Back to Journals » Medical Devices: Evidence and Research » Volume 9
Bilateral posterior cervical cages provide biomechanical stability: assessment of stand-alone and supplemental fixation for anterior cervical discectomy and fusion
Authors Voronov LI, Siemionow K, Havey R, Carandang G, Phillips F, Patwardhan A
Received 1 April 2016
Accepted for publication 5 May 2016
Published 13 July 2016 Volume 2016:9 Pages 223—230
DOI https://doi.org/10.2147/MDER.S109588
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Leonard I Voronov,1,2 Krzysztof B Siemionow,3 Robert M Havey,1,2 Gerard Carandang,1,2 Frank M Phillips,4 Avinash G Patwardhan1,2
1Musculoskeletal Biomechanics Laboratory, Department of Research, Edward Hines Jr VA Hospital, Hines, IL, USA; 2Department of Orthopaedic Surgery and Rehabilitation, Loyola University Chicago, Maywood, IL, USA; 3College of Medicine at Chicago, University of Illinois, Chicago, IL, USA; 4Midwest Orthopedics at Rush, Rush University Medical Center, Chicago, IL, USA
Introduction: Supplemental posterior instrumentation has been widely used to enhance stability and improve fusion rates in higher risk patients undergoing anterior cervical discectomy and fusion (ACDF). These typically involve posterior lateral mass or pedicle screw fixation with significant inherent risks and morbidities. More recently, cervical cages placed bilaterally between the facet joints (posterior cervical cages) have been used as a less disruptive alternative for posterior fixation. The purpose of this study was to compare the stability achieved by both posterior cages and ACDF at a single motion segment and determine the stability achieved with posterior cervical cages used as an adjunct to single- and multilevel ACDF.
Methods: Seven cadaveric cervical spine (C2–T1) specimens were tested in the following sequence: intact, C5–C6 bilateral posterior cages, C6–C7 plated ACDF with and without posterior cages, and C3–C5 plated ACDF with and without posterior cages. Range of motion in flexion–extension, lateral bending, and axial rotation was measured for each condition under moment loading up to ±1.5 Nm.
Results: All fusion constructs significantly reduced the range of motion compared to intact in flexion–extension, lateral bending, and axial rotation (P<0.05). Similar stability was achieved with bilateral posterior cages and plated ACDF at a single level. Posterior cages, when placed as an adjunct to ACDF, further reduced range of motion in both single- and multilevel constructs (P<0.05).
Conclusion: The biomechanical effectiveness of bilateral posterior cages in limiting cervical segmental motion is comparable to single-level plated ACDF. Furthermore, supplementation of single- and multilevel ACDF with posterior cervical cages provided a significant increase in stability and therefore may be a potential, minimally disruptive option for supplemental fixation for improving ACDF fusion rates.
Keywords: cervical spine, posterior fusion, biomechanics, cervical facets, DTRAX Posterior Cervical Cage
Introduction
Anterior cervical discectomy and fusion (ACDF) is commonly performed to treat one- and two-level cervical spondylosis. Favorable fusion rates have been reported; nonunion rate is ∼4% for single level plated ACDF with allograft.1,2 However, fusion success declines with the number of treated levels.3 Reported pseudarthrosis rates are as high as 18% and 37%, in two- and three-level ACDF constructs, respectively.1,4,5 To achieve solid bony fusion, both a favorable bone healing environment and mechanical stability are required.6 These conditions become especially important in patients undergoing multilevel fusion in whom the risk of pseudarthrosis and revision surgery is more prevalent.7
Fusion constructs using ACDF supplemented with posterior fixation are more stable and have been shown to improve fusion rates.8,9 The most commonly used implants, lateral mass screw/rod constructs and transfacet screws, provide effective stabilization, but typically require an open posterior approach with considerable muscle retraction, which has been shown to be associated with significant blood loss, postoperative pain, and morbidity.7,9–13 Fusion with expandable posterior cervical cages placed between the facet joints has been described for the treatment of radiculopathy with favorable results at 1 year.14,15 Bilateral placement of similar devices have been shown to decrease the range of motion (ROM) at the index level, increase foraminal area, and preserve cervical lordosis.16–19
More recently, a nonexpandable titanium alloy posterior cervical cage has become available (DTRAX Posterior Cervical Cage, Providence Medical Technology, Walnut Creek, CA, USA).15,20 To date, no studies have evaluated the biomechanical effects of this cage compared to ACDF or assessed their contribution to stability when used as supplemental posterior fixation in plated ACDF procedures.
