Back to Journals » International Medical Case Reports Journal » Volume 14
Bilateral Exudative Retinal Detachment in a Young Patient with Chronic Renal Failure
Authors Otuka OAI , Eweputanna LI, Okoronkwo NC, Kalu A
Received 23 September 2020
Accepted for publication 13 January 2021
Published 3 March 2021 Volume 2021:14 Pages 139—144
DOI https://doi.org/10.2147/IMCRJ.S283565
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Olufunmi Adebimpe Ijeoma Otuka,1 Lisa Ifenyinwa Eweputanna,2 Nneka Chioma Okoronkwo,3 Anya Kalu1
1Ophthalmology Unit, Department of Surgery, Abia State University Teaching Hospital, Aba, Abia State, Nigeria; 2Department of Radiology, Abia State University Teaching Hospital, Aba, Abia State, Nigeria; 3Department of Paediatrics, Abia State University Teaching Hospital, Aba, Abia State, Nigeria
Correspondence: Olufunmi Adebimpe Ijeoma Otuka
Ophthalmology Unit, Department of Surgery, Abia State University Teaching Hospital, Aba, Abia State, Nigeria
Tel +2348034473250
Email [email protected]
Introduction: Chronic renal failure (CRF) is a multi-systemic disease affecting different organ systems of the body. Ocular manifestations of chronic renal disease include squint, subconjunctival hemorrhage, vitreous hemorrhage, neovascular glaucoma, cataracts and retinal detachment. These result in visual impairment or blindness. In this article, a case of bilateral exudative retinal detachment (ERD) in a pediatric patient with CRF and hypertension is presented.
Methods: The patient is a 16-year-old girl with CRF, grade 3 hypertension, and bilateral ERD. Detailed ophthalmic evaluation including visual acuity, anterior and posterior segments evaluation with +78 DS super field lens and digital slit lamp, intraocular pressure (IOP) measurement using a non-contact tonometer. B-mode ocular and renal ultrasounds scan were done.
Results: With the management of systemic hypertension, and hemodialysis, a slight improvement of vision was noted but this was not sustained as renal replacement therapy was not continued due to financial constraints.
Conclusion: Ocular disturbances may be the pointer to renal compromise. There is a need for thorough systemic review in patients with ocular symptoms and ocular evaluation in all patients with CRF and hypertension.
Keywords: exudative retinal detachment, ERD, chronic renal failure, CRF, hypertension, visual impairment, blindness
Introduction
Chronic renal failure (CRF) is a worldwide health problem, it is more common in adults but can occur in children. The estimated incidences of CRF in Nigeria is 1.7 new cases per million children per year.1 The proportion of primary retinal detachments in children is low when compared to adults, as only 3.2–6.6% of cases of retinal detachment occur in children.2,3 They are mainly post-traumatic but have been reported in 'Coat's disease, leukemia, and sickle cell disease.4
Exudative or serous retinal detachment is even rarer. In severe systemic hypertension, there is vasoconstriction of the ophthalmic and ciliary arteries resulting in choroidal ischemia and hypertensive choroidopathy. There is also a breakdown in the inner blood retinal barrier which is the endothelial tight junction leading to sipping of exudates into the potential space (between the sensory retinal and the retinal pigment epithelium), hence retinal detachment and blindness.
In this article, we present a case of bilateral exudative retinal detachment (ERD) in a pediatric patient with CRF and hypertension. There is a dearth of data on this subject matter in this environment.
Case Presentation
A 16-year-old female with a five-day history of headache, fever, persistent vomiting, and complete loss of vision in both eyes was referred to the Ophthalmology unit from the children's emergency room of Abia State University Teaching Hospital. One month prior to presentation, she noticed a gradual, painless reduction in vision which was ignored.
The eye examination findings were as follows:
Visual acuity was no light perception in both eyes. A left divergent squint was noted with defective inward movement of the left eye. Also noted was a right nasal subconjunctival haemorrhage. Both corneas were clear. Both pupils were markedly dilated and not reactive to light (Figure 1). On fundoscopy/slit lamp examination, there was a white pupil, marked disc, and retina oedema. (Figure 2) Soft and hard exudates were seen, left hazy media, and a macular scar was noted. The intraocular pressure (IOP) were 37mmHg and 28mmHg in the right and left eyes, respectively.
 |
Figure 1 Showing right eye subconjunctival hemorrhage, Left divergent squint and bilaterally markedly dilated pupils. |
 |
Figure 2 Showing leukocoria from retinal detachment. |
A diagnosis of ocular hypertension, and bilateral ERD was made, secondary to CRF and hypertensive complications. Senior Loken syndrome was the differential diagnosis.
A B-mode ocular ultrasound was requested.
