Back to Journals » Cancer Management and Research » Volume 11
Bevacizumab in Combination with Pemetrexed and Platinum Significantly Improved the Clinical Outcome of Patients with Advanced Adenocarcinoma NSCLC and Brain Metastases
Authors Tian Y, Zhai X, Tian H, Jing W, Zhu H, Yu J
Received 12 July 2019
Accepted for publication 14 November 2019
Published 29 November 2019 Volume 2019:11 Pages 10083—10092
DOI https://doi.org/10.2147/CMAR.S222910
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Harikrishna Nakshatri
Yaru Tian,1 Xiaoyang Zhai,2 Hairong Tian,1 Wang Jing,2 Hui Zhu,1,2 Jinming Yu1,2
1Department of Radiation Oncology, Shandong Cancer Hospital and Institute, Shandong University, Jinan, People’s Republic of China; 2Department of Radiation Oncology, Shandong Cancer Hospital and Institute, Shandong First Medical University and Shandong Academy of Medical Science, Jinan, People’s Republic of China
Correspondence: Hui Zhu
Department of Radiation Oncology, Shandong Cancer Hospital and Institute, Shandong First Medical University and Shandong Academy of Medical Science, 440 Jiyan Road, Jinan, Shandong Province 250117, People’s Republic of China
Tel +86 531 6762 6112
Email [email protected]
Jinming Yu
Department of Radiation Oncology, Shandong Cancer Hospital and Institute, Shandong University, 44 Wen Hua Xi Road, Jinan, Shandong Province 250012, People’s Republic of China
Tel +86 531 6762 6971
Email [email protected]
Background: The study aims to evaluate the clinical efficacy and safety of bevacizumab in combination with the first-line pemetrexed-platinum (PP) in patients with advanced adenocarcinoma non-small-cell lung cancer (NSCLC) and brain metastases.
Methods: The clinical data of patients with adenocarcinoma NSCLC and symptomatic or asymptomatic brain metastases were collected in our study. The basic chemotherapy regimen was pemetrexed-platinum (PP). According to whether combined with bevacizumab (B) or not, all enrolled patients were assigned to the B+PP group or the PP alone group.
Results: A total of 71 patients were enrolled in the current study. Twenty-six patients were allocated to the B+PP group and 45 were allocated to the PP group. Overall response rates (ORRs), disease control rates (DCRs) of the thoracic tumors and intracranial metastases and overall survival (OS) were not significantly different between the 2 groups. However, progression-free survival (PFS) and intracranial PFS (iPFS) were significantly prolonged in the B+PP group compared with the PP group. The median PFS was 9.2 and 8.2 months, and the 1-year PFS rates were 47.1% and 15.9%, respectively, in the 2 groups (P=0.029). And, the median iPFS were 24.3 and 10.9 months, and the 1-year iPFS rates were 80.1% and 40.1%, respectively, in the 2 groups (P=0.008). Univariate and multivariate analyses suggested that maintenance therapy and bevacizumab therapy were independent favorable prognostic factors of PFS and iPFS.
Conclusion: The addition of bevacizumab to the first-line pemetrexed and platinum significantly improved clinical outcomes of patients with advanced adenocarcinoma NSCLC and brain metastases.
Keywords: bevacizumab, non-small cell lung cancer, brain metastases, clinical outcome, toxicity
Introduction
Worldwide, lung cancer is the leading cause of cancer incidence and mortality, with 2.1 million new cases and 1.8 million deaths predicted in 20181 Approximately 85% of patients with lung cancer have the subtype of non-small-cell lung cancer (NSCLC). The incidence proportions of brain metastases were highest for lung cancer (19.9%), followed by melanoma (6.9%), renal (6.5%), breast (5.1%), and colorectal (1.8%) cancers.2 Angiogenesis is essential in the development of brain metastases.3,4 It not only constructs the pathway through which the cancer cells (the “seed”) and their primary stromal components (their own “soil”) spread to the brain environment (the “soil”), but can also provide sufficient nutrients to support tumor proliferation. Vascular endothelial growth factor (VEGF) mainly mediates tumor angiogenesis and the progressive growth of the majority of metastatic brain tumors.5–7 These structurally and functionally abnormal blood vessels, exhibiting sluggish blood flow and hyperpermeability, can, therefore, induce tumor interstitial hypertension and impair drug delivery.5 Besides, the blood-brain barrier (BBB) also creates a sanctuary site protecting tumor cells from systemic treatments.