This study tested the following hypotheses:
1. Effectiveness of the DTRAX Posterior Cervical Cage stabilization in limiting motions in flexion–extension (FE), lateral bending (LB), and axial rotation (AR) will be comparable to that of an ACDF construct for a single-level fusion.
2. Supplemental posterior stabilization will significantly increase the effectiveness of the ACDF construct in single- and two-level settings.
Methods
Seven fresh-frozen cadaveric cervical (C2–T1) spine specimens were acquired from an accredited tissue bank. This biomechanical study utilized human cadaveric tissue. While institutional review board approval was not necessary, approval was obtained from the Research and Development committee at the Edward Hines Jr VA Hospital, where testing was performed. Specimen mean age (standard deviation) was 41.1±9.1 years (three male, four female). All specimens were free from osseous abnormalities and previous cervical spinal surgery. After the skin and paravertebral muscles were dissected, individual specimens were potted in aluminum cups with polymethyl methacrylate bone cement. Each specimen was fixed to a kinematic testing apparatus at the caudal end only; the cephalad end was left unconstrained.21,22
The testing apparatus allowed continuous cycling of the specimen between specified maximum moment endpoints (±1.5 Nm) in flexion, extension, LB, and AR. Specimens were subjected to quasi-static flexibility testing at a loading rate of 2.5 Nm/min. The angular motions of the C2 to C7 vertebrae relative to T1 were measured using an optoelectronic motion measurement system (Optotrak® Certus, Northern Digital, Waterloo, Canada). Testing was performed in moment control mode by placing a six-component load cell (Model MC3A-6-1000, AMTI Inc., Newton, MA, USA) under the specimen to measure the applied moments. Continuous loading in each of the three planes of motion was performed. Load-displacement data were collected until two reproducible load-displacement cycles were obtained.
Moment loading in FE and LB was performed using a force applied using a moment arm, while in AR a force couple was used to apply a pure moment (Figure 1). The moment arm length was 50 cm for LB and 60 cm for FE. Due to these long moment arms, the compressive load required to reach 1.5 Nm was ∼2.7 N in FE and 3.0 N in LB. Off-axis moments in all tests averaged less than 0.1 Nm. Fluoroscopic imaging (GE OEC 9800 Plus) was used to document implant placement.
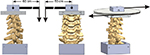  | Figure 1 Schematic of the loading apparatus for flexibility testing in flexion–extension (left image), lateral bending (center image), and axial rotation (right image). |
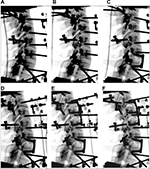
Each of the seven specimens was tested sequentially in the following six conditions: 1) intact (C2–T1), 2) C5–C6 bilateral posterior cages, 3) C6–C7 plated ACDF, 4) C6–C7 plated ACDF + C6–C7 bilateral posterior cages, 5) C3–C5 plated ACDF, and 6) C3–C5 plated ACDF + C3–C5 bilateral posterior cages (Figure 2). This complex study design was intended to fully utilize the donated cadaveric tissue in order to investigate the effectiveness of the implants both in a stand-alone environment as well as in combination for single and two-level fusion constructs. A fluoroscopically guided posterior approach was used to place cages bilaterally between the cervical facet joints of the target level according to the manufacturer’s surgical technique (Figure 3).23 ACDF was performed according to standard surgical procedure. After discectomy, a 5 mm intervertebral cage was inserted and an anterior locking semiconstrained plate was applied (DePuy Synthes, Raynham, MA, USA).