She was placed on gutt 0.005% latanoprost/0.5% timolol, a drop in each eye, nocte; gutt 0.1% brimonidine, a drop in each eye, twice daily; gutt 0.1% diclofenac, vitreolent (0.3% potassium iodide and 0.3% sodium iodide) and 0.1% flurometholone, a drop in each eye, q.i.d.
The latanoprost/timolol and brimonidine are ocular antihypertensives used for the reduction of the IOP. Diclofenac was used for pain and inflammation. Fluorometholone is a low dose steroid used for its anti-inflammatory properties.
She was reviewed by the physicians and the following parameters were noted:
On presentation, her metabolic status showed the level of urea: 40.7 m/l (2.4–8.3), creatinine: 11.92mg/dl (0.6–1.1), potassium: 6.7 (3.4–5.2), chlorine: 91 (96–107), sodium: 133 (138–149), bicarbonate: 14 (24–30). Random blood sugar 245mg/dl; the urine specific gravity was 1.020, PH was 6.0, protein +++. Blood+++.
The systemic blood pressure (BP) was 220/140mmHg (>99 percentile for age). Systemic hypertension was managed with amlodipine 10mg daily, lisinopril 10mg daily, and intravenous furosemide 20mg daily. The hemoglobin level was 7g/dl on admission and she was transfused with 6 units of whole blood all through her stay in the hospital. A dialysis regimen of 3 times weekly was also recommended.
The B-mode ocular ultrasound scan showed normal sized and shaped globes. (Figures 3 and 4) The anterior chambers and the lenses were normal. There were thick echogenic membranes which are V-shaped, attached to the optic disc and ora serrata in the vitreous body on both sides. There were scattered ill-defined collections of slightly echogenic particles as well as a freely mobile membrane in the left vitreous body. The optic nerve shadows were normal. There was limited medial movement of the left eye. The movements of other extraocular muscles were within normal limits. An impression of bilateral retinal detachment with left-sided vitreous hemorrhage was made.
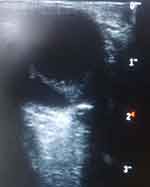 |
Figure 3 B-mode ocular ultrasound of the right eye showing retinal detachment. |
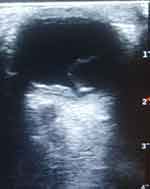 |
Figure 4 B-mode ocular ultrasound of the left eye showing retinal detachment. |
A renal ultrasound scan showed bilateral small kidneys measuring 8.61cm × 3.4cm and 8.31 × 4.28cm on the right and left sides respectively (Figure 5). (Normal kidney sizes for her age are 10.74±1.35cm and 11.0 ±1.15cm on the right and left side, respectively.5) There was a grade 3 increase in renal parenchymal echoes with the loss of corticomedullary echo differentiation. There were no renal cysts, calculi, or calyceal dilatation. Also noted was pelvic ascites. A diagnosis of bilateral chronic renal parenchymal disease with pelvic ascites was made.
 |
Figure 5 Renal ultrasound showing bilateral small echogenic kidneys. |
She was discharged on request one month after admission and was placed on a weekly follow-up at the pediatric outpatient clinic.
In the third month of follow up, the ophthalmic review showed improvement of left eye visual acuity to hand movement. There was a reduction in the IOP to 35mmHg and 23mmHg in the right and left eyes, respectively. This recording was after her fourth hemodialysis. At this time, the systemic BP was 160/100mmHg.
The patient was inconsistent with hemodialysis due to financial constraints. She had only six hemodialysis treatments over seven months, prior to her demise.
Discussion
CRF leads to multiple organ damage. In the eyes, various lesions resulting in impaired vision/blindness have been reported.6,7 Generally, common causes of CRF in this environment are diabetes mellitus, HIV nephropathy, hypertension, and ingestion of nephrotoxic drugs. Among children, acquired disorders such as glomerulonephritis and nephrotic syndrome are common causes of CRF, congenital disorders like posterior urethral valves have also been reported.8,9
ERD is uncommon and is rare in pediatric patients.
