Back to Journals » Journal of Experimental Pharmacology » Volume 11
Bioactive fraction from Lagerstroemia speciosa leaves (DLBS3733) reduces fat droplet by inhibiting adipogenesis and lipogenesis
Authors Karsono AH , Tandrasasmita OM, Tjandrawinata RR
Received 30 July 2018
Accepted for publication 5 March 2019
Published 2 May 2019 Volume 2019:11 Pages 39—51
DOI https://doi.org/10.2147/JEP.S181642
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Bal Lokeshwar
Agung Heru Karsono, Olivia Mayasari Tandrasasmita, Raymond Rubianto Tjandrawinata
Section of Molecular Pharmacology, Department of Research Innovation and Invention, Dexa Laboratories of Biomolecular Sciences, Dexa Medica, Cikarang, West Java, Indonesia
Background: Obesity has become a risk factor for metabolic diseases. One of the cellular characteristics of obesity is the occurrence of adipose cells hyperplasia. Lagerstroemia speciosa is a plant which has been used for the treatment of diabetes. Furthermore, some studies also indicated that L. speciosa possesses antiobesity activity. Its antiobesity activity was examined in the present study through adipogenesis, lipogenesis, and lipolysis pathways.
Aim: DLBS3733, a bioactive fraction of L. speciosa, was explored for its potential benefits to alter obesity through adipogenesis and lipogenesis inhibition and lipolysis induction activity.
Materials and methods: This study was performed using 3T3-L1 cells. mRNA level and protein expressions related to adipogenesis, lipogenesis, and lipolysis pathways were assayed in this study.
Results: Antiadipogenic effects of DLBS3733 (15 μg/mL) were found to be mediated by a significant downregulation of mRNA level of multicomponents involved in adipogenesis which include C/EBPα (CCAAT/enhancer-binding protein alpha) and PPAR-γ (peroxisome proliferator-activated receptor gamma) by 75% and 80.1% (p<0.05), respectively. DLBS3733 was found to inhibit lipogenesis, as shown by the significant reductions of adiponectin excretion and mRNA level of fatty acid synthase, SREBP (sterol regulatory element-binding protein), and ACC-β (Acetyl-CoA carboxylase) by 44.7%, 70.9%, and 83.1%, respectively (p<0.05). In addition, DLBS3733 was found to inhibit fat droplets accumulation in the cells in a dose-dependent manner through Oil-Red O staining. pAMPK protein was upregulated by 75% and ACC-β was downregulated by 88% (p<0.05) which indicates the reduction of lipid synthesis. Meanwhile, DLBS3733 showed an insignificant effect on adipose triglyceride lipase, hormone-sensitive lipase, and carnitine palmitoyl-CoA transferase-1 which indicate that DLBS3733 does not induce lipolysis.
Conclusion: These results demonstrate the inhibitory activity of DLBS3733 on adipogenesis and lipogenesis. DLBS3733 may provide an effective and potential benefit in the prevention of obesity.
Keywords: Lagerstroemia speciosa, obesity, adipogenesis, lipogenesis, adiponectin, ACC-β
Introduction
Obesity is a result of excessive food intake and/or insufficient energy expenditure. Obesity can be measured by the means of body mass index (BMI), which is defined as the ratio of weight to height. People with a BMI of 30 kg/m2 and higher are categorized as obese.1,2 Obesity has been known to be one of the risk factors of adverse metabolic effects on blood pressure, cholesterol, triglycerides (TGs), and insulin resistance which eventually may lead to cardiovascular diseases and diabetes.3 The risks of heart attack, atherosclerosis, cancer, stroke, and type 2 diabetes mellitus have been known to be increased steadily along with increased BMI. To date, obesity has become a global epidemic issue particularly in developed countries.2,4
Obesity can be characterized by an excess body fat accumulation as a consequence of the imbalance between lipogenesis and lipolysis which may further lead to lipotoxicity and alteration in metabolic pathways in both adipose tissue and peripheral organs, such as liver, heart, pancreas, and muscle.5 The cellular characteristics of adipose tissue mass in obesity include cellular hyperplasia (increased cell number) and hypertrophy (increased size of cells). Adipose cells are recognized as the critical regulators of whole-body metabolism and known to be involved in the pathogenesis of metabolic diseases. Differentiation of pre-adipose cell, which is known as adipogenesis, results in the formation of adipose tissue.6,7
Medication that is intended for weight loss is often associated with several adverse effects and weight gain rebound when the use of medication is discontinued.8 Therefore, the use of herbal supplements to alter obesity condition has become popular due to its lower side effects. Various medicinal plants, such as blueberry peel extract9 and green tea extracts10 have been proved to reduce high-fat diet-induced obesity. Our previous researches have been conducted in investigating the molecular activities and mechanisms of various herbals,11–14 which also revealed the potential molecular activities of Lagerstroemia speciosa. DLBS3733 is a bioactive fraction (BAF) derived from L. speciosa leaves produced by Dexa Laboratories of Biomolecular Sciences (DLBS), Dexa Medica, Indonesia. L. speciosa is a common tropical plant grown in Philippines, Malaysia, Vietnam, and other Southeast Asia countries which also include Indonesia. This plant can grow up to 20-m high and locally known as banaba.15 The study on banaba as an antidiabetic agent has extensively been done. In addition, banaba has commonly been used for the treatment of diabetes.16–18 Judy et al, reported that GlucasolTM, a standardized banaba extract, is used as an antidiabetic drug by increasing glucose uptake activity.17 Likewise, our previous results found that the combination of L. speciosa and Cinnamomum burmannii extracts was able to increase insulin signaling and sensitivity.19 Liu et al, have also demonstrated that the extract of L. speciosa increased glucose uptake through the induction of GLUT4 activity.16 Furthermore, L. speciosa can also inhibit adipogenesis, as shown by decreased PPARγ2 expression on samples treated with L. speciosa extract which has been known to prevent obesity. Diabetes and obesity are intrinsically linked, where obesity has been known to increase the risk of diabetes and insulin resistance. The activity of L. speciosa extract to alleviate obesity condition in vivo has previously been studied by Suzuki et al20. In this study, mice administered with banaba extract for 12 weeks exhibited a lower body weight as compared to placebo.