Segmental ROM was analyzed using paired t-tests with Bonferroni correction for multiple comparisons. Significance level was set to alpha =0.05. The following four comparisons were conducted: intact versus C5–C6 cages, C5–C6 cages versus C6–C7 ACDF, C6–C7 ACDF versus ACDF + cages, and C3–C5 ACDF versus ACDF + cages. A stabilization intervention at any level is likely to alter ROM from intact conditions at subsequent spinal levels. Therefore, ROM values after each sequential step were compared to the ROM at that level during the previous protocol step. For example, the C6–C7 ROM after the ACDF (protocol step 3) was compared to the C6–C7 ROM after C5–C6 cages (protocol step 2) rather than the intact C6–C7 ROM from step 1. All comparisons were done separately for FE, LB, and AR, as no comparisons across load-types were intended. The statistical data analyses were performed with the use of the Systat 10.2 software package (Systat Software, Richmond, CA, USA).
Results
The load-displacement curves of both the C5–C6 and C6–C7 levels after instrumentation with ACDF and bilateral posterior cervical cages can be well approximated by straight lines in all three loading modes (Figure 4). As the relationship between angular motion and the moment curve after instrumentation is nearly linear, the stiffness of the segment is equal to the maximum moment divided by the ROM. Thus, the assumption can be made that postinstrumentation comparison of ROM at maximum moments used in the current study is equivalent to comparing segmental stiffness. Assessment of fusion in the clinical setting is determined by ROM measurements, for example, on FE X-ray images, rather than stiffness calculations. Therefore, we report our results as ROM at the index levels for each tested condition.
Comparison of posterior cervical cages and ACDF constructs
Posterior stabilization with bilateral cervical cages at C5–C6 significantly reduced the ROM in all directions when compared to the intact condition: 10.7°±2.6° to 2.5°±1.3° in FE, 6.7°±2.8° to 0.4°±0.3° in LB, and 7.9°±2.8° to 1.1°±1.7° in AR (P<0.05) (Table 1). Plated ACDF at C6–C7 significantly reduced ROM at the treated level compared to the preoperative ROM: 12.3°±2.5° to 2.5°±0.8° in FE, 8.9°±1.5° to 1.6°±0.7° in LB, and 7.1°±1.2° to 1.7°±0.4° in AR (all P<0.05) (Table 1). A statistical analysis comparing posterior cages at C5–C6 and ACDF at C6–C7 revealed no implant group effect for changes in ROM; a similar reduction in ROM was observed in each direction (FE, LB, and AR) for both constructs. However, the percent decreases in LB and AR were larger for the posterior cages compared to ACDF (LB: −94%±3.4% vs −82%±6.1%, AR: −87.2%± 17.8% vs −75.7%±7.1%).
ACDF with supplemental fixation
Plated ACDF at C6–C7 significantly decreased ROM compared to intact in FE, LB, and AR (all P<0.05) (Table 1). ACDF supplemented with posterior cages further significantly reduced motion when compared to plated ACDF alone: 2.5°±0.8° to 0.6°±0.3° in FE, 1.6°±0.7° to 0.1°±0.4° in LB, and 1.7°±0.4° to 0.2°±0.3° in AR (all P<0.005) (Table 2).
In two-level fusion, plated ACDF alone significantly reduced ROM at C3–C5: values decreased from 25.4°±8.1° to 1.7°±0.9° in FE, 27.5°±5.1° to 1.7°±0.6° in LB, and 21.7°±2.0° to 2.1°±0.5° in AR (all P<0.001) (Table 3). Supplemental stabilization with the cages at C3–C5 further significantly reduced ROM when compared to plated ACDF alone: values decreased from 1.7°±0.9° to 0.3°±0.2° in FE, 1.7°±0.6° to 0.2°±0.1° in LB, and 2.1°± 0.5° to 0.3°±0.2° in AR (all P<0.05) (Table 3).
