Back to Journals » Clinical Ophthalmology » Volume 12
Better detection of Demodex mites by Löffler’s alkaline methylene blue staining in patients with blepharitis
Authors Kiuchi K
Received 23 November 2017
Accepted for publication 6 March 2018
Published 16 April 2018 Volume 2018:12 Pages 727—731
DOI https://doi.org/10.2147/OPTH.S157910
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Katsuji Kiuchi
Kiuchi Eye Clinic, Joto-ku, Osaka, Japan
Purpose: To determine whether the Löffler’s alkaline methylene blue staining method is better than no staining in detecting Demodex mites in the eyelashes of patients with blepharitis.
Materials and methods: Eyelashes were collected from 22 patients with blepharitis. The mean age of the patients was 82.5±6.2 years (± SD) with a range from 71 to 93 years. Eyelashes were epilated by forceps and placed individually on microscope slides. The number of Demodex mites was determined by conventional optical microscopy before and immediately after the addition of the methylene blue staining solution.
Results: The mean Demodex count before the addition of the methylene blue solution was 2.9±2.9, and it was 4.4±3.9 after the addition of the methylene blue solution (P<0.01, Wilcoxon test).
Conclusion: The methylene blue staining method is a simple and useful method in detecting the presence and quantifying the number of Demodex mites. We recommend the methylene blue staining method not only for the diagnosis of the presence of Demodex mites but also to evaluate the therapeutic effects of medications to eliminate the mite infestation.
Keywords: Demodex mites, Löffler’s alkaline methylene blue, blepharitis
Introduction
Blepharitis is one of the most common diseases encountered by ophthalmologists in their daily practice. One of the causes of severe marginal blepharitis is the presence of Demodex mites, which are the most common ectoparasites in humans. Among the many species of Demodex, Demodex folliculorum and Demodex brevis have been detected in humans. They can be found on the eyelids, eyelashes, meibomian glands, face, and external ear tract. The incidence of D. folliculorum is higher in patients with blepharitis than in normal individuals,1 and the number of Demodex mites is highly correlated with the age of the individual.2 For diagnosis, it is necessary to prove the existence of Demodex mites from an examination of epilated eyelashes of the patient.
Recently, Kojima et al3 and Randon et al4 reported on a method of counting the number of Demodex mites in situ by confocal microscopy without removing the eyelashes. However, there are many researchers who are still using the previous method of microscopic examination of epilated eye lashes. For this, the epilated eyelashes are placed on glass microscope slides and covered with a coverslip without adding any staining solution.5–7 The slides are then examined by conventional microscopy. In another technique, Kheirkhah et al8 reported adding fluorescein solution to detect and count the number of Demodex mites in the epilated eyelashes. This method has increased the proficiency of detecting and counting the Demodex mites in epilated eyelashes compared to previous methods.8
We reported earlier on the use of the Löffler’s alkaline methylene blue supravital staining method for diagnosing conjunctivitis.9 It is possible to deduce the cause of the infection by the differential distribution of neutrophils and lymphocytes. However, this has not been done for Demodex infections.
Thus, the purpose of this study was to determine the applicability of the Löffler’s alkaline methylene blue staining method for detecting and counting the number of Demodex mites in epilated eyelashes from patients with blepharitis.
Materials and methods
The Löffler’s alkaline methylene blue (Muto Pure Chemicals Co, Ltd, Tokyo, Japan) solution is made by a 10-fold dilution of the original solution with saline, and it was filtered daily through Millex AP Filter, pore size 2.0 μm (Millipore: SLAP 02550, Merck KGaA, Darmstadt, Germany) before use.
To compare the effectiveness of Löffler’s alkaline methylene blue solution staining to fluorescein staining, fluorescein solution was made by moistening a fluorescein strip (FUL-GLO; Akron, Buffalo Grove, IL, USA) with 1 drop of 0.9% NaCl solution.