With the burgeoning biologic knowledge of brain metastases, normalizing tumor vasculature with anti-angiogenic treatment is a new paradigm for combination therapy in clinical applications.8 Bevacizumab, a monoclonal antibody targeting VEGF, has demonstrated significant clinical benefit in the first- and second-line treatment across many cancer types including colorectal cancer, renal cell carcinoma, breast cancer and non-squamous NSCLC.9–16 Multiple studies have confirmed that bevacizumab prolonged PFS in patients with newly diagnosed and progressive glioblastoma, the most common intracranial carcinoma.17–19 Compared with chemotherapy alone, first-line bevacizumab in combination with standard chemotherapy followed by single-agent bevacizumab maintenance significantly improved clinical outcomes with manageable adverse events among patients with advanced non-squamous NSCLC.13–15 However, the efficacy of bevacizumab in patients with brain metastases has not been systematically studied because these patients were generally excluded in the literature to avoid the potential risk of intracranial hemorrhage. However, a retrospective exploratory analysis found that patients with brain metastases from advanced breast, NSCLC, renal and colorectal cancers were at similar risk of developing cerebral hemorrhage, irrespective of bevacizumab therapy.20 What’s more, some prospective trials (PASSPORT, ATLAS, BeTa, ERACLE and PRONOUNCE) included patients with brain metastases and reported a low rate of central nervous system (CNS) hemorrhage.21–25 Pemetrexed plus cisplatin was better than gemcitabine plus cisplatin in patients with lung adenocarcinoma in the phase III study and was one of the standard chemotherapy regimens in the clinic.26 There is a lack of studies involving bevacizumab for the brain metastases of NSCLC. Therefore, we conducted this retrospective study to explore the clinical efficacy and safety of first-line regimen of bevacizumab plus pemetrexed and platinum in NSCLC patients with brain metastases and further support clinical application of bevacizumab.
Materials and Methods
Patients
Patients with advanced adenocarcinoma NSCLC and brain metastases at diagnosis in Shandong Cancer Hospital and Institute (Jinan, Shandong, China) were enrolled in this retrospective study. The eligible criteria were as follows: histologically or cytologically diagnosed with adenocarcinoma NSCLC; no previous treatment; stage IV with brain metastases confirmed by a brain-computed tomography (CT) or magnetic resonance imaging (MRI) scan; Karnofsky performance status (KPS) score ≥70; symptomatic (headache, dizziness, nausea, vomit, visual disturbance et al) or asymptomatic. Exclusion criteria were predominantly squamous histology; severe hemoptysis (more than one-half teaspoon of bright red blood in recent 3 months); tumors invading major blood vessels; medically uncontrolled hypertension; current or recent (within 10 days of first-line therapy) therapeutic use of anticoagulants; and regular use of aspirin. This study was approved by the Ethics Committee of Shandong Cancer Hospital and Institute. All procedures involving patients were conformed to Declaration of Helsinki. All patients provided written informed consent before their participation in this study.
Treatments
Patients received either PP (pemetrexed 500mg/m2 on day 1 and cisplatin 30 mg/m2 on days 1–3 or carboplatin 400 or 500 mg on day 1) or B+PP (bevacizumab 7.5mg/kg on the day before PP). PP and bevacizumab were administered intravenously and repeated every 21 days for a total of 4 to 6 cycles. After the first-line therapy, patients who had a responsive or stable disease could receive maintenance therapy including pemetrexed monotherapy, tyrosine kinase inhibitors (TKIs), BP (bevacizumab plus pemetrexed) and BT (bevacizumab plus TKIs). During the first-line therapy or after disease progression, thoracic radiation and brain radiation were also available. The total dose of thoracic radiation was 45–60 Gy delivered at 1.8–2.0 Gy per fraction for 5 days weekly. Whole brain radiation therapy (WBRT) (30–60 Gy at 1.8–3.0 Gy per fraction) with/without additional localized boost (10–20 Gy).