  | Table 3 Effectiveness of posterior cervical cages as a supplement for two-level ACDF constructs Note: *Paired t-test. Abbreviations: ACDF, anterior cervical discectomy and fusion; ROM, range of motion. |
Discussion
The current study demonstrated that plated ACDF and bilateral posterior cages offer comparable postoperative segmental stability; both techniques significantly decreased cervical ROM in FE, LB, and AR. The percent reduction in LB and AR was higher for the posterior cage construct compared to the plated ACDF. This is likely due to the more lateral position of the implants relative to the axis of rotation in LB and AR. The plated ACDF is closer to the axis of rotation and as such has a lesser ability to resist the LB and AR motions. Supplementation of one- and two-level plated ACDF constructs with bilateral posterior cervical cages further significantly decreased cervical ROM in all tested modes.
ACDF supplementation with transfacet screws was previously evaluated using a protocol similar to that reported herein.10 Traynelis et al assessed FE, LB, and AR in eight cadaveric specimens before and after applying stand-alone plated ACDF and with the addition of unilateral and bilateral transfacet screws. Reported reduction in ROM values for the C6–C7 segment with concurrent bilateral transfacet screws is similar to those reported for posterior cages in the current study.
Kasliwal et al evaluated clinical and radiographic outcomes in patients who underwent revision surgery for pseudarthrosis following ACDF using a cervical interfacet spacer similar to the device reported herein.24 The authors report a 20-month follow-up on 19 patients. Patient-reported outcomes using Visual Analog Scale for neck and arm pain and Neck Disability Index showed significant improvement from baseline based on improvement of at least three points on Visual Analog Scale and 7.5 points on Neck Disability Index. There were no significant changes in cervical lordosis or C2–C7 sagittal vertical alignment.
One previous study analyzed the biomechanics of a construct similar in concept to the cages investigated in the current study. Leasure and Buckley evaluated foraminal decompression and segmental ROM after posterior bilateral placement of an expandable screw and washer system between the facet joints.16 The results demonstrated a significant reduction in cervical ROM in flexion, LB, and AR after implantation. Although the implant design differed from the one evaluated in the current study, these results show that distracting and mechanically locking the translation of the interarticular facet surfaces relative to each other contribute to reduction of cervical segmental ROM.
As with all biomechanical cadaveric studies, this investigation has limitations. Notably, kinematic evaluation of the tested constructs provides evidence for the immediate postoperative effects of the implants and does not reflect the possible consequence of long-term cyclical loading experienced in vivo. Stand-alone constructs for ACDF and posterior cages were performed at different levels. C5–C6 and C6–C7 segments are similar in their intervertebral disc anatomy and facet morphologies. Their kinematic behavior is similar as evidenced by the intact ROM values of the two levels in FE, LB, and AR (FE: 10.7 vs 11.4, P=0.556; LB: 6.7 vs 8.2, P=0.235; AR: 7.9 vs 7.5, P=0.795). These two levels are a natural choice as controls for each other as they come from the same spine specimen and allow a paired comparison of construct data. Evaluating the two constructs at the same (C5–C6 or C6–C7) levels would have required a substantially larger number of specimens to account for the biologic variability between specimens. Furthermore, a sequential testing mode was employed in order to fully utilize each specimen.