ERD in the setting of CRF has been reported by Borowitz et al;10 in a 31-year-old female by Guven et al;11 and in a 20-year-old male by Chang et al12 and Dahal et al.6 It results from fluid accumulation in the potential subretinal space due to the breaking down of the blood-retinal barrier. This may result from inflammatory, infectious, neoplastic, vascular, and degenerative lesions.13 These result in increased permeability of the choroidal vessels, disorders of the retinal pigment epithelium, or changes in osmolarity and in a consequent fluid shift between different spaces. High levels of urea (>60mg/dl) affect the serum osmolarity and intravascular/extravascular compartments so that the fluid moves into the subpigment epithelium and subsequently into the subretinal space.11
On fundoscopy evaluation, leukocoria was noted because there was detachment of the retina from the underlying vascularized choroid. Soft exudates seen were due to the death of retinal cells secondary to compromise of blood supply to the retina. As macrophages pick up the retina pigment cells they appear as black exudates on fundoscopy. With very low hemoglobin levels as seen in CRF, there is anterior and posterior optic neuropathy. Retinopathic features seen include retinal hemorrhage, hard and soft exudates, pale optic disc, pale arterioles, and distended veins.7
Visual disturbance was the first clinical sign manifesting one month prior to presentation. Visual acuity was no light perception in both eyes on presentation. This is in corroboration with a work by Pociej-Marciak et al.14 amongst others, in which sudden visual loss was the first presentation in patients with CRF. Initial visual disturbance is likely due to hypertensive choroidopathy, with complete loss of vision, retinal detachment, and macular scar come into play.14
Left divergent squint and bilateral pupillary dilatation were noted. Squints and pupillary dilatation occur in the eye in systemic hypertension6,7,10,14 as a result of affectation of the oculomotor nerve usually at the cavernous sinus.
Sub conjunctival hemorrhage is hemorrhage between the conjunctiva and episclera due to a break in the subconjunctival vessels.15 Causes include hypertension and vomiting as noted in this case, trauma, cough, and contact lens usage in young patients, hemorrhagic conjunctivitis, diabetes, and arteriosclerosis.15
The left vitreous media was hazy as a result of a vitreous hemorrhage. Vitreous hemorrhage in patients with CRF has been noted in previous studies.6,11 It is due to bleeding into the vitreous chamber is as a result of mechanical disruption of vessels due to retinal detachment.6,16
The IOP reduced from 37mmHg and 28mmHg to 35mmHg and 23mmHg on the right and left eyes, respectively, on follow-up of the ocular examination. This improvement followed anti-glaucoma therapy, hemodialysis and reduction in systemic BP. This is in keeping with studies that have shown improvement in ocular parameters with control of BP and hemodialysis.10,11,17
This improvement is however minimal. Though other surgical interventions such as laser, trabeculectomy, trabeculectomy and use of drainage devices may lead to a further reduction in IOP, these were not explored due to financial constraints. It is postulated that reduction in IOP is due to changes in oncotic pressure and ultrafiltration as a result of dialysis.17 Anti-glaucoma drugs could also be contributory to the marginal reduction in IOP noted in this patient. The exact pathophysiology of elevated IOP is not known in ocular hypertension. In primary open-angle glaucoma, myocilin (MYOC) gene mutation has been found and determined to cause protein misfolding, making trabecular meshwork cells dysfunctional, with a subsequent decrease in outflow facility and elevation of IOP. In glaucoma, optic nerve ischemia from vascular dysfunction and mechanical dysfunction via cribriform plate compression of neuronal axons have been postulated as the pathophysiology of raised IOP.18
In adolescents 13 years and older, systemic hypertension is defined as BP of 130/80 mmHg or higher. The prevalence of hypertension in children is 6%. Childhood hypertension is correlated with hypertension and the risk of cerebrovascular disease (CVD) in adulthood. It is also associated with hyperlipidemia and insulin resistance, as well as target organ (eye, kidney, and heart) damage.19,20
Current studies have shown that the effect of systemic hypertension on the retinal, optic nerve head, and choroidal circulation produce three distinct and independent manifestations: hypertensive retinopathy, hypertensive optic neuropathy, and hypertensive choroidopathy.21 Poorly controlled systemic hypertension causes worsening of microvascular disease of the eye.20,21 Though there are a number of ocular conditions in systemic hypertension, the exact causal relationships are yet to be fully defined.21
Senior Loken syndrome though considered as a differential was ruled out because of the sudden onset of eye symptoms and absence of a renal cyst on ultrasound.
Limitation of Study
This study was grossly limited by financial constraints. Investigations like optical coherence tomography and fundal photograph were not carried out due to lack of funds. Drugs and renal replacement therapy regimen were not adhered to also due to financial constraints.
Conclusion
There are varied ocular complications of CRF and hypertension. Awareness is needed for an early check-up in these patients.
Recommendation
We recommend regular ophthalmic evaluation for all chronic kidney patients to avert irreversible loss of vision. Renal replacement therapy should be undertaken as soon as possible to prevent ocular complications of chronic renal disease. Multidisciplinary management by ophthalmologists, nephrologist, radiologists, and pathologists should be undertaken where available.
Ethical Issues
Ethical approval was obtained from the research and ethics committee of the Abia State University Teaching Hospital, Aba. Informed written consent was obtained from the patient’s parent. Consent was also obtained to use her pictures/images.