Assessments of Response and Toxicity
Responses of primary tumors and brain metastases were evaluated using the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.0. From the initial treatment, tumors evaluation was conducted every 2 cycles for 4–6 cycles, then every 2 months for the remainder of the treatment course and every 3 to 6 months after the completion of treatment. AEs were graded according to the National Cancer Institute-Common Toxicity Criteria (NCI-CTC) version 3.0.
Endpoints
The primary endpoint was OS and the secondary endpoints were PFS, iPFS and AEs. OS was measured from the initial diagnosis to the date of death from any cause or the date of the last known follow-up. PFS was defined as the duration from the initial diagnosis to the date of disease failure, the date of death or the last known follow-up. Intracranial PFS (iPFS) was from the diagnosis of brain metastases to the date of the intracranial progression, the date of death or the last known follow-up. AEs were evaluated in all patients of the 2 groups.
Statistical Analysis
Comparisons of patient characteristics, response rates and AEs between the 2 groups were performed using Chi-square test and Fisher’s exact test. The Kaplan–Meier method was used to calculate OS, PFS and iPFS. The survival difference between the 2 groups was compared using log-rank test. Using the Kaplan–Meier method, univariate survival analysis was performed to determine associations between survival (OS, PFS and iPFS) and clinical characteristics. All clinical variables were brought into a multivariate model in which a Cox proportional hazards model was used to determine significant factors. Two-sided P values <0.05 were considered statistically significant in all reported results and confidence intervals (CIs) are at the 95% level.
Results
Patient Characteristics
Between February 2013 and December 2017, approximately 5680 patients were diagnosed with stage IV adenocarcinoma NSCLC in our cancer center, and approximately 1620 patients had confirmed brain metastases. Since pemetrexed was expensive, and bevacizumab was not covered by medical insurance, only a number of patients received the first-line pemetrexed or in combination with bevacizumab. As a result, 76 NSCLC patients with confirmed brain metastases in our cancer center were evaluated. The follow-up rate was 93.4% with 5 patients lost during follow-up. Seventy-one patients had fully eligible information and were enrolled in the study finally. The median follow-up time was 14.5 months (range, 1.2–47.4 months) for the total patients and 14.8 months (range, 1.5–47.4 months) for the living patients. For the 2 cohorts of patients, the median age was 55 and their age ranged from 27 to 76. Nine patients were 65 years and older. Thirty-four (47.9%) were male while 37 (52.1%) were female. According to the treatment modality, 26 (36.6%) patients were allocated to the B+PP group and 45 (63.4%) patients were allocated to the PP group.
Table 1 summarizes patient characteristics of the 2 groups. The distribution of all characteristics was balanced. Thirty-nine patients were assessable for epidermal growth factor receptor (EGFR) mutation status and 21(53.8%) patients were EGFR mutation positive. More patients in the PP group have symptomatic brain metastases than the B+PP group (42.2% v 34.6%, P=0.527). Table 2 is the summarization of treatment details. Most treatment characteristics were not significantly differ except for brain radiation between the 2 groups. The rate of patients receiving brain radiation was significantly higher in the PP group compared with B+PP group (68.9% v 38.5%, P=0.012). One reason was that more patients in the PP group had symptomatic brain metastases than the other group.
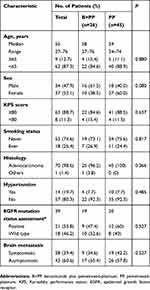 |
Table 1 Patient Characteristics |
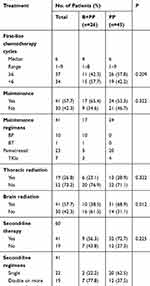 |
Table 2 Treatment Details |
Response
Among all patients in the 2 groups, ORR was 47.9% and DCR was 81.7%. ORRs and DCRs were not significantly different between the 2 groups (ORRs, 53.8% v 44.4%, P=0.445; DCRs, 88.5% v 88.5%, P=0.422, for the B+PP and PP groups, respectively, Table S1). For intracranial metastases, ORR was 50.7% and DCR was 91.5% for the total patients. Intracranial response rates were 53.8% and 48.9% in the B+PP and PP groups, respectively (P=0.565, Table S2). Among patients who received B+PP, 3 (11.5%) achieved a complete response of the intracranial metastases. DCRs of the intracranial tumors were 96.2% and 88.9% in the 2 groups, respectively (P=0.537).