In the present study, the effect of DLBS3733 and its mechanism of action were investigated in differentiated mouse 3T3-L1 adipocytes, a basic cell model to assess adipogenesis and adipocyte metabolism in vitro. This study mainly focused on the effect of DLBS3733 on adipogenesis, lipogenesis, and lipolysis in vitro using 3T3-L1 preadipocyte cells and the differentiated 3T3-L1 adipocyte cells, and further investigated the related molecular mechanisms. This study was done by measuring the viability and accumulation of fat droplets in the treated cells, as well as measuring major adipogenic transcriptional factors in adipogenesis pathways which include PPARγ and C/EBPα gene expressions. Lipogenesis pathway was studied by measuring FASn, SREBP-1, and ACC-β gene expressions. Furthermore, lipolysis pathway was observed by measuring ATGL, HSL, and CPT-1 gene or protein expressions.
Materials and methods
Materials
The chemicals and solvents used to prepare DLBS3733 were purchased from Merck (Darmstadt, Germany). Ellagic acid reference standard was purchased from ChromaDex (Irvine, USA).
DLBS3733 preparation
DLBS3733 is a BAF derived from the aqueous extract of L. speciosa leaves, which was obtained from Temanggung, Central Java, Indonesia. This plant has been identified by Herbarium Bogoriense, Research Center of Biology, Indonesian Institute of Sciences with certificate No. 1261/IPH.1.02/If.8/XII/2009. The dried cut of L. speciosa leaves was macerated in water with a ratio of 1:12 w/v at 70°C for 2 hrs, followed by filtration. The filtrate was concentrated under low pressure at 60°C using rotary evaporator (Büchi, Flawil, Switzerland). The concentrate was then dried in a conventional oven at 70°C for 24 hrs and stored in a well-closed container at 25–30°C.
The identification of DLBS3733 was done by thin layer chromatography (TLC) with Silica Gel 60 F254 plate (Merck, Darmstadt, Germany) as the stationary phase and the mixture of toluene-ethyl acetate-formic acid-methanol (30:30:8:4, v/v) as the mobile phase. The eluent was allowed to move along the TLC plate for a distance of 8 cm. The chromatogram was then observed under UV λ 254 and 366 nm with Rf 0.5–0.6 as a black band without derivatization (Camag, Muttenz, Switzerland). Ellagic acid in DLBS3733 was quantified using high-pressure liquid chromatography, Waters® (Milford, USA) under UV λ 280 nm with Symmetry C18 (Merck, Darmstadt, Germany), 5 µm 4.6×250 mm as the column and acetonitrile-0.1% phosphoric acid in water (v/v) as the mobile phase. Elution system was isocratic with 25% of acetonitrile and 75% of 0.1% phosphoric acid in water (v/v). The content of the marker compound in DLBS3733 was not less than 1%.
Cell culture and differentiation
The pre-adipocyte Mus musculus cell line 3T3-L1 (ATCC® CL-173™) was purchased from American Type Culture Collection (Rockville, MD, USA). 3T3-L1 cells, which are commonly used for the study of adipogenesis and obesity-related characteristics, were cultured in DMEM supplemented with 10% bovine serum and 1% penicillin/streptomycin (Gibco, Grand Island, USA). In a six-well plate, adipocyte differentiation was initiated with 0.5 mM 3-isobutyl-1-methylxanthine, 1 mM dexamethasone and 20 µg/mL insulin (Sigma, MO, USA) added in culture medium for 48 hrs. Subsequently, the differentiation medium was replaced with culture medium supplemented with 20 µg/mL insulin. There were four different 3T3-L1 stages used in this study: pre-adipose cell (undifferentiated 3T3-L1 cell), early adipose cell (differentiated 3T3-L1 cell into adipocyte cell for 3 days), mature adipose cell (maturated early adipose cell for 10 days), and advanced adipose cell (maturated early adipose cell for 20 days, fully differentiated adipose cell).