When evaluating biomechanical results, it is important to note that kinematics vary depending on the cervical level and so comparisons are best made before and after surgeries at the same level.10 The mean ROM in FE after the two-level fusion (C3–C5) was less than that of the mean single-level fusion at C6–C7. This was true for both ACDF and ACDF with posterior cages. This seemingly disparate result may be due to a combination of factors. As the FE testing was not performed using pure moments, C6–C7 could be subjected to a slightly higher (1.46 vs 1.5 Nm) moment than the upper cervical levels. However, a more likely explanation deals with differences in location of the segmental center of rotation (COR) and facet joints between the upper and lower cervical spines. The distance between the segmental COR and the fusion implant has a great effect on the stability provided by the implant. At C6–C7, the COR is positioned just posterior to the center of the upper endplate of C7 and coincident with the caudal surface of the interbody cage providing a poor mechanical advantage to resist FE motion. At C3–C4 and C4–C5, the COR is considerably more caudal providing improved mechanics for the ACDF to resist FE motion.25
As with any implant system, it is important to understand how sagittal alignment may be affected by the use of single and multilevel instrumentation. The focus of this study was evaluation of motion reduction with both posterior cervical cages and ACDF. As such, evaluation of sagittal alignment after each construct was beyond the scope of the study. Future analysis of biomechanical data and corroboration with clinical findings will provide insight into the effects of these fusion techniques on sagittal balance.
This study is the first to evaluate the role of bilateral cervical cages placed between the facet joints as a posterior supplement to plated ACDF at one and two levels. The results of the current study support the role of these implants to significantly increase stability in single and multilevel ACDF constructs. This suggests a role for the use of these implants when added stability is required, such as in situations in which ACDF has a higher risk of pseudarthrosis, or in the treatment of an established pseudarthrosis following ACDF.
Conclusion
The biomechanical effectiveness of bilateral posterior cages in limiting cervical segmental motion is comparable to single-level plated ACDF. Supplementation of plated ACDF with these implants further increases cervical spine stability in single and multilevel ACDF constructs. These findings provide a biomechanical rationale for undertaking further studies to assess the performance of posterior cervical cages under repeated loading that simulates postoperative activity until biologic fusion occurs.
Acknowledgments
Funding for this study was provided by the Rehabilitation Research and Development Service, Department of Veterans Affairs (Grant 1-I01-RX-001269-01-A2), Washington DC, USA, and Providence Medical Technology, Walnut Creek, CA, USA. The authors wish to thank Robyn Capobianco for assistance with manuscript preparation.
Disclosure
Dr Siemionow and Dr Phillips are consultants for Providence Medical Technology, and report no other conflicts on interest in this work. Dr Voronov, R Havey, G Carandang, and Dr Patwardhan report no conflicts of interest in this work, and had full control of all data.
References
Fraser JF, Härtl R. Anterior approaches to fusion of the cervical spine: a metaanalysis of fusion rates. J Neurosurg Spine. 2007;6(4):298–303. | ||
Kaiser SP, Gardner MJ, Liu J, Routt MLC, Morshed S. Anatomic determinants of sacral dysmorphism and implications for safe iliosacral screw placement. J Bone Joint Surg Am. 2014;96(14):e120. | ||
Veeravagu A, Cole T, Jiang B, Ratliff JK. Revision rates and complication incidence in single- and multilevel anterior cervical discectomy and fusion procedures: an administrative database study. Spine J. 2014;14(7):1125–1131. | ||
Jiang L, Liu XG, Yuan HS, et al. Diagnosis and treatment of vertebral hemangiomas with neurologic deficit: a report of 29 cases and literature review. Spine J. 2014;14(6):944–954. | ||