Survival
For the entire cohort of patients, the median survival was 21.2 months, and the 1-year and 2-year OS rates were 86.2% and 38.9%, respectively (Figure 1). Overall survival was not significantly different between the 2 groups (P=0.460, Figure 2). The median survival was 21 months, and the 1-year and 2-year OS rates were 68.8% and 45.9%, respectively, in the B+PP group. In the PP group, the median survival was 33.4 months, and the 1-year and 2-year OS rates were 85.9% and 39.5%, respectively.
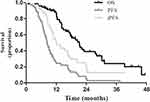 |
Figure 1 OS, PFS and iPFS for the entire cohort of patients. |
 |
Figure 2 OS between the B+PP group and the PP group. Median OS, 21.0 v 21.0 months; log-rank test, P=0.460; Hazard Ratio=1.34. |
The median PFS was 8.4 months and the 1-year and 2-year PFS rates were 24.3% and 5.3%, respectively for the entire cohort of patients (Figure 1). Patients receiving B+PP had significantly longer PFS than those receiving PP (P=0.029, Figure 3). The median PFS was 9.2 and 8.2 months, the 1-year PFS rates were 47.1% and 15.9%, and 2-year PFS rates were 17.6% and 0%, respectively, in the 2 groups.
 |
Figure 3 PFS between the B+PP group and the PP group. Median PFS, 9.2 v 8.2 months; log-rank test, P=0.029; Hazard Ratio=0.56. |
The median iPFS for the intracranial metastases was 12 months and the 1-year and 2-year PFS rates were 49.8% and 21.0%, respectively (Figure 1). As shown in Figure 4, iPFS was significantly longer in the B+PP group than the PP group (P=0.008). In the 2 groups, the median iPFS were 24.3 and 10.9 months, the 1-year iPFS rates were 80.1% and 40.1%, and 2-year iPFS rates were 60.1% and 13.2%, respectively.
 |
Figure 4 iPFS between the B+PP group and the PP group. Median iPFS, 24.3 v 10.9 months; log-rank test, P=0.008; Hazard Ratio=0.40. |
Prognostic Factors
Univariate and multivariate analyses indicated that all clinical factors, including age at diagnosis, sex, KPS score, smoking status, brain metastases, EGFR mutation status, first-line chemotherapy cycles, maintenance therapy, thoracic radiation, brain radiation and bevacizumab therapy, were not associated with overall survival (Table 3). However, for PFS, univariate analysis suggested that maintenance therapy and bevacizumab therapy were significant favorable prognostic factors. Multivariate analysis revealed that maintenance therapy (P=0.003), brain radiation (P=0.030) and bevacizumab therapy (P=0.004) were favorable prognostic factors of PFS (Table 4). Similarly, as shown in Table 4, univariate analysis indicated that maintenance therapy and bevacizumab therapy were significantly associated with favorable iPFS. Multivariate analyses also indicated that maintenance therapy (P=0.001) and bevacizumab therapy (P=0.012) were favorable prognostic factors of iPFS (Table 4).
 |
Table 3 Univariate and Multivariate Analyses of the Prognostic Factors for OS in NSCLC Patients with Brain Metastases |
 |
Table 4 Univariate and Multivariate Analyses of the Prognostic Factors for PFS and iPFS in NSCLC Patients with Brain Metastases |
Toxicities
As shown in Table 5, the incidence of any grade AE was significantly higher in the B+PP group than the PP group (73.1% v 46.7%, P=0.031). For the total patients, the incidence of any grade AE was 56.3%. None of the patients suffered from thrombosis, hemorrhage or proteinuria after bevacizumab treatment. However, there was no significant difference in the prevalence of grade ≥3 AEs between the 2 groups (7.7% v 4.4%, P=0.970). One patient in the B+PP group experienced drug withdrawal due to intolerable toxicity. None of the total patients died of AEs.
 |
Table 5 Adverse Events |
Discussion
In the current study, the addition of bevacizumab to the first-line PP chemotherapy significantly improved PFS and intracranial PFS compared with chemotherapy alone. Univariate and multivariate analyses indicated that maintenance therapy and bevacizumab therapy are independent favorable factors of PFS and iPFS. Our study supported that bevacizumab was beneficial and safe for patients with advanced adenocarcinoma and brain metastases.