Cytotoxicity assay
3T3-L1 cells were cultured in 96-well plate until confluent (80%) using culture medium (DMEM, 10% BS, 1% p/s). The medium was then replaced with a free-serum medium for 24 hrs, continued by 24 hrs of incubation with various concentrations of DLBS3733 (25, 50, and 100 µg/mL). Cell viability was observed using CellTiter 96® AQueous Non-Radioactive Cell Proliferation Assay Reagents (Promega, Fitchburg, WI, USA) according to the manufacturer’s protocol.
mRNA isolation and gene expression study
The following genes, ie, PPARγ, C/EBP-α, Adiponectin, ACC-β, FASn, SREBF, were suggested to be involved in adipogenesis and lipogenesis. Total mRNA was isolated using RiboEx reagent (GeneAll, Seoul, Korea), and mRNA concentration was quantified using NanoDrop 2000c spectrophotometer (Thermo Scientific, Waltham, MA, USA). Reverse transcription of total mRNA (1 µg) was done using GoScript reagent (Promega, Fitchburg, WI, USA). Gene expression study was carried out using gene-specific primers (Table 1). Gene amplification was done using PCR, GoTaq (Promega, Fitchburg, WI, USA) or real-time PCR, SsoFastTM EvaGreen® Supermix (Biorad, Hercules, California, USA). PCR and qPCR were performed using T3000 Thermocycler (Biometra, Göttingen, German) and Mini Opticon MJ MiniTM (Biorad, Hercules, California, USA), respectively.
 | Table 1 Gene-specific primers |
pAMPK protein expression assay
pAMPK and CPT-1 proteins were suggested to be involved in the activation of the AMPK pathway. Total protein was isolated using RIPA buffer (50 mM Tris pH 8, 150 mM NaCl, 0.5% SDS, 1% NP-40, 2 mM EDTA and 1x protease inhibitor) and measured using Lowry method. The pAMPK and CPT-1 protein expression were observed using pAMPK ELISA kit (Abcam, Cambridge, UK) and CPT-1 ELISA kit (Cusabio, Wuhan, China). All experiments were done according to the manufacturer’s protocol.
Oil-red O (ORO) assay
ORO assay was performed to evaluate the fat droplet amount within adipose cell. 3T3-L1 cells that have been differentiated to adipose cell were stained with ORO dye (Sigma, MO, USA). ORO was diluted with ddH2O 3:2, mixed and incubated at room temperature for 20 mins. After incubation, ORO was filtered with a 0.2 µm filter membrane. Cells were fixated using 10% formalin in PBS (Sigma, MO, USA) for 1 hr and subsequently washed with 60% isopropanol (Sigma, MO, USA). Furthermore, ORO was applied to the cells for 10 mins and then washed using ddH2O and allowed to dry at room temperature. The fat droplets were stained red. ORO retentions were extracted using 60% isopropanol for 10 mins and quantified using spectrophotometer at 520 nm.
Statistical analysis
The data were presented as the mean of at least two replicates experiments and standard error (SE) of mean. Significant differences between experimental values were determined using student unpaired t-test (Abacus Concepts, Piscataway, USA) with p<0.05 considered as significant.
High-performance liquid chromatography (HPLC) conditions
Chromatographic analysis was performed using a Waters HPLC system equipped with an Alliance e2695 separation module, an autosampler injector, and a 2489 UV detector. Chromatographic data were processed by Empower 3 software. Chromatographic separation was achieved using Waters SunFire – C18 column (250 mm ⨰ 4.6 mm i.d., 5 um) with the mobile phase consisted of methanol and 0.2% phosphoric acid at a flow rate of 1.0 ml/min. Separation was carried out under the condition of gradient elution for 30 mins run time. Detection was set at 254 nm with 20 µL injection volume. The retention time of ellagic acid was approximately 11 mins under this chromatographic condition.
Results
Effect of DLBS3733 on 3T3-L1 cells viability
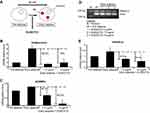
In vitro toxicity of DLBS3733 using 3T3-L1 preadipocytes did not show any significant effect on 3T3-L1 cell viability at concentrations up to 50 μg/mL. At all concentrations studied, the extension of inhibition did not exceed the IC50 (Figure 1). These results indicate that the use of DLBS3733 at concentrations below 50 µg/mL is considered as safe.
 | Figure 1 The effect of DLBS3733 on preadipocytes viability. Values are presented as mean ± SD. The data shown are representative of at least two independent experiments. |
Effect of DLBS3733 on phosphorylation of AMPK in preadipocytes
AMP-activated protein kinase (AMPK) is an important regulator of sugar and fat metabolism. In this experiment, the expression of pAMPK protein was upregulated by 69% and 75% following DLBS3733 treatment at concentrations of 7.5 and 15 µg/mL, respectively. These results indicate the stimulatory effect of DLBS3733 in AMPK phosphorylation. Furthermore, it was observed that DLBS3733 downregulated ACC-β gene expression (Figure 2). ACC-β gene expression was downregulated by 75% and 88% following DLBS3733 treatment at concentrations of 7.5 and 15 µg/mL, respectively. The downregulation of ACC-β gene expressions may also decrease the level of Malonyl CoA and may further increase CPT-1 with the consequence of fatty acid oxidation. However, AMPK pathway activation by DLBS3733 was only observed in 3T3-L1 preadipocytes (Figure 2).
Effect of DLBS3733 on adipogenesis inhibition
Adipogenesis is one of the key pathways that increase the mass of adipose tissue. To investigate the effect of DLBS3733 on adipogenesis inhibition, 3T3-L1 cells were differentiated into adipocytes for 3 days and DLBS3733 was added during the differentiation process. In ORO staining assay, the differentiation of preadipocytes into adipocytes is associated with an increased number of ORO-stained cells due to lipid accumulation. Adipogenic transcription factors, such as CCAAT/enhancer binding protein-beta (C/EBPβ), nuclear receptor peroxisome proliferator-activated receptor gamma (PPARγ), and CCAAT/enhancer binding protein-alpha (C/EBPα), play a key role in the complex transcriptional cascade that occurs during adipogenesis.21 The secretion of adiponectin was steadily increased during adipogenesis.