Wang JC, McDonough PW, Kanim LE, Endow KK, Delamarter RB. Increased fusion rates with cervical plating for three-level anterior cervical discectomy and fusion. Spine. 2001;26(6):643–646; discussion 646–647. | ||
Steinmann JC, Herkowitz HN. Pseudarthrosis of the spine. Clin Orthop. 1992;(284):80–90. | ||
Stauff MP, Knaub MA. Pseudoarthrosis following anterior cervical surgery: Diagnosis, treatment options, and results. Semin Spine Surg. 2006;18(4):235–244. | ||
DuBois CM, Bolt PM, Todd AG, Gupta P, Wetzel FT, Phillips FM. Static versus dynamic plating for multilevel anterior cervical discectomy and fusion. Spine J. 2007;7(2):188–193. | ||
Clavenna AL, Beutler WJ, Gudipally M, Moldavsky M, Khalil S. The biomechanical stability of a novel spacer with integrated plate in contiguous two-level and three-level ACDF models: an in vitro cadaveric study. Spine J. 2012;12(2):157–163. | ||
Traynelis VC, Sherman J, Nottmeier E, et al. Kinetic analysis of anterior cervical discectomy and fusion supplemented with transarticular facet screws. J Neurosurg Spine. 2014;20(5):485–491. | ||
Klekamp JW, Ugbo JL, Heller JG, Hutton WC. Cervical transfacet versus lateral mass screws: a biomechanical comparison. J Spinal Disord. 2000;13(6):515–518. | ||
Takayasu M, Hara M, Yamauchi K, Yoshida M, Yoshida J. Transarticular screw fixation in the middle and lower cervical spine. Technical note. J Neurosurg. 2003;99(1 Suppl):132–136. | ||
Memtsoudis SG, Hughes A, Ma Y, Chiu YL, Sama AA, Girardi FP. Increased in-hospital complications after primary posterior versus primary anterior cervical fusion. Clin Orthop Relat Res. 2011;469(3):649–657. | ||
McCormack BM, Bundoc RC, Ver MR, Ignacio JMF, Berven SH, Eyster EF. Percutaneous posterior cervical fusion with the DTRAX facet system for single-level radiculopathy: results in 60 patients. J Neurosurg Spine. 2013;18(3):245–254. | ||
McCormack BM, Eyster EF, Chiu J, Siemionow K. Minimally disruptive posterior cervical fusion with DTRAX cervical cage for single level radiculopathy – results in 10 patients at 1-year. Spine Res. 2016;2(1):1–5. | ||
Leasure JM, Buckley J. Biomechanical evaluation of an interfacet joint decompression and stabilization system. J Biomech Eng. 2014;136(7). | ||
Tan LA, Gerard CS, Anderson PA, Traynelis VC. Effect of machined interfacet allograft spacers on cervical foraminal height and area. J Neurosurg Spine. 2014;20(2):178–182. | ||
Tan LA, Straus DC, Traynelis VC. Cervical interfacet spacers and maintenance of cervical lordosis. J Neurosurg Spine. 2015;22(5):466–469. | ||
Goel A, Shah A. Facetal distraction as treatment for single- and multilevel cervical spondylotic radiculopathy and myelopathy: a preliminary report. J Neurosurg Spine. 2011;14(6):689–696. | ||
Siemionow K, Janusz P, Glowka P. Cervical cages placed bilaterally in the facet joints from a posterior approach significantly increase foraminal area. Eur Spine J. Epub 2016 Feb 11. | ||
Brody MJ, Patel AA, Ghanayem AJ, et al. The effect of posterior decompressive procedures on segmental range of motion after cervical total disc arthroplasty. Spine. 2014;39(19):1558–1563. | ||
Wojewnik B, Ghanayem AJ, Tsitsopoulos PP, et al. Biomechanical evaluation of a low profile, anchored cervical interbody spacer device in the setting of progressive flexion-distraction injury of the cervical spine. Eur Spine J. 2013;22(1):135–141. | ||
Siemionow K, McCormack BM, Menchetti PPM. Tissue sparing posterior cervical indirect decompression and fusion in foraminal stenosis. In: Cervical Spine: Minimally Invasive and Open Surgery. New York/Berlin/Heidelberg: Springer; 2015:135–148. | ||
Kasliwal MK, Corley JA, Traynelis VC. Posterior cervical fusion using cervical interfacet spacers in patients with symptomatic cervical pseudarthrosis. Neurosurgery. 2016;78(5):661–668. | ||
Hipp J, Wharton N. Quantitative motion analysis (QMA) of motion-preserving and fusion technologies for the spine. In: Yue JJ, Bertagnoli R, McAfee PC, An HS, editors. Motion Preservation Surgery of the Spine. Advanced Techniques and Controversies. Philadelphia, PA: Elsevier/Saunders; 2008:85–96. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.