Brain metastasis is a serious obstacle in the treatment of solid tumors and indicates poor prognosis, always determining a fatal outcome of these cancers. With improved systemic therapy and prolonged survival, and the early detection of asymptomatic metastases, the frequency of brain metastases is increasing. Although large-randomized phase III trials showed that bevacizumab produced significant clinical benefits and was well tolerated in patients with advanced non-squamous NSCLC. Nevertheless, most studies concerning bevacizumab excluded brain metastases to avoid the fetal CNS hemorrhage.13–15 The lack of the current trials of bevacizumab in treating metastatic brain tumors of NSCLC gave us a fresh impetus to conduct this retrospective study.
In our study, compared with the PP group, bevacizumab plus PP improved tumor responses and significantly prolonged PFS and iPFS in patients with adenocarcinoma and brain metastases. In the B+PP group, ORRs were 53.8% and 50.7% for thoracic tumors and intracranial metastases, respectively. The median PFS, 1-year and 2-year PFS rates were 9.2 months, 47.1% and 17.6%, respectively, and the median intracranial PFS, 1-year and 2-year PFS rates were 23.4 months, 80.1% and 60.1%, respectively. These results were consistent with BRAIN, a phase II prospective study, which demonstrated encouraging efficacy and tolerable safety profile of bevacizumab with the first-line paclitaxel and carboplatin (PC) in NSCLC patients with asymptomatic untreated brain metastases.27 Of the total 67 patients, 6-month PFS rate was 56.5%, the median PFS was 6.7 months and the median OS was 16.0 months. Investigator-assessed ORR was 62.7%, with 61.2% in intracranial lesions and 64.2% in extracranial lesions. What’s more, the PRONOUCE study, which enrolled 54 (15%) NSCLC patients with brain metastasis, also indicated that PFS without grade 4 toxicity was also favorable in the first-line B+PC group compared with the PC group.25 The ARIES observational cohort study included 150 (7.6%) NSCLC patients with brain metastases showed a median PFS of 6.6 months in the first-line bevacizumab-containing group.28 Thus, bevacizumab could achieve a good response and disease control both in the thoracic tumors and the intracranial metastases.
However, there was no significant difference in overall survival between the 2 groups (21 v 21 months, P=0.460). We speculate that the higher percentage of patients receiving the first-line chemotherapy cycles, brain radiation and the second-line therapy in the PP group may be involved in this issue. Brain radiation therapy, including WBRT and SRS (stereotactic radiosurgery), has been used as the primary nonsurgical therapeutic modality for brain metastases.8 And the combination of brain radiation therapy with systemic chemotherapy and targeted therapy also improved the control of brain metastases and clinical outcomes in NSCLC patients with brain metastases. Maintenance therapy, with either chemotherapy or TKIs, is an important part of the whole course treatment, and has significantly improved overall survival in patients with advanced NSCLC according to multiple large studies.29–33 In the current study, the differences in the maintenance and the second-line therapy between the 2 groups may also influence the overall survival. However, the second-line bevacizumab therapy was unlikely to affect the overall survival, since only 3 and 4 patients in the 2 groups received bevacizumab-containing regimens as the second-line therapy.
Asian population had a more favorable survival outcome compared with a mainly white population with advanced NSCLC.34 The median OS, median PFS and ORR in our study were 21 months, 9.2 months and 53.8% in the B+PP group, which is comparable with BEYOND.14 In BEYOND, the median OS, median PFS and ORR were 24.3 months, 9.2 months and 54%, respectively, in the B+PC group. It is suspected that the higher EGFR positive mutation rate in Chinese patients (27% in BEYONG and 53.8% in our study) may partly account for the better clinical outcome compared with the West.