The antiadipogenic effect of DLBS3733 in 3T3-L1 cells was analyzed by measuring the expression of differentiation markers which include PPARγ, C/EBP-α, and adiponectin. As shown in Figure 3, adipogenesis gene expressions in early adipocyte sample were significantly higher than the preadipocytes. PPARγ, C/EBP-α, and adiponectin gene expressions levels in early adipocytes were increased by 1.43-, 1.26- and 4.16-fold compared to preadipocytes, respectively. These results indicate that adipogenesis in preadipocytes was increased. Meanwhile, the addition of DLBS3733 at concentrations of 7.5 and 15 µg/mL during cell differentiation reduced the mRNA levels of PPARγ, C/EBPα and adiponectin (Figure 3) by 46.9%, 45.2%, 83.2% and 60.1%, 90.5%, 75%, respectively, as compared to early adipocytes as the control. The downregulation of PPAR-γ and C/EBP-α gene expressions occurred in a dose-dependent manner.
Comparison of DLBS3733 effect on lipogenesis and lipolysis
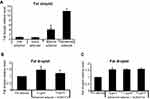
Four different 3T3-L1 cells were used in this study, which include preadipocytes, early adipocytes, mature adipocytes, and advanced adipocytes. Fat droplet was one of the parameters observed, which can be the hallmark of DLBS3733 activity on lipogenesis and lipolysis. Fat droplets accumulation was increased during preadipocytes differentiation to advanced adipocytes which takes 23 days to generate mature advanced adipocytes. During this period, the highest fat droplets accumulation was found. In this study, fat droplets profile for each 3T3-L1 stage was observed (Figure 4A) using ORO staining to measure fat droplets accumulation. As seen in Figure 4A, fat droplets accumulation levels were 1.0-, 0.8-, 4.1- and 11.7-fold for preadipocytes, early adipocytes, mature adipocytes, and advanced adipocytes, respectively. Preadipocytes were used as control samples.
The effect of DLBS3733 on lipogenesis and lipolysis in 3T3-L1 cells was measured by calculating the fat droplets of ORO-stained cells. To investigate the effects of DLBS3733 on the inhibition of lipogenesis, 3T3-L1 preadipocytes were differentiated into adipocytes for 3 days. Early adipocytes were then maintained in a medium containing insulin for 10 days, continued by medium containing insulin with the presence or absence of DLBS3733 at concentrations of 7.5 and 15 µg/mL for 10 days. In the absence of DLBS3733, early adipocytes were differentiated to advanced adipocytes after 23 days which was marked by an increased fat droplets accumulation. Conversely, DLBS3733 treatment during the maturation period successfully decreased fat droplet level by 15.4% (Figure 4B). This result indicates the lipogenesis inhibitory effect of DLBS3733.
In addition, the effect of DLBS3733 on lipolysis was also investigated using a similar protocol as described previously. In this observation, DLBS3733 treatment was done on day 23 for 24 hrs. As shown in Figure 4C, DLBS3733 treatment did not affect fat droplet level which indicates that DLBS3733 might not possess bioactivity on lipolysis induction.
Effect of DLBS3733 on lipogenic-related genes
The maturation of adipose cells is interpreted by the accumulation of lipid droplets. It has also been known that fat cells reached size fluctuations under insulin stimulation. Lipid droplets synthesis, which is known as lipogenesis, and lipid accumulation were increased during cell maturation. To investigate the effects of DLBS3733 in the inhibition of lipogenesis in 3T3-L1 preadipocytes, cells were differentiated into adipocytes for 3 days. Afterwards, the early adipocyte cells were maintained in a medium containing insulin with the presence or absence of DLBS3733 (7.5 and 15 µg/mL) for the next 10 days with medium exchange every 2–3 days. To quantify the effect of DLBS3733 on major lipogenic transcriptional factor expressions in 3T3-L1 cells, ORO staining assay and PCR analysis were conducted. ORO dye was used to stain the fat droplets on day 13.
The anti-lipogenic effect of DLBS3733 in 3T3-L1 cells was measured by calculating the fat droplets of ORO-stained cells, as well as measuring the expression of lipogenic markers which include SREBP, FASn, and ACC-β. As seen in Figure 5A and B, higher fat droplets by 2.12-fold were observed in mature adipocyte as compared to preadipocytes as the control samples. This indicates that lipogenesis occurred during maturation. In other words, the fat droplets were developed more in adipocytes during maturation.
Early adipose treated with DLBS3733 at concentrations of 7.5 and 15 µg/mL showed fewer fat droplets as compared to the normal mature adipocytes as the control by 22.6% and 31.1%, respectively. It suggests that DLBS3733 treatment during adipogenesis and maturation successfully inhibited fat droplets synthesis (Figure 5A and B). During adipogenesis and maturation, the lipogenic genes expressions were significantly increased by 1.66-, 1.23-, and 1.41-fold for ACC-β, FASn and SREBP genes, respectively, as compared to preadipocytes which indicate that lipogenesis occurred during the maturation process. Meanwhile, DLBS3733 treatment at concentrations of 7.5 and 15 µg/mL, respectively, reduced the mRNA expression of lipogenic-specific genes which include ACC-β (6.02% and 83.1%), SREBP-1 (37.6% and 70.91%) and FASn (36.6% and 44.7%), in a dose-dependent manner (Figure 5C–F). Mature adipocytes were used as control samples. Based on ORO staining assay and lipogenesis gene markers assays, we suggest that DLBS3733 exhibited lipogenesis inhibitory effect.