Consent for Publication
This was obtained from the patient’s parent.
Acknowledgment
Appreciation: Dr. Evans Ogbonna and Dr Tunji Oluleye for their support all through this work.
Author Contributions
All authors made a significant contribution to the work reported, they all participated in the conception, study design, execution, acquisition of data, analysis and interpretation, took part in drafting, revising and critically reviewing the article; they gave final approval of the version to be published and have agreed on the journal to which the article has been submitted. They agree to be accountable for all aspects of the work.
Funding
There was no external funding for this report.
Disclosure
The authors report no conflicts of interest for this work.
References
1. Michael IO, Gabriel OE. Chronic renal failure in children of Benin Nigeria. Saudi J Kidney Dis Transpl. 2004;15:79–83.
2. Meler P, Kindesalter N. Retinal detachment in children: differential diagnosis and current therapy. Klin Monbi Augenheilkd. 2008;225(9):779–790.
3. Meler P. Pediatric retinal detachment. Klin Monbi Augenheilkd. 2019;236(1):74–87.
4. Adegbehingbe B. Blindness from bilateral bullous retinal detachment: tragedy of a Nigerian family. Afr Health Sci. 2008;8(1):50–53.
5. Dahnert W. Chapter 10 – Urogenital tract. In: Radiology Review Manual.
6. Dahal P, Gouli S. Ocular findings in chronic renal failure. J Coll Med Sci Nepal. 2015;10(2):18–26. doi:10.3126/jcmsn.v10i2.12949
7. Bajracharya L, Shah DN, Raut KB, Koirala S. Ocular evaluation in patients with chronic renal failure – a hospital based study. J Coll Med Sci Nepal. 2008;10(4):209–214.
8. Ulasi II, Ijoma CK. The enormity of chronic kidney disease in Nigeria: the situation in a teaching hospital in south-east Nigeria. J Trop Med. 2010;2010:1–6. doi:10.1155/2010/501957
9. Anochie I, Eke F. Chronic renal failure in children: a report from Port Harcourt, Nigeria (1985–2000). Pediatr Nephrol. 2003;18(7):692–695. doi:10.1007/s00467-003-1150-0
10. Borowicz D, Bielecka M, Wojda T, Kedziora W, Biela K. Bilateral exudative retinal detachment in a patient with end-stage renal disease- a case report. Ophthalmol J. 2019;4:22–27. doi:10.5603/OJ.2018.0037
11. Guven S, Kucukevcilioglu M, Durukan AH, Ayyildiz O, Ozge G. Atypical ocular sign of chronic renal failure: bilateral massive serous retinal detachment. EC Ophthalmol. 2018;9(6):368–371.
12. Chang Y-S, Weng S-F, Chang C, Wang -J-J. Risk of serous retinal detachment in patients with end-stage renal disease on dialysis. PLoS One. 2017;12(6):1–11.
13. Amer R, Nalci H, Yalcindag N. Exudative retinal detachment. Surv Ophthalmol. 2017;62(6):723–769.
14. Pociej-Marciak W, Karska-Basta I, Kuźniewski M, Kubicka-Trząska A, Romanowska-Dixon B. Romanowska-dixon B. sudden visual deterioration as the first symptom of chronic kidney failure. Case Rep Ophthalmol. 2015;6(3):394–400. doi:10.1159/000442182
15. Tarlan B, Kiratti H. Subconjunctival haemorrhage: risk factors and potential indicators. Clin Ophthalmol. 2013;1163–1170.
16. Berdahi JP, Mruthyunjaya P. Vitreous Hemorrhage: Diagnosis and Treatment. EyeNet; 2007.
17. Chelala E, Fadlallah DA. Effect of haemodialysis on visual acuity, intraocular pressure and macular thickness in patients with chronic kidney disease. J Clin Ophthalmol. 2015;9:109–114.
18. Souzeau E, Burdon KP, Dubowsky A, et al. Higher prevalence of myocilin mutations in advanced glaucoma in comparison with less advanced disease in an Australian disease registry. Ophthalmology. 2013;120(6):1135–1143. doi:10.1016/j.ophtha.2012.11.029
19. Riley M, Hernandez AK, Kuznia AL. High blood pressure in children and adolescents. Am Fam Physician. 2018;98(8):486–494.
20. Schmiedar RE. End organ damage in hypertension. Dtsch Arztebl Int. 2010;107(49):866–873. doi:10.3238/arztebl.2010.0866
21. Chatterjee S, Chattopadhya S, Hope-ROSS M, Lip PL. Hypertension and the eye: changing perspectives. J Hum Hypertens. 2002;16(10):667–675. doi:10.1038/sj.jhh.1001472
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