Toxicity, especially hemorrhage, was the major concern of bevacizumab therapy. Our study suggested that bevacizumab was safe in treating NSCLC patients with brain metastases. The incidence of grade ≥3 AEs was similar between the 2 groups. Intracranial, pulmonary and gastrointestinal hemorrhage did not occur in the entire cohort of patients. A retrospective study demonstrated that the risk of developing intracranial hemorrhage was independent of bevacizumab therapy among patients with brain metastases from advanced breast cancer, NSCLC, renal and colorectal cancer.20 What’s more, bevacizumab was safe and produced clinical benefit in the form of relieved symptoms and reduced corticosteroid requirements among 6 patients with active brain metastases from NSCLC.35 An evidence-based review also showed that there was no significant increased risk of intracranial hemorrhage in NSCLC patients with brain metastases treated with anti-VEGF therapy.36 The incidence of intracranial hemorrhage was relatively low for NSCLC patients with brain metastases in prospective trials.16,21,22,27,28 Our study, in consistent with previous studies, demonstrates that bevacizumab has tolerable toxicity profile in the treatment of NSCLC patients with brain metastases.
There are also some limitations in our study. First, as a retrospective study, some important clinical information, such as the baseline lung and heart function, which could influence the clinical outcome of the treatment, was not fully introduced. Second, the patient population was too small to be representative of the patients of the whole country. Third, possible selection biases could also exist, as patients with a history or high risk of hypertension, bleeding or thrombosis tended to be included in the PP group, which could influence the final survival results. All possible confounding factors could not be controlled despite the adjustment of a Cox regression model.
Conclusion
In conclusion, our study indicates that the addition of bevacizumab to pemetrexed-platinum was associated with the better local control of both primary tumors and intracranial metastatic lesions, and prolonged PFS and intracranial PFS in NSCLC patients with symptomatic or asymptomatic brain metastases. Adverse events were manageable with no pulmonary or intracranial hemorrhage observed after bevacizumab therapy. Consistent with previous analyses and early phase studies, our study demonstrated that bevacizumab in combination with the first-line chemotherapy could produce clinical benefit and was tolerable for NSCLC patients with brain metastases. In this respect, bevacizumab can be considered for NSCLC patients with brain metastases, and prospective trials concerning bevacizumab in the treatment of advanced NSCLC should also include patients with brain metastases.
Abbreviations
NSCLC, non-small-cell lung cancer; VEGF, vascular endothelial growth factor; AE, adverse event; CNS, central nervous system; OS, overall survival; PFS, progression-free survival; CT, computed tomography; MRI, magnetic resonance imaging; KPS, Karnofsky performance status; PP, pemetrexed-platinum; B+PP, bevacizumab plus PP; EGFR, epidermal growth factor receptor; TKI, tyrosine kinase inhibitor; BP, bevacizumab plus pemetrexed; BT, bevacizumab plus TKI; WBRT, whole brain radiation therapy; SRS, stereotactic radiosurgery; ORR, overall response rate; DCR, diseases control rate; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; BBB, blood-brain barrier; RECIST, Response Evaluation Criteria in Solid Tumors; NCI-CTC, National Cancer Institute-Common Toxicity Criteria; HR, hazard ratio; CI, confidence interval.
Author Contributions
Yaru Tian, Hairong Tian, Xiaoyang Zhai and Wang Jing contributed to the acquisition, analysis and interpretation of the clinical data. They also drafted the manuscript. Hui Zhu and Jinming Yu contributed to the conception and design of the study, and made revisions for the manuscript critically for important intellectual content. All authors approved the final manuscript to be published and agreed to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi:10.3322/caac.v68.6