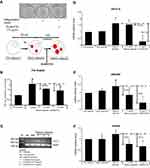
Effect of DLBS3733 on lipolysis-related genes
Lipolysis is the process of lipid breakdown, which is the important key process of weight loss. Adipose triglyceride lipase (ATGL) is the rate-limiting enzyme of lipolysis in adipocytes. Other important lipase that involved in catalyzing lipolysis is hormone-sensitive lipase (HSL), which is implicated in hormonal regulation by insulin. mRNA expressions of lipolysis enzymes (HSL and ATGL) were increased during adipogenesis and maturation, which also resulted in an increased fat droplets accumulation (Figure 6). Surprisingly, DLBS3733-treated cells have significantly resulted in decreased HSL and ATGL mRNA expressions during cell maturation.
ATGL and HSL gene expressions were found to be increased in mature and advanced adipocytes. Preadipocytes were used as the control samples. As shown in Figure 6, ATGL gene expressions in advanced adipocytes were upregulated by 52%. However, increased gene expressions were not detected in advanced adipose treated with DLBS3733. ATGL and HSL gene expressions were downregulated by 6.6% and 43% in a dose-dependent manner after DLBS3733 treatment at a concentration of 7.5 μg/mL and 31.6% and 64.5% reduction after treated with higher concentration (15 µg/mL) (Figure 6C and D), respectively. These results indicate that lipolysis was likely not occurred due to DLBS3733 treatment. In addition to the genes, CPT-1, a protein which plays a major role in the β-oxidation process, was also observed and showed consistent results in ATGL and HSL gene expressions. CPT-1 protein level in advanced adipose cells was increased by 26.5% compared to preadipocytes, and decreased to 9.4% and 23.8% compared to advanced adipocytes after DLBS3733 treatment at concentrations of 7.5 and 15 μg/mL, respectively. This study indicates that DLBS3733 affected lipogenesis and exhibited no effect on lipolysis and β-oxidation. Fat droplets were accumulated more from pre-adipose to advanced adipose, and lipolysis may further be initiated, and its activity was increased from pre-adipose to advanced adipose. However, when the DLBS3733 was introduced, lipogenesis and lipolysis activities were restrained. Therefore, the markers of lipolysis and β-oxidation were decreased (Figure 6C–E).
Discussion
Adipocytes differentiation and fat accumulation are associated with the development and occurrence of obesity. Adipose tissue consists of preadipocytes and mature adipocytes. Preadipocytes possess the ability to propagate and differentiate into mature adipocytes, where the number of mature adipocytes determines the number of fat cells that exists in an organism throughout its lifespan.22 Changes in preadipocyte cells to mature adipocytes require time and certain stages of development. Therefore, the analysis in the study was carried out at several time points to represent the stages of fat droplet development and gene expression changes in 3T3-L1 cells from preadipocytes to mature adipocytes. The differentiation process of adipocyte is known as adipogenesis, which is induced by C/EBP-α and PPARγ genes.23,24 Following adipocytes differentiation, the cell signal adipokines may be secreted by adipose tissue. Reduction of adipose tissue has recently been proposed to be a potential candidate in body weight management. Based on the results of the present study, DLBS3733 exhibited its ability to inhibit adipogenesis, as confirmed by the reduction of adipogenesis-related genes which include PPARγ, C/EBP-α, and adiponectin. PPARγ and C/EBPα have been known to play major roles in adipogenesis.25,26 In addition, other study on the extract of olive seeds also showed a similar mechanism of this activity, where it exhibited antiadipogenic activity by reducing the expression of PPARγ.27 Meanwhile, adiponectin is one of the adipokines that is secreted by adipocytes during differentiation.28,29 Liu et al, reported that decreased PPARγ expression did not occur by the presence of insulin in the adipogenesis induction process.16 In this study, insulin was a part of adipogenesis induction medium. The presence of DLBS3733 was found to down-regulate PPARγ expression, which further showed the ability of DLBS3733 to decrease PPARγ expression by the presence of insulin in induction media. Since the expansion of adipose tissue is the root cause of obesity, inhibition of adipogenesis may become a potential option to reduce obesity.