Eyelashes were collected from 22 patients whose mean age was 82.5±6.2 years (± SD) with a range from 71 to 93 years (Table 1).
  | Table 1 Characteristics of patients, diagnosis, and the Demodex count before and after the addition of the Löffler’s alkaline methylene blue solution |
The procedures used in this study were approved by the Institutional Review Board of the Kiuchi Eye Clinic. The procedures used in this study conformed to the tenets of the Declaration of Helsinki. Written informed consent was obtained from all patients. All patients had blepharitis. Under 12× magnification of slit-lamp biomicroscopy, a total of 8 lashes were epilated randomly from the superior eyelids of both eyes by forceps and placed separately on the center of a glass microscope slide. Each eyelash was covered with a coverslip without adding any solution and examined by a conventional optical microscope. Later, one drop of the Löffler’s alkaline methylene blue solution was placed at the edge of the coverslip, and the preparation examined by a conventional optical microscope immediately. The number of Demodex mites was counted by the same examiner first without and then with the methylene blue solution.
Wilcoxon tests were used to determine the significance of the differences of the number of Demodex mites before and after adding the Löffler’s alkaline methylene blue solution.
Results
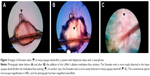
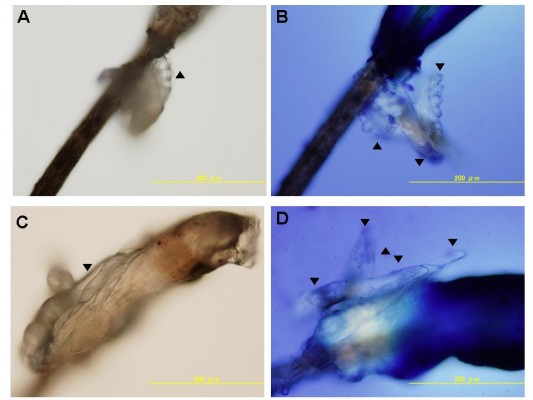
Photographs of Demodex mites attached to the eyelashes of a patient with blepharitis are shown in Figure 1. The photographs of the eyelashes in Figure 1A and C were taken without any staining, and the photographs in Figure 1B and D were taken after staining with the Löffler’s alkaline methylene blue solution. The Demodex mites are easier to detect with the blue background.
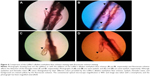
The Demodex mites were also more easily detected in heavy opaque dandruff (▼; Figure 2) after the methylene blue staining. The Demodex count before the addition of the methylene blue solution was 2.9±2.9, and it was 4.4±3.9 (± SD) after the methylene blue staining (P<0.01; Figure 3).
The results of our study showed that adding the Löffler’s alkaline methylene blue solution and fluorescein solutions significantly improved the contrast of Demodex mites in the epilated eyelashes. The better detection was due to the blue background provided by the Löffler’s alkaline methylene blue solution and yellow background provided by fluorescein solution (Figure 4).
In 1 of the 22 cases of blepharitis, Demodex mites could not be detected (Case 1), and in 7 cases (Cases 2–8), the number of Demodex mites detected was the same with and without the methylene blue staining. However, in 14 cases (Cases 9–22), the Demodex mites could be detected better after methylene blue staining than without any staining (Table 1).
Discussion
Blepharitis generally has a good prognosis and responds well to antibacterial eye ointments or adrenal steroid-containing eye ointments in many cases. However, cases with severe blepharitis are resistant to these ophthalmic ointments, and it is possible that one of the causes of the infections is Demodex mites.
There are many reports on the treatments of Demodex infections. For example, the application of 4% pilocarpine HCL gel,1,10 tea tree oil,2,4,6,11 cleansing with baby shampoo,10 antibiotic ointment,5 and oral ivermectin in combination with topical permethrin have been reported to eliminate the Demodex mites.7 In many cases, the number of Demodex mites count is evaluated by direct microscopic examination of the epilated eyelashes without adding any solution. Kheirkhah et al8 reported that a more accurate count of the number of Demodex mites was obtained after the addition of fluorescein solution than without adding any solution. This was probably because of the yellow background provided by fluorescein solution improved the contrast.
The results of our study showed that adding the Löffler’s alkaline methylene blue solution significantly increased the number of Demodex mites counted in the epilated eyelashes. This capability coupled with the blue background provided by the Löffler’s alkaline methylene blue solution allowed a better visibility of the Demodex mites. Because eyelashes, Demodex mites, epithelial tissues, and eye discharges can be distinguished by their different colors, using the Löffler’s alkaline methylene blue solution was probably the basis for detecting the Demodex mites more clearly than with the fluorescein solution (Figure 4).