2. Barnholtz-Sloan JS, Sloan AE, Davis FG, Vigneau FD, Lai P, Sawaya RE. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the metropolitan detroit cancer surveillance system. J Clin Oncol. 2004;22:2865–2872. doi:10.1200/JCO.2004.12.149
3. Eichler AF, Chung E, Kodack DP, Loeffler JS, Fukumura D, Jain RK. The biology of brain metastases—translation to new therapies. Nat Rev Clin Oncol. 2011;8:344. doi:10.1038/nrclinonc.2011.58
4. Fidler IJ, Yano S, Zhang RD, Fujimaki T, Bucana CD. The seed and soil hypothesis: vascularisation and brain metastases. Lancet Oncol. 2002;3:53–57. doi:10.1016/S1470-2045(01)00622-2
5. Jain RK. Normalizing tumor vasculature with anti-angiogenic therapy: a new paradigm for combination therapy. Nat Med. 2001;7:987–989. doi:10.1038/nm0901-987
6. Ellis LM, Hicklin DJ. VEGF-targeted therapy: mechanisms of anti-tumour activity. Nat Rev Cancer. 2008;8:579. doi:10.1038/nrc2403
7. Kerbel RS. Tumor angiogenesis. N Engl J Med. 2008;358:2039–2049. doi:10.1056/NEJMra0706596
8. Owonikoko TK, Arbiser J, Zelnak A, et al. Current approaches to the treatment of metastatic brain tumours. Nat Rev Clin Oncol. 2014;11:203. doi:10.1038/nrclinonc.2014.25
9. Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi:10.1056/NEJMoa032691
10. Giantonio BJ, Catalano PJ, Meropol NJ, et al. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (folfox4) for previously treated metastatic colorectal cancer: results from the eastern cooperative oncology group study e3200. J Clin Oncol. 2007;25:1539–1544. doi:10.1200/JCO.2006.09.6305
11. Hainsworth JD, Sosman JA, Spigel DR, Edwards DL, Baughman C, Greco A. Treatment of metastatic renal cell carcinoma with a combination of bevacizumab and erlotinib. J Clin Oncol. 2005;23:7889–7896. doi:10.1200/JCO.2005.01.8234
12. Gray R, Bhattacharya S, Bowden C, Miller K, Comis RL. Independent review of e2100: a phase III trial of bevacizumab plus paclitaxel versus paclitaxel in women with metastatic breast cancer. J Clin Oncol. 2009;27:4966–4972. doi:10.1200/JCO.2008.21.6630
13. Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. doi:10.1056/NEJMoa061884
14. Zhou C, Wu YL, Chen G, et al. Beyond: a randomized, double-blind, placebo-controlled, multicenter, phase III study of first-line carboplatin/paclitaxel plus bevacizumab or placebo in chinese patients with advanced or recurrent nonsquamous non-small-cell lung cancer. J Clin Oncol. 2015;33:2197–2204. doi:10.1200/JCO.2014.59.4424
15. Reck M, von Pawel J, Zatloukal P, et al. Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: avail. J Clin Oncol. 2009;27:1227–1234. doi:10.1200/JCO.2007.14.5466
16. Crino L, Dansin E, Garrido P, et al. Safety and efficacy of first-line bevacizumab-based therapy in advanced non-squamous non-small-cell lung cancer (sail, mo19390): a phase 4 study. Lancet Oncol. 2010;11:733–740. doi:10.1016/S1470-2045(10)70151-0
17. Gilbert MR, Dignam JJ, Armstrong TS, et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014;370:699–708. doi:10.1056/NEJMoa1308573
18. Chinot OL, Wick W, Mason W, et al. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med. 2014;370:709–722. doi:10.1056/NEJMoa1308345
19. Wick W, Gorlia T, Bendszus M, et al. Lomustine and bevacizumab in progressive glioblastoma. N Engl J Med. 2017;377:1954–1963. doi:10.1056/NEJMoa1707358
20. Besse B, Lasserre SF, Compton P, Huang J, Augustus S, Rohr UP. Bevacizumab safety in patients with central nervous system metastases. Clin Cancer Res. 2010;16:269–278. doi:10.1158/1078-0432.CCR-09-2439
21. Socinski MA, Langer CJ, Huang JE, et al. Safety of bevacizumab in patients with non-small-cell lung cancer and brain metastases. J Clin Oncol. 2009;27:5255–5261. doi:10.1200/JCO.2009.22.0616
22. Johnson BE, Kabbinavar F, Fehrenbacher L, et al. Atlas: randomized, double-blind, placebo-controlled, phase IIIB trial comparing bevacizumab therapy with or without erlotinib, after completion of chemotherapy, with bevacizumab for first-line treatment of advanced non-small-cell lung cancer. J Clin Oncol. 2013;31:3926–3934. doi:10.1200/JCO.2012.47.3983