Differentiated adipocytes store fatty acids in the cytoplasm in the form of TGs by the involvement of various enzymes such as fatty acid synthase (FASn) and acetyl-coenzyme A (acetyl-CoA) carboxylase (ACC-β). Glucose induces ACC-β enzyme expression that stimulates insulin secretion to promote lipogenesis as the synthesis of fatty acid from acetyl-CoA and TG biosynthesis. ACC-β is also known as the rate-limiting enzyme of lipogenesis that catalyzes the carboxylation of acetyl-CoA to produce malonyl-CoA. Fatty acids are synthesized from malonyl-CoA through the processes catalyzed by FASn and stearoylcoenzyme A desaturase-1 (SCD-1). Suppression of genes involved in the fatty acid synthesis, such as ACC-β, FAS, and SCD-1, leads to the reduction of adipocyte TG synthesis and accumulation.30 Excessive lipids accumulation is recognized as the risk factor for the development of obesity. Several factors have been known to regulate lipogenesis pathway. Insulin induces sterol regulatory element-binding protein 1 (SREBP-1) gene expression, which is a lipogenic gene and major transcriptional regulator of cholesterol, fatty acid, and TG synthesis. In fatty acid synthesis, FASn acts as the key enzyme involved in long-chain saturated fatty acid synthesis.31–33 We demonstrate a positive result of DLBS3733 as a potential natural anti-obesity agent through the reduction of fat droplets accumulation, as well as the downregulation of lipogenic genes expression which include ACC-β, FASn, and SREBP-1.
Several studies have shown that FASn can be a potential target in reducing obesity. Study conducted by Berndt et al,34 suggested an allegation that increased FASn expression may be directly related to the occurrence of obesity and type 2 diabetes, while in other study, administration of FASn inhibitors (C75 and cerulenin) led to weight loss.35 Loftus et al, stated that the administration of FASn inhibitors in mice was thought to affect food intake.36 However, it was suspected that there was another mechanism, as some mice that tolerate the inhibitors still experience weight loss. Energy expenditure was thought to be the other mechanism underlying this condition.37 Our result suggests that DLBS3733 tends to decrease FASn expression; therefore, it may possess a similar effect to FASn inhibitors C75 and cerulenin.
In addition, obesity can also be reduced by decreasing adipogenesis and/or lipid synthesis (lipogenesis) and/or increasing lipid breakdown (lipolysis). Lipolysis is a catabolic pathway that promotes the mobilization of metabolic fuel from adipose to peripheral tissues as the response to appropriate energy demands. AMPK is considered as the main sensor that maintains energy homeostasis.38,39 This protein is expressed ubiquitously and has a major role in fatty acid and glucose metabolisms. The activation of AMPK can lead to ACC-β inhibition activity through the phosphorylation or down-regulation of ACC-β gene expression. This inhibition may result in the suppression of carnitine palmitoyl-CoA transferase-1 (CPT-1) which further leads to increased fatty acid oxidation.40–43 Gaidhu et al, reported that mice treated with AICAR, an AMPK activator, with chronic doses showed a decreased adipose cells count and increased number of mitochondria.44
In this study, we also observed AMPK activation in 3T3-L1 preadipocytes with DLBS3733 treatment which showed an increased phosphorylated AMPK protein. Down-regulation of ACC-β gene expression was also observed but not related to AMPK activation (data not shown). This phenomenon seems to be relevant to lipogenesis inhibition, thus we suggest that DLBS3733 may only be effective as an AMPK activator of preadipocytes. Further studies are required to confirm its activities on AMPK.
Another strategy to induce weight loss can be achieved by increasing lipid breakdown which includes β-oxidation or lipolysis. CPT-1 is known as a rate-limiting enzyme for β-oxidation, hence, it was frequently considered as one of the important markers of lipid degradation. Results showed that DLBS3733 treatment exhibited no effect on lipolysis enzyme (HSL and ATGL). ATGL initiates lipolysis by specifically removing free fatty acids to produce a diacylglycerol, which is then hydrolyzed by HSL.45 Interestingly, DLBS3733 decreased HSL and ATGL mRNA levels and CPT-1 protein level, indicating that lipogenesis, but not lipolysis, were occurred during maturation which indicates that fat droplets were decreased but lipolysis enzyme did not increase. To re-confirm the effect of DLBS3733 on lipolysis, advanced adipocyte 3T3-L1 (fully differentiated adipocytes) were treated with DLBS3733. Furthermore, after 24 hrs of incubation, result showed no fat droplet reduction was found. This result confirmed that DLBS3733 did not induce lipolysis. However, it is important to note that lipolysis is a responsive process in which it requires any trigger or signal, such as physical exercise and lower energy state, which is difficult to assess in an in vitro models. The effect of downregulated HSL and ATGL gene expressions in DLBS3733-treated samples may require further research. The downregulation may possibly due to the stronger effect of lipogenesis caused by DLBS3733, which is enough to decrease the fat droplet and finally impacts HSL and ATGL metabolisms.
DLBS3733 possesses good potential in this study. However, the BAF itself is not a single compound. The chromatogram showed 11.063% of ellagic acid compounds in DLBS3733 (Figure 7). Ellagic acid is a naturally occurring substance that is commonly found in strawberries, raspberries, blackberries, cherries, and walnuts. Panchal et al, reported that rats induced with metabolic syndrome were attenuated by ellagic acid trough normalization of protein levels of Nrf2, NF-κB and CPT1.46 Further research on DLBS3733 is still required.
 | Figure 7 Chromatogram of DLBS3733, ellagic acid was detected for 11.063%. |
Conclusion
In conclusion, DLBS3733 exhibited the ability to suppress fat accumulation by inhibiting the process of adipogenesis and lipogenesis. Both activities are useful in preventing the formation of body fat or the fat itself. Therefore, DLBS3733 may become a potential option to reduce body fat and may further use to control obesity in the future.
Acknowledgments
This research was fully supported by Dexa Medica. All authors are employed by Dexa Medica. Authors would like to thank Irfan Agustian Darfiansyah for preparing DLBS3733, Tia Mariana for her contribution in the cell culture works and Prof. Dr. Heni Rachmawati and Isabela Anjani for their assistance in editing the manuscript.
