripheral blood neutrophils in vitro and reduces certain inflammatory parameters in vivo. Inflammopharmacology. 1999;7(4):377–386. | |
Daels-Rakotoarison AD, Greisser B, Trotin F, et al. Effects of Rosa canina fruit extract on neutrophil respiratory burst. Phytother Res. 2002;16:157–161. | |
Rein E, Kharazmi A, Winther K. A herbal remedy, Hyben Vital (stand. powder of a subspecies of Rosa canina fruits), reduces pain and improves general wellbeing in patients with osteoarthritis – a double-blind, placebo-controlled, randomised trial. Phytomedicine. 2004;11:383–391. | |
Winther K. Rose hip, in the form of Hyben Vital, has no impact on coagulation, platelet function and fibrinolysis. In: Proceedings of the Third International Exhibition and Conference on Neutraceuticals and Foods for Vitality, Palexpo Exhibition and Conference, Geneva, Switzerland, May 3–5, 2000. | |
Nam DE, Lee MJ, Kang N, Park G, Lee J. A comparative study of rose hip extracts on osteoarthritis in cartilage cells. J Korean Soc Food Sci Nutr. 2012;41(12):1663–1670. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.