23. Herbst RS, Ansari R, Bustin F, et al. Efficacy of bevacizumab plus erlotinib versus erlotinib alone in advanced non-small-cell lung cancer after failure of standard first-line chemotherapy (beta): a double-blind, placebo-controlled, phase 3 trial. Lancet. 2011;377:1846–1854. doi:10.1016/S0140-6736(11)60545-X
24. Galetta D, Cinieri S, Pisconti S, et al. Cisplatin/pemetrexed followed by maintenance pemetrexed versus carboplatin/paclitaxel/bevacizumab followed by maintenance bevacizumab in advanced nonsquamous lung cancer: the goim (gruppo oncologico italia meridionale) eracle phase III randomized trial. Clin Lung Cancer. 2015;16:262–273. doi:10.1016/j.cllc.2014.12.002
25. Zinner RG, Obasaju CK, Spigel DR, et al. Pronounce: randomized, open-label, phase III study of first-line pemetrexed + carboplatin followed by maintenance pemetrexed versus paclitaxel + carboplatin + bevacizumab followed by maintenance bevacizumab in patients ith advanced nonsquamous non-small-cell lung cancer. J Thorac Oncol. 2015;10:134–142. doi:10.1097/JTO.0000000000000366
26. Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008;26:3543–3551. doi:10.1200/JCO.2007.15.0375
27. Besse B, Le Moulec S, Mazieres J, et al. Bevacizumab in patients with nonsquamous non-small cell lung cancer and asymptomatic, untreated brain metastases (brain): a nonrandomized, phase II study. Clin Cancer Res. 2015;21:1896–1903. doi:10.1158/1078-0432.CCR-14-2082
28. Lynch TJ
29. Ciuleanu T, Brodowicz T, Zielinski C, et al. Maintenance pemetrexed plus best supportive care versus placebo plus best supportive care for non-small-cell lung cancer: a randomised, double-blind, phase 3 study. Lancet. 2009;374:1432–1440. doi:10.1016/S0140-6736(09)61497-5
30. Belani CP, Brodowicz T, Ciuleanu TE, et al. Quality of life in patients with advanced non-small-cell lung cancer given maintenance treatment with pemetrexed versus placebo (h3e-mc-jmen): results from a randomised, double-blind, phase 3 study. Lancet Oncol. 2012;13:292–299. doi:10.1016/S1470-2045(11)70339-4
31. Paz-Ares LG, de Marinis F, Dediu M, et al. Paramount: final overall survival results of the phase III study of maintenance pemetrexed versus placebo immediately after induction treatment with pemetrexed plus cisplatin for advanced nonsquamous non-small-cell lung cancer. J Clin Oncol. 2013;31:2895–2902. doi:10.1200/JCO.2012.47.1102
32. Lopez-Chavez A, Young T, Fages S, et al. Bevacizumab maintenance in patients with advanced non-small-cell lung cancer, clinical patterns, and outcomes in the eastern cooperative oncology group 4599 study: results of an exploratory analysis. J Thorac Oncol. 2012;7:1707–1712. doi:10.1097/JTO.0b013e318265b500
33. Patel JD, Socinski MA, Garon EB, et al. Pointbreak: a randomized phase III study of pemetrexed plus carboplatin and bevacizumab followed by maintenance pemetrexed and bevacizumab versus paclitaxel plus carboplatin and bevacizumab followed by maintenance bevacizumab in patients with stage iiib or iv nonsquamous non-small-cell lung cancer. J Clin Oncol. 2013;31:4349–4357. doi:10.1200/JCO.2012.47.9626
34. Soo RA, Loh M, Mok TS, et al. Ethnic differences in survival outcome in patients with advanced stage non-small cell lung cancer: results of a meta-analysis of randomized controlled trials. J Thorac Oncol. 2011;6:1030–1038. doi:10.1097/JTO.0b013e3182199c03
35. De Braganca KC, Janjigian YY, Azzoli CG, et al. Efficacy and safety of bevacizumab in active brain metastases from non-small cell lung cancer. J Neurooncol. 2010;100:443–447. doi:10.1007/s11060-010-0200-2
36. Sandler A, Hirsh V, Reck M, von Pawel J, Akerley W, Johnson DH. An evidence-based review of the incidence of CNS bleeding with anti-VEGF therapy in non-small cell lung cancer patients with brain metastases. Lung Cancer. 2012;78:1–7. doi:10.1016/j.lungcan.2012.07.004
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