Disclosure
The authors reported no conflict of interest in this work.
References
1. Friedenberg RM. Obesity. Radiology. 2002;225:629–632. doi:10.1148/radiol.2253020937
2. Caballero B. The global epidemic of obesity: an overview. Epidemiol Rev. 2007;29:1–5. doi:10.1093/epirev/mxm012
3. Cascio G, Schiera G, Liegro ID. Dietary fatty acids in metabolic syndrome, diabetes and cardiovascular diseases. Curr Diabetes Rev. 2012;8:2–17.
4. Gomez-Hernandez A, Beneit N, Diaz-Castroverde S, et al. Differential role of adipose tissue in obesity and related metabolic and vascular complications. Int J Endocrinol. 2016;1216783.
5. Saponaro C, Gaggini M, Carli F, Gastaldelli A. The subtle balance between lipolysis and lipogenesis: a critical point in metabolic homeostasis. Nutrients. 2015;7:9453–9474. doi:10.3390/nu7020948
6. Lee JY, Hashizaki H, Goto T, et al. Activation of peroxisome proliferator-activated receptor-α enhances fatty acid oxidation in human adipocytes. Biochem Biophys Res Commun. 2011;407:818–822. doi:10.1016/j.bbrc.2011.03.106
7. Charo NL, Ceschan MIR, Galigniana NM, et al. Organization of nuclear architecture during adipocyte differentiation. Nucleus. 2016;7:249–269. doi:10.1080/19491034.2016.1197442
8. Abdollahi M, Afshar-Imani B. A review on obesity and weight loss measures. Middle East Pharmacy. 2003;11:6–10.
9. Song Y, Park HJ, Kang SN, et al. Blueberry peel extracts inhibit adipogenesis in 3T3-L1 cells and reduce high-fat diet-induced obesity. PLoS One. 2013;8:e69925. doi:10.1371/journal.pone.0069925
10. Yang X, Yin L, Li T, et al. Green tea extracts reduce adipogenesis by decreasing expression of transcription factors C/EBPα and PPARγ. Int J Clin Exp Med. 2014;7:4906–4914.
11. Tandrasasmita OM, Lee JS, Baek SH, Tjandrawinata RR. Induction of cellular apoptosis in human breast cancer by DLBS1425, a Phaleria macrocarpa compound extract, via down-regulation of PI3-kinase/AKT pathway. Cancer Biol Ther. 2010;10:1–11.
12. Tjandrawinata RR, Arifin PF, Tandrasasmita OM, et al. DLBS1425, a Phaleria macrocarpa (Scheff.) Boerl. extract confers anti proliferative and proapoptosis effects via eicosanoid pathway. J Exp Ther Oncol. 2010;8:187–201.
13. Tjandrawinata RR, Nailufar F, Arifin PF. Hydrogen potassium adenosine triphosphatase activity inhibition and downregulation of its expression by bioactive fraction DLBS2411 from Cinnamomum burmannii in gastric parietal cells. Int J Gen Med. 2013;6:807–815. doi:10.2147/IJGM.S50134
14. Tandrasasmita OM, Sutanto AM, Arifin PF, Tjandrawinata RR. Anti-inflammatory, antiangiogenic, and apoptosis-inducing activity of DLBS1442, a bioactive fraction of Phaleria macrocarpa, in a RL95-2 cell line as a molecular model of endometriosis. Int J Womens Health. 2015;7:161–169. doi:10.2147/IJWH.S74552
15. Klein G, Kim J, Himmeldirk K, et al. Antidiabetes and anti-obesity activity of Lagerstroemia speciosa. Adv Access. 2007;4:401–407.
16. Liu F, Kim J, Li Y, et al. An extract of Lagerstroemia speciosa L. has insulin-like glucose uptake-stimulatory and adipocyte differentiation-inhibitory activities in 3T3-L1 cells. J Nutr. 2001;131:2242–2247. doi:10.1093/jn/131.9.2242
17. Judy WV, Hari SP, Stogsdill WW, et al. Antidiabetic activity of a standardized extract (GlucosolTM) from Lagerstroemia speciosa leaves in type II diabetics: a dose-dependence study. J Ethnopharmacol. 2004;87:115–117.
18. Hou W, Li Y, Zhang Q, et al. Triterpene acids isolated from Lagerstroemia speciosa leaves as α-glucosidase inhibitors. Phytother Res. 2008;23:614–618. doi:10.1002/ptr.2661
19. Tandrasasmita OM, Wulan DD, Nailufar F, et al. Glucose-lowering effect of DLBS3233 is mediated through phosphorylation of tyrosine and upregulation of PPARγ and GLUT4 expression. Int J Gen Med. 2011;4:345–357.
20. Suzuki Y, Unno T, Ushitani M, et al. Antiobesity activity of extracts from Lagerstroemia speciosa L. leaves on female KK-Ay mice. J Nutr Sci Vitaminol. 1999;45:791–795.