One of the drawbacks of this procedure was that it was not possible to compare the results of the different types of stains, for example, fluorescein, methylene blue, and oil drops. In addition, the slides could not be preserved because the eyelashes were not fixed. However, as shown in Figure 2, digital images can be made with smartphones which will allow long-term preservation of the images.12 These images can also be shown to a patient to obtain informed consent for treatments.13 Further studies are required to compare the results of Demodex counting using the Löffler’s alkaline methylene blue solution to those with other methods with the highest yield for Demodex detection in eyelashes of the patients with blepharitis.
Conclusion
In conclusion, the use of the Löffler’s alkaline methylene blue solution is simple and can be used for an easier detection of Demodex mites. It can be used not only for the diagnosis but also for the evaluation of the therapeutic effects of the treatment.
Disclosure
The author reports no conflicts of interest in this work.
References
Türk M, Öztürk I, Şener AG, Küçükbay S, Afşar I, Maden A. Comparison of incidence of Demodex folliculorum on the eyelash follicule in normal people and blepharitis patients. Türkiye Parazitol Derg. 2007;31(4):296–297. | ||
Koo H, Kim TH, Kim KW, Wee SW, Chun YS, Kim JC. Ocular surface discomfort and Demodex: effect of tea tree oil eyelid scrub in Demodex blepharitis. J Korean Med Sci. 2012;27(12):1574–1579. | ||
Kojima T, Ishida R, Sato EA, et al. In vivo evaluation of ocular demodicosis using laser scanning confocal microscopy. Invest Ophthalmol Vis Sci. 2011;52(1):565–569. | ||
Randon M, Liang H, El Hamdaoui M, et al. In vivo confocal microscopy as a novel and reliable tool for the diagnosis of Demodex eyelid infestation. Br J Ophthalmol. 2015;99(3):336–341. | ||
Kawakita M, Kawashima M, Ibrahim O, Murato D, Tsubota K. [Demodex-related marginal blepharitis in Japan]. Nippon Ganka Gakkai Zasshi. 2010;114(12):1025–1029. Japanese. | ||
Nicholls SG, Oakley CL, Tan A, Vote BJ. Demodex treatment in external ocular disease: the outcomes of a Tasmanian case series. Int Ophthalmol. 2016;36:691–696. | ||
Damian D, Rogers M. Demodex infestation in a child with leukaemia: treatment with ivermectin and permethrin. Int J Dermatol. 2003;42(9):724–726. | ||
Kheirkhah A, Blanco G, Casas V, Tseng SC. Fluorescein dye improves microscopic evaluation and counting of Demodex in blepharitis with cylindrical dandruff. Cornea. 2007;26(6):697–700. | ||
Kiuchi K. Rapid alkaline methylene blue supravital staining for assessment of anterior segment infections. Clin Ophthalmol. 2016;10:1971–1975. | ||
Inceboz T, Yaman A, Over L, Ozturk AT, Akisu Ç. Diagnosis and treatment of demodectic blepharitis. Türkiye Parazitol Derg. 2009;33(1):32–36. | ||
Gao YY, Di Pascuale MA, Li W, et al. In vitro and in vivo killing of ocular Demodex by tea tree oil. Br J Ophthalmol. 2005;89(11):1468–1473. | ||
Roy S, Pantanowitz L, Amin M, et al. Smartphone adapters for digital photomicrography. J Pathol Inform. 2014;5(1):24. | ||
Kimura M, Enoki E, Maenishi O, Ito A, Chikugo T. [Capture of histopathological images by medical students using the digital cameras of cell phones and smart phones during histopathology classes]. Igakukyouiku. 2013;44(2):85–87. Japanese. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


 ; A and C, respectively). After addition of the Löffler’s alkaline methylene blue solution, 3 and 5 Demodex mites can be seen on the eyelash (
; A and C, respectively). After addition of the Löffler’s alkaline methylene blue solution, 3 and 5 Demodex mites can be seen on the eyelash ( ; B and D, respectively). The Demodex mites are easily detected on the blue background of the methylene blue dye. Photographs taken by conventional optical microscope magnification at 200×.
; B and D, respectively). The Demodex mites are easily detected on the blue background of the methylene blue dye. Photographs taken by conventional optical microscope magnification at 200×.