21. Cristancho AG, Lazar MA. Forming functional fat: a growing understanding of adipocyte differentiation. Nat Rev Mol Cell Biol. 2011;28:722–734. doi:10.1038/nrm3198
22. Armani A, Mammi C, Marzolla V, et al. Cellular models for understanding adipogenesis adipose dysfunction obesity. J Cell Biochem. 2010;110:564–572. doi:10.1002/jcb.22598
23. Otto TC, Lane MD. Adipose development: from stem cell to adipocyte. Crit Rev Biochem Mol Biol. 2005;40:229–242. doi:10.1080/10409230591008189
24. Feng S, Reuss L, Wang Y. Potential of natural products in the inhibition of adipogenesis through regulation of PPARγ expression and/or its transcriptional activity. Molecules. 2016;21:1278. doi:10.3390/molecules21101278
25. Siersbaek R, Nielsen R, Mandrup S. PPARγ in adipocyte differentiation and metabolism – novel insights from genome-wide studies. FEBS Lett. 2010;584:3242–3249. doi:10.1016/j.febslet.2010.06.010
26. Lefterova MI, Haakonsson AK, Lazar MA, Mandrup S. PPARγ and the global map of adipogenesis and beyond. Trends Endocrinol Metab. 2014;25:293–302. doi:10.1016/j.tem.2014.04.001
27. Veciana-Galindo C, Cortes-Castell E, Toro-Montell A, et al. Anti-adipogenic activity of an olive seed extract in mouse fibroblasts. Nutricion Hospitalaria. 2015;31:2747–2751. doi:10.3305/nh.2015.31.6.8997
28. Kadowaki T, Yamauchi T. Adiponectin and adiponectin receptors. Endocr Rev. 2002;26:439–451. doi:10.1210/er.2005-0005
29. Achari AE, Jain SK. Adiponectin, a therapeutic target for obesity, diabetes, and endothelial dysfunction. Int J Mol Sci. 2017;18:1321. doi:10.3390/ijms18061321
30. Ntambi JM, Young-Cheul K. Adipocyte differentiation and gene expression. J Nutr. 2000;130:3122–3126. doi:10.1093/jn/130.12.3122S
31. Moreno-Indias I, Tinahones FJ. Impaired adipose tissue expandability and lipogenic capacities as ones of the main causes of metabolic disorders. J Diabetes Res. 2015;12:1–12.
32. Luo L, Liu M. Adipose tissue in control of metabolism. J Endocrinol. 2016;231:R77–R99. doi:10.1530/JOE-16-0211
33. Yilmaz M, Caliborn KC, Hotamisligil GS. De novo lipogenesis products and endogenous lipokines. Diabetes. 2016;65:1800–1807. doi:10.2337/db16-0251
34. Berndt J, Kovacs P, Ruschke K, et al. Fatty acid synthase gene expression in human adipose tissue: association with obesity and type 2 diabetes. Diabetologia. 2007;50:1472–1480. doi:10.1007/s00125-007-0689-x
35. Mobbs CV, Makimura H. Block the FAS, lose the fat. Nat Med. 2000;8:335–336. doi:10.1038/nm0402-335
36. Loftus TM, Jaworsky DE, Frehywot GL, et al. Reduced food intake and body weight in mice treated with fatty acid sintase inhibitor. Science. 2000;288:2379–2381.
37. Kumar MV, Shimokawa T, Nagy TR. Differential effects of a centrally acting fatty acid synthase inhibitor in lean and obese mice. Pnas. 2002;99:1921–1925. doi:10.1073/pnas.042683699
38. Hardie DG, Hawley SA. AMP-activated protein kinase: the energy charge hypothesis revisited. BioEssays. 2001;23:112–119. doi:10.1002/bies.10009
39. Hardie DG. AMP-activated protein kinase: an energy sensor that regulates all aspects of cell function. Genes Dev. 2011;25:1895–1908. doi:10.1101/gad.17420111
40. Atkinson LL, Fischer MA, Lopaschuk GD. Leptin activates cardiac fatty acid oxidation independent of changes in the AMP-activated protein kinase-acetyl-CoA carboxylase-malonyl-CoA Axis. J Biol Chem. 2002;227:29424–29430. doi:10.1074/jbc.M203813200
41. Towler MC, Hardie DG. AMP-activated protein kinase in metabolic control and insulin signaling. Circ Res. 2007;100:328–341. doi:10.1161/01.RES.0000256090.42690.05
42. Daval M, Foufelle F, Ferré P. Functions of AMP-activated protein kinase in adipose tissue. J Physiol. 2006;574:55–62. doi:10.1113/jphysiol.2006.111484
43. Ceddia RB. The role of AMP-activated protein kinase in regulating white adipose tissue metabolism. Mol Cell Endocrinol. 2013;366:194–203. doi:10.1016/j.mce.2012.06.014
44. Gaidhu MP, Frontini A, Hung S, et al. Chronic AMP-kinase activation with AICAR reduces adiposity by remodeling adipocyte metabolism and increasing leptin sensitivity. J Lipid Res. 2011;52:1702–1711. doi:10.1194/jlr.M015354
45. Kim JH, Kim OK, Yoon HG, et al. Anti-obesity effect of extract from fermented Curcuma longa L. through regulation of adipogenesis and lipolysis pathway in high-fat diet-induced obese rats. Food Nutr Res. 2016;60:30428. doi:10.3402/fnr.v60.30428
46. Panchal SK, Ward L, Brown L. Ellagic acid attenuated high-carbohydrate, high-fat diet-induced metabolic syndrome in rats. Eur J Nutr. 2013;52:559. doi:10.1007/s00394-013-0494-x
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.